Abstract
The behavioral sensitization produced by repeated amphetamine treatment may represent the neural adaptations underlying some of the features of psychosis and addiction in humans. Chromatin modification (specifically histone hyperacetylation) was recently recognized as an important regulator of psychostimulant-induced plasticity. We have investigated the effects of treatment with the histone deacetylase (HDAC) inhibitors butyric acid (BA, 630 mg/kg, i.p) and valproic acid (VPA, 175 mg/kg, i.p.) on the psyhcostimulant locomotor sensitization induced by amphetamine (AMPH, 2.0 mg/kg, i.p.). Neither BA nor VPA had locomotor effects alone, but both significantly potentiated the amphetamine-induced behavioral sensitization in mice. At the molecular level, VPA and amphetamine produced an increase of histone H4 acetylation in the striatum as detected by Western blot analysis, while co-treatment with VPA and AMPH produced an additive effect on histone H4 acetylation. We then administered the HDAC inhibitors after treatment with amphetamine for 8 days to establish locomotor sensitization. We found that repeated administration of VPA or BA for 6 days inhibited the expression of sensitized response following amphetamine challenge. Finally, in a context-specific model we studied the effect of HDAC inhibitors on amphetamine-induced association of the treatment environment (associative learning). We found that VPA and BA enhance the context-specificity of expression of amphetamine sensitization. Thus, HDAC inhibitors differentially modulate the induction and expression of amphetamine-induced effects. Together, these results suggest that dynamic changes in chromatin modification may be an important mechanism underlying amphetamine-induced neuronal plasticity and associative learning.
Keywords: Behavioral sensitization, Associative learning, Histone deacetylase inhibitor, Chromatin modification, Amphetamine
1. Introduction
Repeated administration of psychostimulants such as amphetamine or cocaine, induces an enhanced behavioral response to subsequent drug exposure, a phenomenon known as behavioral sensitization that can persist for months [33,35]. Psychostimulant-induced behavioral sensitization in rodents provides a model of addictive behaviors such as those associated with craving and relapse, as well as for psychotic complications of psychostimulant abuse [18,34,35]. Neural sensitization itself appears to be a non-associative process, but contextual learning powerfully modulates both the induction and the expression of behavioral sensitization [35]. There are two learning processes by which psychostimulant behavioral sensitization can be modified: (i) An occasion-setting mechanism [2,37] can prevent the expression of behavioral sensitization in contexts in which the drug is not expected. (ii) Excitatory Pavlovian associations can increase drug-induced psychomotor response when animals are placed in environments where the drug is expected [2,38,39]. These two associative processes may combine to modulate the expression of sensitization [2,3].
Persistent behavioral sensitization after repeated psychostimulant treatment indicates that drug-induced short- and long-term changes in gene expression may be involved [28,29]. The transcription factors are among the relatively short-term molecular adaptations associated with the reward behavioral and psychostimulant sensitization. However, an additional mechanism such as chromatin modification involving histone acetylation is increasingly recognized as an important regulator of gene expression [17]. The acetylation state of histones is determined by the action of two families of enzymes: histone acetyltransferase (HAT), which catalyzes the transfer of an acetate group onto the histone tail, and histone deacetylase (HDAC), which catalyzes the removal of these acetates. Acetylation and deacetylation of core histone tails have been associated primarily with transcriptional activation and gene silencing, respectively [11]. Several studies suggest that dynamic histone acetylation and deacetylation processes are also active in postmitotic neurons [12, 20, 21, 27]. Recently, it has been shown that both acute and chronic cocaine administration are able to induce specific histone modification at different gene promoters in the striatum [6,20,22]. Furthermore, treatment with HDAC inhibitors alter locomotor and rewarding responses to cocaine [20].
We hypothesized that dynamic changes in chromatin modification (histone acetylation/deacetylation) contribute both to psychostimulant-induced molecular neuroplasticity and associative learning. The aim of our study was to evaluate the effects of HDAC inhibitors (valproic acid and butyric acid) (1) on the induction and maintenance/expression of behavioral sensitization, and (2) on associative learning of the testing environment that underlies amphetamine-induced sensitization.
