Abstract
Background
The purpose of this study was to establish the repeatability of left-ventricular (LV) dyssynchrony and function parameters measured from serial gated myocardial perfusion SPECT (GMPS) studies.
Methods
Thirty patients, who met standard criteria for cardiac resynchronization therapy (CRT), were prospectively enrolled. One hour after resting injection, a standard GMPS was performed, and repeated 30 minutes later after repositioning the patient. The two serial studies were processed blinded from each other by an experienced operator, and processed side-by-side by another experienced operator using iterative reconstruction, Butterworth filtering, and the Emory Cardiac Toolbox with phase analysis. Phase standard deviation, phase histogram bandwidth, LV ejection fraction, end-systolic volume, and end-diastolic volume were calculated and compared.
Results
All measured parameters were highly correlated (r > .90) between the serial studies without significant difference by paired t test. The variations of the parameters measured by side-by-side processing were significantly smaller than those measured by blinded processing.
Conclusion
These results indicated high repeatability of LV dyssynchrony and function parameters when measured serially by GMPS, especially when the serial studies were processed side-by-side. The measured variations of these parameters can be used to evaluate changes in LV dyssynchrony and function measured by GMPS before and after CRT.
Keywords: Phase analysis, left-ventricular dyssynchrony, left-ventricular function, gated myocardial perfusion SPECT
INTRODUCTION
Cardiac resynchronization therapy (CRT) has been shown to improve quality of life, functional class, exercise capacity, left ventricular ejection fraction (LVEF), and survival probability in selected patients with LV systolic dysfunction and symptomatic heart failure. Based on prior clinical trials, criteria for CRT consist of NYHA class III-IV heart failure, LVEF <35%, and QRS duration on the surface electrocardiogram (ECG) >120 ms.1-6 However, when selected based on the above criteria, up to 40% of CRT recipients do not respond.7 Therefore, studies have explored the utility of directly measuring LV mechanical dyssynchrony in order to optimize patient selection for CRT.8-10
Tissue Doppler Imaging (TDI) has been used to measure LV dyssynchrony and predict CRT response.11-13 A recent study has shown that serial assessment of LV mechanical dyssynchrony by Tissue Doppler Imaging (TDI) before and after CRT can be used to predict long-term outcome.14 However, the measurement variability in echo-based parameters of dyssynchrony was very high when tested in a recent multi-center PROSPECT trial (Predictors of Response to Cardiac Resynchronization Therapy).15
Phase analysis of gated myocardial perfusion SPECT (GMPS) (SyncTool™, Emory University, Atlanta, GA, USA) has been used for the assessment of LV dyssynchrony.16 This technique approximates the change of LV wall thickness using continuous Fourier harmonic functions so that it has sufficient temporal resolution to analyze LV dyssynchrony.17 This technique was shown to appropriately discriminate between normal controls and various patient cohorts (left bundle branch block, right bundle branch block, ventricular paced rhythms, and LV dysfunction with LVEF <40%) who were expected on average to have different degrees of LV dyssynchrony.18 Moreover, phase analysis showed good correlation with TDI in a study of 75 heart failure patients,19 and good sensitivity and specificity (>70%) for the prediction of response to CRT in another study of 42 heart failure patients.20 Importantly, serial assessment of LV dyssynchrony before and immediately after CRT by phase analysis of GMPS using a single injection of radiotracer has been shown feasible and is likely to provide valuable clinical information.21 A previous study has shown high reproducibility (the variation of a measurement by a single operator in different times or by different operators) of phase analysis, which indicated that different operators with different image processing settings could obtain highly correlated (r > .95) LV dyssynchrony parameters from the same GMPS studies.22 However, the repeatability (the variation of successive measurements) of phase analysis has not yet been evaluated. The variability in these parameters is expected to be higher between serial GMPS studies than when re-processing the same GMPS studies. Determining the limits of variability in the LV dyssynchrony and function parameters between serial GMPS studies is essential, if serial GMPS assessment of LV dyssynchrony and function is used to assess CRT response. Accordingly, the purpose of this study was to determine the repeatability of the LV dyssynchrony and function parameters, which characterizes the variation of these parameters when measured using similar image processing settings from serial GMPS studies.
MATERIALS AND METHODS
Patient Studies
Thirty patients were prospectively enrolled in this study from the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China. These patients were hospitalized because of severe heart failure symptoms. Table 1 shows the characteristics of the patients when they were hospitalized. All patients were in NYHA III or IV heart failure, LVEF <35%, and QRS >120 ms, eligible for CRT based on conventional criteria. After hospitalization and medical treatment, 8 of the 30 patients had improved cardiac function. When they were discharged, they were in NYHA class II heart failure with slightly improvement in LVEF. The GMPS studies were performed on these patients during hospitalization.