2. Materials and Methods
2.1. Animal and drug treatments
All experiments were performed in accordance with NIH and EU guidelines (directive 86/609/EEC) on the ethical use of animals using the experimental protocol approved by the IACUC at Boston University Medical Centre. Male C57BL/6 mice (weight, 25–30 g) were obtained from the Charles River Laboratories (MA, USA) and from the National Animal Centre (Kuopio, Finland) and were maintained in temperature and humidity-controlled rooms with 12-h light-dark cycle (light from 7:00 am to 7:00 pm). Prior to behavioral testing (which was conducted during the light phase of the light-dark cycle), all mice were handled for 4 days. The mice were injected intraperitoneally (0.1 ml/10 g body weight) with d-amphetamine sulphate (2.0 mg/kg), sodium valproate (175 mg/kg), and sodium butyrate (630 mg/kg) (Sigma, MO). The induction of behavioral sensitization (a co-treatment paradigm) was performed in US and repeated in Estonia. The expression of behavioral sensitization was performed only in US. A context-specific study was performed in Estonia. Western blott analyses was done in US.
2.2. Locomotor activity
Horizontal locomotor activity was assessed in standard polypropylene cages (15 × 25 cm) placed into adjustable frames equipped with five infrared photocell beams (San Diego Instruments, CA) that traverse each cage in a plane above its floor. Ambulation (sequential breaks in two adjacent beams) was recorded and analysed on a computer [7].
2.3. Co-treatment of HDAC inhibitors with amphetamine during the induction phase
For the co-treatment paradigm, mice were treated with butyric acid (BA) or valproic acid (VPA) or saline (SAL) 15 min prior to amphetamine (AMPH) or saline treatment. Mice were randomly assigned into one of the following four treatment groups: (1) SAL+SAL; (2) SAL+AMPH; (3) VPA or BA+SAL and (4) VPA or BA+AMPH. On days 1, 3, 5 and 8 of treatment the locomotor activity of animals was recorded 120 min after the treatment. On days 2, 4, 6 and 7 after treatment mice were left in the locomotor test environment for 120 min without the measurement of ambulation.
2.4. Apart treatment paradigm
For the apart treatment paradigm, mice were treated for 8 days with amphetamine (2.0 mg/kg, i.p.) and placed in the test cages for 120 min. Then, all mice were randomly assigned to the following three treatment groups: (1) SAL; (2) VPA (175 mg/kg); or (3) BA (630 mg/kg) and injected i.p. once a day, for 6 days. During the treatment session all mice were left for 120 min in the locomotor test environment. On day 15th, all groups were tested for locomotor activity for 120 min after challenge with amphetamine (2.0 mg/kg, i.p.).
2.5. Context-specific model
There were a total of sixteen groups in these experiments, which came from two types of pre-treatment groups: the Paired and Unpaired. Both pre-treatment groups were divided into following eight subgroups: (1) SAL+SAL, (2) SAL+AMPH, (3) VPA+SAL, (4) VPA+AMPH; and (5) SAL+SAL, (6) SAL+AMPH, (7) BA+SAL, (8) BA+AMPH. Animals in the Paired group were transported to the locomotor test environment, where they received the respective treatment. After the daily treatment the Paired mice were left in the locomotor test environment for 120 min and then returned to their home cages. Animals in the Unpaired group were transported from their home cages to the “Third world” environment, where they received treatments. We defined the “Third world” as standard polypropylene cages (15 × 25 cm) with wire lids, pine wood-shavings on the floor and located in a quiet room, distinct from the locomotor test environment. After treatment all mice returned to their home cages. These procedures for the Paired and Unpaired groups were repeated for 8 days, once a day.
Three days after the last pre-treatment day (day 12) the animals in both groups were transported to the locomotor test environment, where they received a single saline i.p. injection. Horizontal locomotor activity was recorded for 20 min after the saline treatment. Then all of the mice received a subsequent injection of amphetamine (2.0 mg/kg i.p.) and locomotor activity was recorded for another 120 min.
2.6. Western Immunoblotting
Mice were sacrificed 90 minutes after the last injection of amphetamine, HDAC inhibitor or saline. Striata were removed quickly in a +4°C room and stored at −80°C until processed. Nuclear proteins were isolated from striata as described earlier [41]. For Western blot, nuclear protein pellets (10–20 μg) were resuspended and electrophoresed in a 10–20% gradient Tris-glycine gel. After blotting, the membranes were incubated with antibodies against either histone H4 (#07-108 1:1,000, Upstate, NY) or its acetyl isoforms (#06-761 AcH4, 1:1,000, Upstate, NY), and finally developed by incubating with peroxidase-conjugated anti-rabbit antibody (1:5,000, Vector Laboratories, CA). Immunoblots were quantified using a UVP bioimaging acquisition and image analysis system. The optical density (OD) of the acetylated histone H4 immunoreactivity was normalized to that of the total histone H4 immunoreactivity, and was expressed as the ratio of acetylated histone H4 over total histone H4.
2.7. Statistical Analysis
Behavioral data of all experiments were analyzed using the parametric statistics with one-way ANOVA, or repeated two-way ANOVA followed by post hoc Bonferroni test, as appropriate. All data expressed as mean ± S.E.M., significance was set at p<0.05.
3. Results
3.1. The effects of HDAC inhibitors on histone H4 acetylation in striatum
The main goal of the present study was to assess the effects of HDAC inhibitors on amphetamine-induced behavioral sensitization. We first determined the doses for each HDAC inhibitor that would inhibit HDAC activity without affecting basal locomotor activity. We focused on histone H4 acetylation because our pilot studies showed that repeated treatment with VPA or amphetamine produced the most consistent changes in histone H4 acetylation compared to histone H3 acetylation (data not shown). Of note, the anti-acetylated histone H4 antibody in this study recognizes a single acetylation site on the H4 histone tail (Lysine 12) and repeated treatment with VPA and BA likely accumulates acetylated histone H4 associated with the increased number of gene promoter.
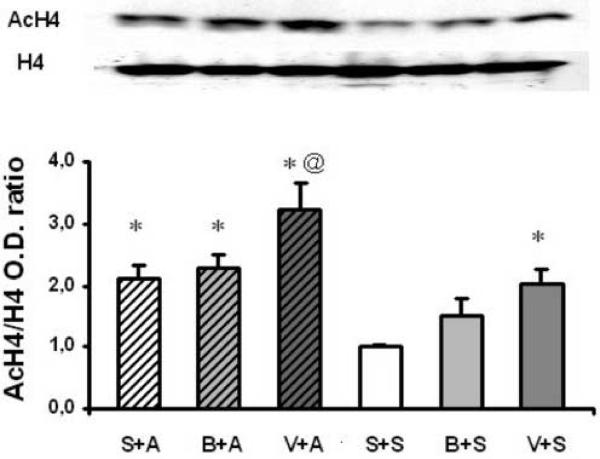
We first characterized hyperacetylation of histone H4 after treatment with VPA (175 mg/kg, i.p.), BA (630 mg/kg, i.p.) or amphetamine (2.0 mg/kg, i.p.) alone, or after combined treatment of amphetamine with VPA or BA. Following the repeated co-treatment paradigm for 8 days, VPA significantly enhanced acetylation of histone H4 in striatum as detected by Western blot (Figure 1, one-way ANOVA, compared VPA+SAL vs. SAL+SAL, Bonferroni post-test, p < 0.05). However, BA at the dose tested showed a trend to increase, but failed to produce statistically significant histone H4 hyperacetylation. Interestingly, repeated treatment with amphetamine significantly increased acetylation of histone H4 in striatum (compared SAL+AMPH vs. SAL+SAL one-way ANOVA, Bonferroni post-test, p < 0.05). Importantly, the combined treatment of VPA with amphetamine produced additive effects on histone H4 acetylation that was higher than that from the treatment with VPA or amphetamine alone (compared VPA+AMPH vs. SAL+AMPH or VPA+SAL, one-way ANOVA, Bonferroni post-test, p < 0.05). Thus, repeated treatment with VPA or amphetamine alone induces histone H4 hyperacetylation and co-treatment with VPA and amphetamine produces a additive effect on histone H4 acetylation in mouse striatum.
Figure 1.
Repeated treatment with valproic acid and amphetamine produce additive global increase of histone H4 acetylation in the striatum. Mice were injected (i.p.) with saline (SAL+SAL), amphetamine (2.0 mg/kg, SAL+AMPH), VPA (175 mg/kg, VPA+SAL), BA (630 mg/kg, BA+SAL) or co-administration with amphetamine and VPA (VPA+AMPH) or amphetamine and BA (BA+AMPH), daily for 8 days and sacrificed 90 minutes after the last injection at day 8. Histone H4 acetylation was analyzed by Western blot using antibodies specific for acetylated H4 histone. The immunoreactivity for acetylated histone H4 was normalized to total (non-acetylated and acetylated) histone H4 immunoreactivity. Upper panel show a representative blot of acetylated H4 histone (AcH4) and total histone (H4). Bar graph shows quantitative densitometric analysis of AcH4 after normalizing to total H4. Data are expressed as ratio of AcH4/H4 (O.D.) and represent as the mean ± S.E.M.; n = 6, * p<0.05 oneway ANOVA compared to the saline-treated group (S+S). Δ p<0.05, Student t-test compared the V+A group with the S+A or V+S group.