Table 1.
Clinical characteristics of the study population (n = 30)
| Age (yrs) | 60.5 ± 18.2 |
| Men | 14 (46.7%) |
| Ischemic cardiomyopathy | 10 (33.3%) |
| Previous infarction | 6 (20%) (4 anterior, 2 inferior) |
| Idiopathic dilated cardiomyopathy | 20 (66.7%) |
| Previous infarction | 6 (20%) |
| QRS duration (ms) | 145 ± 14 |
| Echo LVEF (%) | 29.1 ± 3.6 |
| NYHA class | 3.43 ± .50 |
Data are presented as mean ± standard deviation, or as number (%).
LVEF, Left ventricular ejection fraction; NYHA, New York Heart Association.
A protocol using a single resting injection of Tc-99m sestamibi, followed by two serial resting GMPS scans performed 30 minutes apart was used. One hour after resting injection of 25 mCi of Tc-99m sestamibi, a standard GMPS resting scan was acquired using a GE Infinia system with low-energy high-resolution collimators. The patient was then taken off the table. The scan was repeated 30 minutes later after repositioning the patient. For both scans, images were acquired over a 180° noncircular orbit from 45° right anterior oblique to 45° left posterior oblique, with 30 seconds per projection, 60 projections, 64 × 64 matrix, and 140 keV ± 20% energy window for emission images. No attenuation correction was performed.
Image Processing
All patient studies were reconstructed by ordered subsets expectation maximization (OSEM) with 3 iterations and 10 subsets. A Butterworth filter with a cutoff frequency of .4 cycles · cm-1 and a power of 10 was used to filter the summed images. For gated images, the cutoff frequency of the Butterworth filter was reduced to .35 cycles · cm-1, as the gated images contained more noise than the summed images. Image reconstruction was totally automatic. The reconstructed images were submitted to oblique reorientation (adjusting centers and angles of the LV to generate gated short-axis images) and LV region-of-interest (ROI) determination (determining LV center, radius, apex slice, and base slice in the Emory Cardiac Toolbox). Oblique reorientation and LV ROI determination involved manual image processing depending on the operator's preference.
Two image processing settings, which represented typical clinical settings, were evaluated. The first setting was independent processing, where an experienced operator processed the two serial GMPS studies separately in different weeks. The operator was blinded from his first processing when he processed the second study. This setting represented a typical clinical setting to compare two GMPS studies that were processed independently. The second setting was side-by-side processing, where another experienced operator processed the two serial GMPS studies together (one as “stress” and the other as “rest”, similar to a typical stress/rest GMPS scan) to minimize potential inconsistency in oblique reorientation and LV ROI determination between the two serial GMPS studies. This setting represented a desirable clinical setting to compare two GMPS studies after re-alignment.
Once the images were processed, LV dyssynchrony [phase standard deviation (PSD) and phase histogram bandwidth (PHB)] and function parameters [LVEF, LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV)] were calculated by the Emory Cardiac Toolbox, and then compared between the two serial GMPS studies.
Statistical Analysis
The LV dyssynchrony and function parameters between the two serial GMPS studies were compared using the paired t test and Pearson's correlation analysis.
The differences in LV dyssynchrony and function parameters between the two serial GMPS studies were expected to be normally distributed. No significant differences in these parameters were expected between the serial images of the same patients. Therefore, the mean (μ) and standard deviation (σ) of the differences in these parameters should represent the measurement variability. The variability of the LV dyssynchrony and function parameters measured by independent processing and side-by-side processing was compared using the F test.
Once μ and σ are determined, changes in LV dyssynchrony and function (for example, due to CRT) on serial GMPS studies in any particular patient can be evaluated by one-sample z test as follows.
| (1) |
where x is the difference in LV dyssynchrony and function parameters of the patient between the two serial studies. With the z score beyond ± 1.645 or ± 1.96, there will be 95% confidence (based on two-side or one-side z test, respectively) that significant changes in LV dyssynchrony and function have occurred.