3.2. The effect of HDAC inhibitors on the induction of behavioral sensitization
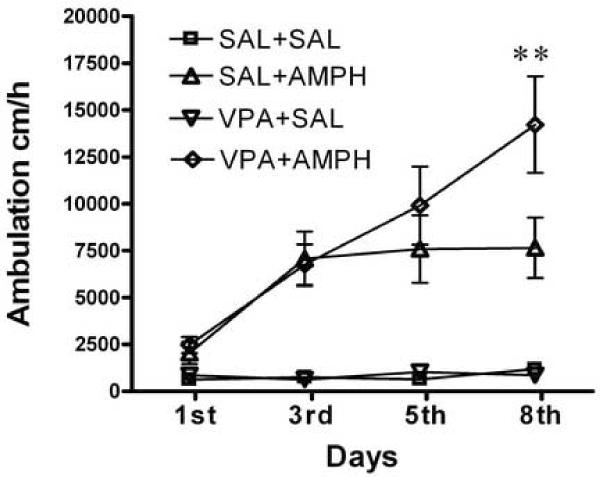
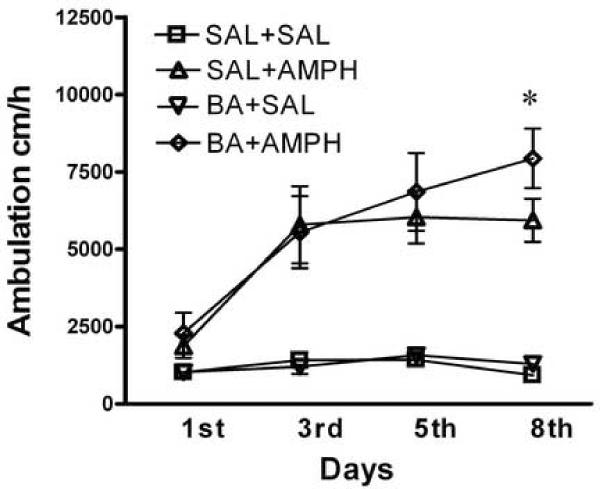
We next examined the effects of VPA and BA on the induction of amphetamine-induced behavioral sensitization. Mice were treated with amphetamine (2.0 mg/kg, i.p.) alone or co-treated with amphetamine and VPA (175 mg/kg, i.p.) or BA (630 mg/kg, i.p.) daily for 8 days, and their locomotor responses were monitored on the days 1, 3, 5 and 8 (Figure 2 A, B). On the first day, acute treatment with amphetamine increased locomotor activity, while acute administration of VPA or BA did not. Co-administration of amphetamine with VPA or BA did not significantly affect amphetamine-induced locomotor activity in mice on the first day compared to the amphetamine alone group. Following the daily injection of amphetamine (SAL+AMPH) for 7 days, mice displayed a nearly 3-fold increase in responses across days (repeated measures ANOVA, F=10.5, p < 0.0001, followed by Bonferroni post-test, 1st day vs. 8th day, p < 0.001), indicating a robust sensitization. The saline-treated mice (SAL+SAL) did not show any sensitization across test sessions (repeated measures ANOVA, F=2.5, p = 0.09), nor did the repeated treatment with BA or VPA alone (VPA+SAL repeated measures ANOVA, F=1.4, p = 0.26; BA+SAL, F=2.3, p = 0.12). However, repeated co-treatment with VPA and amphetamine induced behavioral sensitization (repeated measures ANOVA, F=11.6, p < 0.0001, followed by Bonferroni post-test, 1st vs. 8th day, p = 0.001). Importantly, the locomotor activity of the VPA+AMPH group was significantly higher than that of the SAL+AMPH mice on the 8th day (two-way ANOVA with repeated measurments, treatment F3,144 = 29.3, p < 0.0001, followed by Bonferroni post-test, p < 0.01). There was also significantly enhanced locomotor activity in mice co-treated with BA and AMPH compared with the SAL+AMPH group on the 8th day (Fig. 2 B, two-way ANOVA with repeated treatments, treatment F3,104 = 19.9, p < 0.0001, followed by Bonferroni post-test, p < 0.05). These co-treatment experiments were repeated twice in two lab settings and similar results were obtained. This suggests that repeated co-administration of HDAC inhibitors and amphetamine enhanced the induction of amphetamine sensitization in mice.
Figure 2.
Induction of behavioral sensitization. Mice were treated daily for 8 days and ambulation was recorded for 120 min immediately after treatment on day 1, 3, 5 and 8. Panel A: Mice were treated with saline SAL+SAL, amphetamine (2.0 mg/kg i.p.) SAL+AMPH, valproic acid (175 mg/kg i.p.) VPA+SAL, or valproic acid and amphetamine VPA+AMPH. Panel B: Mice were treated with saline SAL+SAL, amphetamine (2.0 mg/kg i.p.) SAL+AMPH, butyric acid (630 mg/kg i.p.) BA+SAL, or butyric acid and amphetamine BA+AMPH. Data expressed as mean±S.E.M, n=8–12 (A), n=9 (B). Two-way ANOVA with repeated treatment, followed by Bonferroni post-test. * p<0.05, BA+AMPH vs. SAL+AMPH, and ** p<0.01, VPA+AMPH vs. SAL+AMPH.
3.3. HDAC inhibitors effect on the expression of behavioral sensitization
Next, we studied the effect of VPA or BA on the expression of amphetamine-induced behavioral sensitization in an apart-treatment paradigm. All mice received daily treatment with amphetamine (2.0 mg/kg, i.p.), for 8 days. Then, mice were randomly divided into three groups and treated daily with saline or with VPA (175 mg/kg, i.p.) or BA (630 mg/kg, i.p.), for 6 days. On the day 15th all mice were challenged with amphetamine and the locomotor activity was estimated.
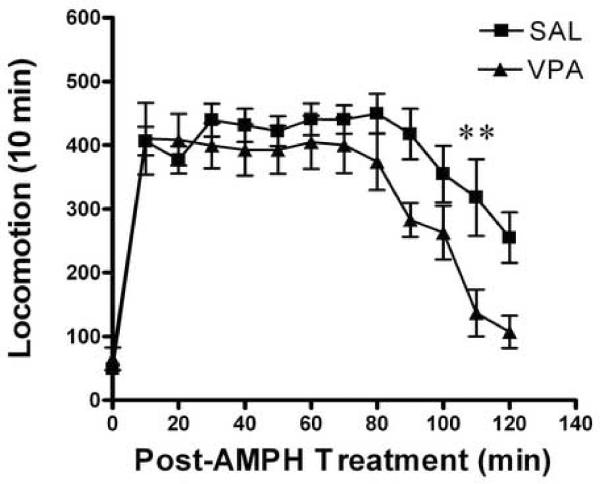
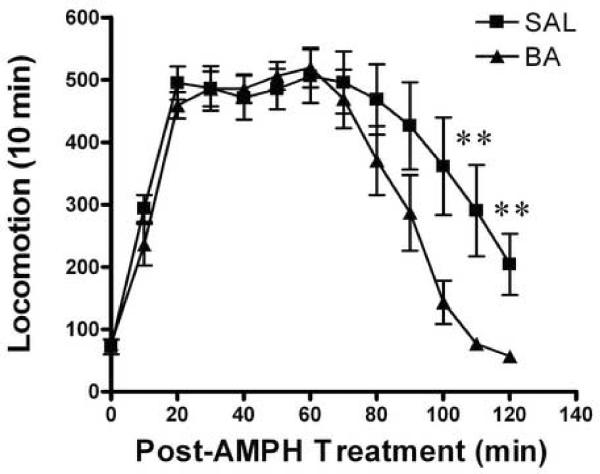
The time course of the locomotor response is depicted in Figure 3. There was a significant difference on locomotor responses between the saline and the VPA or BA treated mice after amphetamine challenge (SAL vs. VPA, two-way ANOVA with repeated measurment, treatment groups F1,156 = 17.62, p < 0.0001 and SAL vs. BA, treatment groups F1,182 = 17, p < 0.0001). The post-test analysis suggests that there was difference in the locomotor activity between the groups on the second hour after the amphetamine challenge (Bonferroni post-test, SAL vs. BA post-injection interval 90–110 min, p < 0.01; SAL vs. VPA post-injection interval 100–110 min, p < 0.01). These data indicate that after development of amphetamine sensitization, repeated VPA or BA treatment inhibits the expression of sensitized response to amphetamine.
Figure 3.