RESULTS
The measured differences in the LV dyssynchrony and function parameters between the serial GMPS studies are shown in Table 2 (independent processing) and Table 3 (side-by-side processing). Paired t test showed no significant difference in any parameter between the serial studies. The measured parameters were highly correlated between the serial studies. The standard deviations of difference in PSD and PHB were significantly (P < .01 for all parameters by the F test) smaller by side-by-side processing than by independent processing, indicating that inconsistent oblique reorientation and LV ROI determination between the serial GMPS studies introduced a considerable amount of variation in the LV dyssynchrony and function parameters. The coefficients of variability of the LV dyssynchrony and function parameters were calculated for each patient as the standard deviation of the measured parameters divided by the mean of the measured parameters. The average coefficients of variability in the 30 patients were also improved from about 20% to less than 10% for all LV dyssynchrony and function parameters when side-by-side processing was used instead of independent processing. These results indicated high repeatability of LV dyssynchrony and function measurement when using a side-by-side image processing setting to align the serial GMPS studies.
Table 2.
Differences in LV dyssynchrony and function parameters between independently processed serial gated myocardial perfusion SPECT studies
| N = 30 | PSD | PHB | LVEF | LVESV | LVEDV |
|---|---|---|---|---|---|
| μ | 1.79° | .00° | –.03% | 3.10 mL | 5.23 mL |
| σ | 10.24° | 28.55° | 5.14% | 13.06 mL | 17.96 mL |
| P | .347 | .988 | .972 | .204 | .121 |
| r | .915 | .920 | .970 | .989 | .982 |
| CV | 22.9% | 20.0% | 9.4% | 8.5% | 13.5% |
PSD, Phase standard deviation; PHB, phase histogram bandwidth; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume.
μ = mean difference, σ = standard deviation of the difference, P < .05 = statistical significance by paired t test, r = correlation coefficient.
CV = average coefficient of variability. Coefficient of variability for each patient study was defined as the standard deviation of the measurements divided by the mean of the measurements. Here, CV is the average coefficient of variability in all 30 patients.
Table 3.
Differences in LV dyssynchrony and function parameters between side-by-side processed serial gated myocardial perfusion SPECT studies
| N = 30 | PSD | PHB | LVEF | LVESV | LVEDV |
|---|---|---|---|---|---|
| μ | .58° | 2.03° | –.37% | 2.20 mL | 2.73 mL |
| σ | 5.11° | 13.77° | 3.92% | 8.42 mL | 10.94 mL |
| P | .541 | .425 | .612 | .163 | .182 |
| r | .979 | .976 | .982 | .996 | .994 |
| CV | 8.8% | 8.7% | 9.2% | 8.7% | 5.4% |
PSD, Phase standard deviation; PHB, phase histogram bandwidth; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume.
μ = mean difference, σ = standard deviation of the difference, P < .05 = statistical significance by paired t test, r = correlation coefficient.
CV = average coefficient of variability. Coefficient of variability for each patient study was defined as the standard deviation of the measurements divided by the mean of the measurements. Here, CV is the average coefficient of variability in all 30 patients.
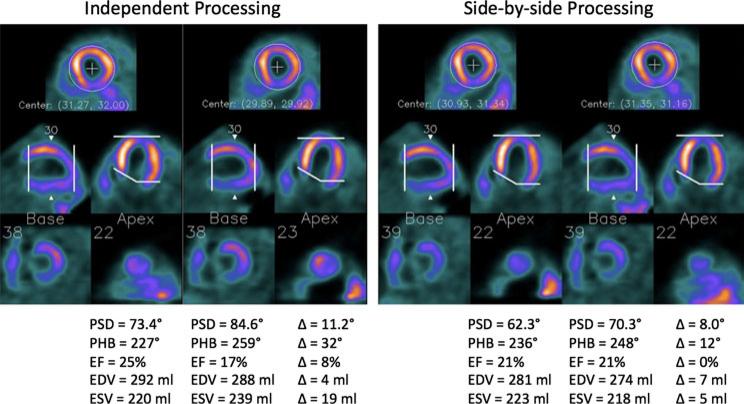
Figure 1 shows the two serial GMPS studies of a patient example by independent and side-by-side processing. The two serial GMPS studies of the patient, when processed independently, had inconsistent oblique reorientation (different orientation of the horizonal long-axis images) and LV ROI determination (different apical slice selection). Such inconsistency resulted in higher variation in the LV dyssynchrony and function parameters than that obtained by side-by-side processing, which better aligned the two images and matched the apical and basal slice selection.
Figure 1.
Two serial GMPS studies of a patient example by independent and side-by-side processing. The two serial GMPS studies of the patient, when processed independently in different weeks, had inconsistent oblique reorientation and apical slice selection. Such inconsistency resulted in higher variation in the LV dyssynchrony and function parameters than that given by side-by-side processing, which better aligned the two images and matched the apical and basal slice selection.