Expression of behavioral sensitization. All mice challenged with amphetamine (2.0 mg/kg, i.p.) on the day 15, following induction of sensitization for 8 days and after saline or VPA (175 mg/kg, i.p.) or BA (630 mg/kg, i.p.) daily treatment for 6 days. The locomotor activity was estimated for 120 min immediately after amphetamine challenge. Panel A: saline (SAL) or valproic acid (VPA) pretreated mice. Panel B: saline (SAL) or butyric acid (BA) pretreated mice. Data expressed as mean±S.E.M, n=7 (A), n=8 (B). ** p< 0.01, two-way ANOVA with repeated treatment, followed by Bonferroni post-test.
3.4. HDAC inhibitor effect on associative learning processes underlying amphetamine-induced sensitization
To evaluate whether HDAC inhibitors influence the amphetamine-associated learning processes, we tested the effect of HDAC inhibitors on amphetamine-induced association of the testing environment, a context-specific model. All mice were divided into two groups: Paired and Unpaired. Both groups were further divided into four subgroups: SAL+SAL, SAL+AMPH, VPA (or BA)+SAL, and VPA (or BA)+AMPH, and treated respectively for 8 days. For the Paired group, the environment for the amphetamine treatment and the locomotor test were same. In contrast, for the Unpaired group, the environment for amphetamine treatment was made in the “Third world”(see methods section) while the locomotor test was made in the regular “testing” environment. Specifically, after daily treatment the Paired mice were left in the locomotor “test” environment for 2 hours and then removed back to home cages. In contrast, the Unpaired mice were treated with amphetamine in the Third world and then immediately moved back to the home cages after treatment.
3.4.1. Saline challenge
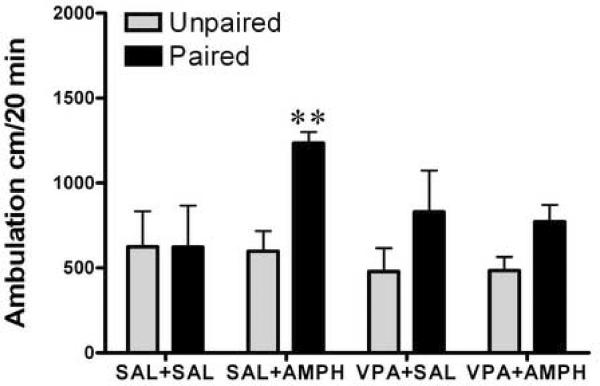
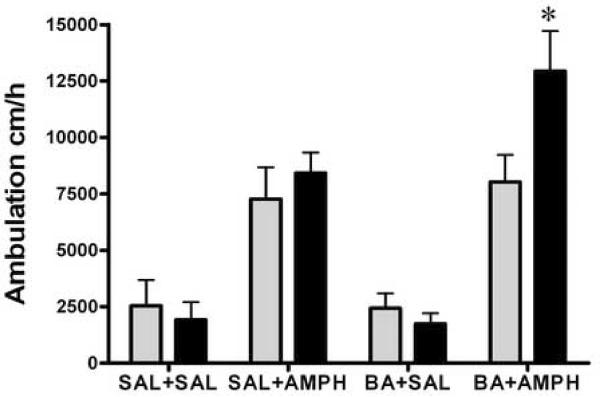
To study the effects of HDAC inhibitors on a facilitatory conditioned response we evaluated saline challenge response to the locomotor “testing” environment the Paired and Unpaired groups. After the induction of amphetamine sensitization for 8 days, both the Paired and Unpaired groups underwent a washout period for three days. This was followed (on the 12th day) by a 20-min saline challenge test in the locomotor test environment, which was novel to the Unpaired groups but not to the Paired groups. Figure 4 shows that, after the saline challenge, there was a significant difference in the locomotor activities between the Paired and Unpaired groups (Fig. 4 A, two-way ANOVA; treatment groups F3,72 =1.9, p = 0.13, Paired and Unpaired groups F1,72 =9.6, p = 0.003; interaction F3,72 =1.61, p = 0.19 and Fig. 4 B, treatment groups F3,50 =3.27, p < 0.029, Paired and Unpaired groups F1,50 =28, p < 0.0001, interaction F3,50 =2.7, p = 0.059). The Paired SAL+AMPH mice exhibited substantially larger locomotor activities in response to the saline injection in the test environment than the Unpaired SAL+AMPH group (Bonferroni post-tests p < 0.01). Interestingly, the Paired VPA+AMPH mice did not show an increase in the locomotor activity after the saline challenge, compared to the Unpaired VPA+AMPH mice. However, we found that while there was some reduction in locomotor response to the saline challenge in the Paired BA+AMPH group compared to the Unpaired group, there was still a significant difference in the BA+AMPH groups between the Unpaired- and Paired groups (Bonferroni post-tests p < 0.05). Thus, the facilitatory conditional response was absent in the Paired VPA+AMPH mice but not in the Paired BA+AMPH group after the saline challenge.