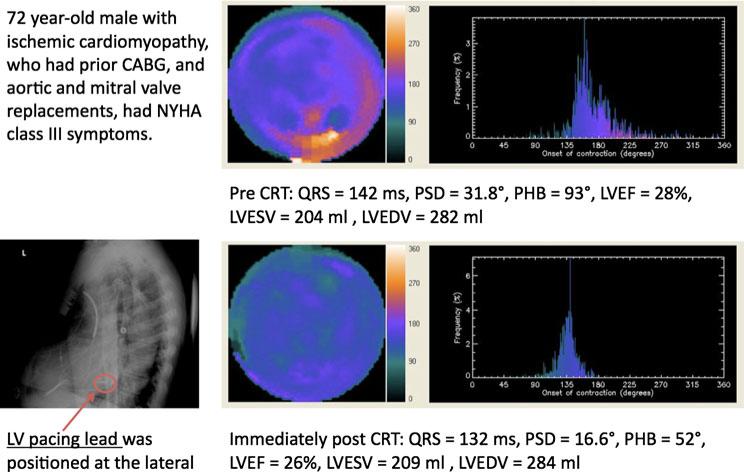
Figure 2 shows an example of using serial GMPS to assess patient response to CRT. A 72-year-old male with ischemic cardiomyopathy underwent CRT for NYHA class III symptoms, severe LV dysfunction, and wide QRS duration. The upper and lower panels show the phase analysis results from the pre- and post-CRT scans, respectively. Left ventricular (LV) dyssynchrony parameters significantly improved following CRT with z scores of –3.09 and –3.12 for phase standard deviation (PSD) and phase histogram bandwidth (PHB), respectively. Comparison of the phase polar maps before and after CRT shows that phase delay was reduced remarkably after CRT in the lateral wall, where the LV pacing lead was positioned. LV function parameters did not change significantly after CRT with z scores of –.42, .33, and –.07 for LV ejection fraction (LVEF), LV endsystolic volume (LVESV), and LV end-diastolic volume (LVEDV), respectively.
Figure 2.
Serial assessment of LV dyssynchrony and function using phase analysis of GMPS before (upper panel) and after (lower panel) CRT. Left ventricular (LV) dyssynchrony parameters significantly improved after CRT with z scores of –3.09 and –3.12 for phase standard deviation (PSD) and phase histogram bandwidth (PHB), respectively, based on the measured variability of these parameters in this study. Comparing the phase polar maps before and after CRT, it was observed that phase delay was reduced remarkably after CRT in the lateral wall, where the LV pacing lead was positioned. LV function parameters changed insignificantly after CRT with z scores of –.42, .33, and –.07 for LV ejection fraction (LVEF), LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV), respectively, based on the measured variability of these parameters in this study.
DISCUSSION
This study evaluated the repeatability of LV dyssynchrony and function measurement using serial GMPS studies of 30 heart failure patients who were eligible for CRT. The measured LV dyssynchrony and function parameters were highly correlated between the serial GMPS studies. The limits of variability of these parameters were established in this study, so that it can be applied to serial GMPS evaluation of CRT response. This study also showed that inconsistent oblique reorientation and LV ROI determination introduced a considerable amount of variation in the LV dyssynchrony and function parameters when comparing the two serial GMPS studies. Thus, processing serial GMPS studies side-by-side is desirable, since this approach will reduce variation resulting from image processing, and it can measure smaller changes in LV dyssynchrony and function in serial GMPS studies.
A recent study using serial assessment of LV dyssynchrony with TDI before and after CRT in 100 heart failure patients showed that acute improvement in TDI parameters predicted a sustained response with 100% negative predictive value and 95% positive predictive value.14 While these data provided a basis for the measurement of changes in dyssynchrony parameters soon after CRT, it must be noted that the use of echocardiography parameters for this purpose has been limited by high variability in measurement, when applied in large, multicenter clinical trials.15 Our data indicated much smaller variability in the LV dyssynchrony parameters measured by phase analysis of GMPS, even when the variability was derived from two separate, serial GMPS studies. If proven in larger multicenter studies, this will be an advantage of the phase analysis approach to dyssynchrony measurement, compared to echocardiography.
It must be noted that the limits of variability derived from this study pertain to serial GMPS using identical acquisition parameters. These limits would, therefore, be not applicable if the acquisition parameters differed between studies. Similarly, these limits of variability cannot be extrapolated to patients with normal LV function.