Figure 4.
Saline challenge test. Both the Unpaired and the Paired mice received an injection of saline and locomotion was estimated immediately for 20 min in the locomotion test environment, following daily treatment for 8 days with saline (SAL), amphetamine (AMPH, 2.0 mg/kg, i.p.), valproic acid (VPA, 175 mg/kg, i.p.), and VPA+AMPH, n=8–12 (Panel A); or butyric acid (BA, 630 mg/kg, i.p.), and BA+AMPH, n=6–9 (Panel B). Data expressed as mean±S.E.M. Two-way ANOVA followed by Bonferroni post-test, ** p< 0.01, SAL+AMPH Unpaired vs. Paired, and # p< 0.05, BA+AMPH Unpaired vs. Paired.
3.4.2. Amphetamine challenge
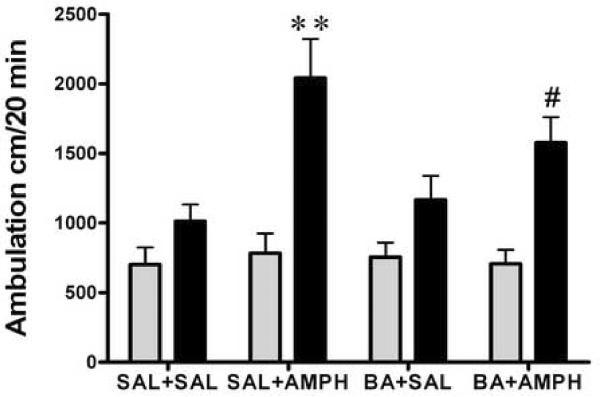
Immediately after the saline challenge, all groups were administered amphetamine (2.0 mg/kg, i.p.) and the locomotor activity was recorded for 120 min. Amphetamine induced a significant expression of the sensitization in the Paired groups (Fig. 5 A, one-way ANOVA, F3,35= 11.9, p = 0.0001; Fig. 5 B, F3,28 = 12.8, p = 0.04). The Paired SAL+AMPH groups demonstrated a robust sensitization, exhibiting significantly more locomotor activities than saline treated mice (SAL+AMPH vs. SAL+SAL, Bonferroni post-test p < 0.05). Also the Paired VPA+AMPH or the Paired BA+AMPH mice showed substantial expression of sensitization (Bonferroni post-test VPA+AMPH vs. VPA+SAL p < 0.001, BA+AMPH vs. BA+SAL p < 0.001, and SAL+AMPH vs. BA+AMPH p < 0.05). There was no expression of locomotor sensitization in VPA (or BA)+SAL groups. However, most of the Unpaired groups failed to express locomotor sensitization (Fig. 5 A, one–way ANOVA, F3,35=1.9, p = 0.149; Fig 5 B, one–way ANOVA, F3,28=6.2, p = 0.004, Bonferroni post-test, BA+AMPH vs. SAL+SAL, or BA+SAL p < 0.05).
Figure 5.
Amphetamine challenge test. Both the Unpaired and the Paired mice received an injection of amphetamine (2.0 mg/kg i.p.) immediately after saline challenge and locomotion was estimated for 120 min in the locomotion test environment, following daily treatment for 8 days with saline (SAL), amphetamine (AMPH, 2.0 mg/kg, i.p.), valproic acid (VPA, 175 mg/kg, i.p.), VPA+AMPH, n=8–12 (Panel A); butyric acid (BA, 630 mg/kg, i.p.) and BA+AMPH, n=6–9 (Panel B). Data expressed as mean±S.E.M. Two-way ANOVA followed by Bonferroni post-test, * p< 0.05, BA+AMPH Unpaired vs. Paired, and ** p< 0.01, VPA+AMPH Unpaired vs. Paired.