CONCLUSION
Serial GMPS measurements of LV dyssynchrony and function in CRT-eligible heart failure patients are highly repeatable when acquisition and processing parameters are carefully controlled. The derived limits of variability could be used to evaluate changes in LV dyssynchrony and function following CRT.
Acknowledgment
This study was supported in part by an NIH-funded research project (1R01HL094438-01A1; PI: Ji Chen, PhD), and an American Society of Nuclear Cardiology Foundation grant (PI: Prem Soman, MD). Several authors (Chen, Garcia, and Folks) receive royalties from the sale of the Emory Cardiac Toolbox. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict-of-interest practice.
References
- 1.Abraham WT, Hayes DL. Cardiac resynchronization therapy for heart failure. Circulation. 2003;108:2596–603. doi: 10.1161/01.CIR.0000096580.26969.9A. [DOI] [PubMed] [Google Scholar]
- 2.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, Boehmer JP, Higginbotham MB, De Marco T, Foster E, Yong PG. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–9. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 3.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 4.McAlister F, Ezekowitz J, Wiebe N, Rowe B, Spooner C, Crumley E, Hartling L, Kaul P, Nichol G, Klassen T. Cardiac resynchronization therapy for congestive heart failure. Evid Rep Technol Assess (Summ) 2004;106:1–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 7.Leclercq C, Kass DA. Retiming the failing heart: Principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol. 2002;39:194–201. doi: 10.1016/s0735-1097(01)01747-8. [DOI] [PubMed] [Google Scholar]
- 8.Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 9.Brecker SJ, Xiao HB, Sparrow J, et al. Effects of dual-chamber pacing with short atrioventricular delay in dilated cardiomyopathy. Lancet. 1992;340:1308–12. doi: 10.1016/0140-6736(92)92492-x. [DOI] [PubMed] [Google Scholar]
- 10.Leclercq C, Faris O, Tunin R, et al. Systolic Improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–3. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 11.Achilli A, Sassara M, Ficili S, et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42:2117–24. doi: 10.1016/j.jacc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–40. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, van Erven L, Boersma E, et al. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol. 2003;92:1238–40. doi: 10.1016/j.amjcard.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Bleeker GB, Mollema SA, Holman ER, Van d, Ypenburg C, Boersma E, et al. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy: Analysis in patients with echocardiographic evidence of left ventricular dys-synchrony at baseline. Circulation. 2007;116:1440–8. doi: 10.1161/CIRCULATIONAHA.106.677005. [DOI] [PubMed] [Google Scholar]
- 15.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, Iskandrian AE. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–95. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Faber TL, Cooke CD, Garcia EV. Temporal resolution of multi-harmonic phase analysis of ECG-gated myocardial perfusion SPECT studies. J Nucl Cardiol. 2008;15:383–91. doi: 10.1016/j.nuclcard.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trimble MA, Smalheiser S, Bornes-neto S, Chen J, et al. Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging in patients with left ventricular dysfunction and conduction disturbances. J Nucl Cardiol. 2007;14:298–307. doi: 10.1016/j.nuclcard.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 19.Henneman MM, Chen J, Ypenburg C, Dibbets P, Stokkel M, van der Wall EE, Garcia EV, Bax JJ. Phase analysis of gated myocardial perfusion SPECT compared to tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–14. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 20.Henneman MM, Chen J, Dibbets P, Stokkel M, Bleeker GB, Ypenburg C, van der Wall EE, Schalij MJ, Garcia EV, Bax JJ. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104–11. doi: 10.2967/jnumed.107.039925. [DOI] [PubMed] [Google Scholar]
- 21.Friehling M, Chen J, Saba S, Garcia EV, Adelstein E, Martin L, Follansbee WP, Soman P. Feasibility of a novel protocol for serial assessment of left ventricular synchrony by gated SPECT before and after cardiac resynchronization therapy with a single injection of radiotracer (abstract). J Nucl Cardiol. 2009;16:675. [Google Scholar]
- 22.Trimble MA, Velazquez EJ, Adams GL, Honeycutt EF, Pagnanelli RA, Barnhart HX, Chen J, Iskandrian AE, Garcia EV, Borges-Neto S. Repeatability and reproducibility of phase analysis of gated SPECT myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun. 2008;29:374–81. doi: 10.1097/MNM.0b013e3282f81380. [DOI] [PMC free article] [PubMed] [Google Scholar]