An occasion-setting mechanism can block the expression of neural sensitization in contexts where a drug of abuse is not expected, and can enhance the expression of neuronal sensitization in contexts where the drug is expected [2]. To evaluate whether HDAC inhibitors affect the occasion-setting mechanism, we compared the Paired and Unpaired mice after amphetamine challenge (Fig. 5 A, two-way ANOVA, treatment groups F3,72 = 7, p < 0.0004, Paired and Unpaired groups F1,72 = 2.6, p = 0.11; interaction F3,72 = 2.94, p < 0.039 and Fig. 5 B, treatment groups F3,50 = 20.95, p < 0.0001, Paired and Unpaired groups F1,50 = 1.7, p = 0.2, interaction F3,50 = 2.1, p < 0.11). Paired and Unpaired subgroups SAL+SAL, BA+SAL or VPA+SAL did not vary significantly from one another after amphetamine challenge. Also, there was no significant difference between the Paired and Unpaired SAL+AMPH treated mice (Bonferroni post-test p > 0.05). However, the Paired VPA+AMPH group demonstrated significantly higher locomotor activity than the Unpaired VPA+AMPH mice (Bonferroni post-test p < 0.01). Similarly, we also detected significantly higher locomotor response in the Paired BA+AMPH group compared to the Unpaired BA+AMPH group (Bonferroni post-test p < 0.05). Thus, after the amphetamine challenge, there was a significant difference on the sensitization response between the Unpaired and Paired mice co-treated with HDAC inhibitors.
4. Discussion
We hypothesized that HDAC inhibition-induced chromatin modification contributes to both amphetamine-induced behavioral sensitization and associative-learning processes. This hypothesis is supported by the following findings in the present study: (i) repeated treatment with BA or VPA did not have locomotor effects by themselves but significantly potentiated amphetamine-induced behavioral sensitization in mice; (ii) repeated BA and VPA administration after the induction of amphetamine sensitization (apart treatment paradigm) inhibited the expression of sensitized response to amphetamine upon the challenge; (iii) repeated co-treatment with amphetamine and VPA (but not BA), during the induction of behavioral sensitization, inhibited the facilitatory effect of Pavlovian associations (environment setting effect); (iiii) only the context trained mice exposed to VPA or BA (Paired VPA (or BA) + AMPH mice) demonstrated significantly greater expression of sensitization than the non-context trained mice (Unpaired VPA or BA + AMPH group) after the amphetamine challenge. We also found that repeated treatment with VPA or amphetamine alone induces histone H4 hyperacetylation and co-treatment with VPA and amphetamine produces additive effects on histone H4 acetylation in the mouse striatum.
Chromatin modification was recently recognized as an important regulator of a gene expression in the brain [27]. In the nucleus, DNA is packed tightly into chromatin, comprising a group of highly basic proteins, the histones. Unmodified histones are linked tightly to DNA, preventing interactions of other proteins, including RNA polymerase II, which is required for transcription [10]. Transcription factors regulate target genes by recruiting to the gene promoter's enzymes that modify the core histones by regulating their acetylation (HAT and HDAC), phosphorylation and methylation. HDAC catalyzes the removal of acetate from the modified lysine residues located in the N-terminal tail regions of the core histones H2, H3, and H4. HDAC inhibitors are the group of agents that increase the acetylation of the core histones and may be a critical determinant of gene expression. Two non-specific HDAC inhibitors (VPA and BA) were tested in the present study. Previous studies showed that VPA inhibits the majority of class I and II HDAC at CI50, ranging from 0.7 to 1.3 mM [13]. It has been also demonstrated that the inhibitory effect of VPA on HDAC initiates within hours after exposure, with a rapid return to baseline acetylation levels of histones. BA is another non-specific HDAC inhibitor with CI50 also in the milli-molar range and is typically rapidly eliminated from the body. In addition to their inhibition of HDAC, both VPA and BA have some other significant actions [25,32]. VPA increases the level of γ-amino butyric acid (GABA), an inhibitory neurotransmitter, enhances the sensitivity of GABA receptors in brain and consequently is widely used as an anticonvulsant and mood stabilizer [24,30]. BA does not have any reported effects on the GABAergic system. BA is used as an effective treatment in a wide range of human diseases, including cancers, β-thalassemia and bowel inflammatory pathologies [14,25,31]. Thus, VPA and BA differ in many aspects of their pharmacological actions, but share the common molecular action of HDAC inhibition. The demonstration that these two disparate drugs have similar behavioral effects strongly argues that their shared inhibition of HDAC, rather than other actions, is responsible for modulation of the amphetamine-induced behavioral sensitization observed in this study.
4.1. HDAC inhibitor treatment modulates the induction and expression of behavioral sensitization
In the present study, a single co-administration of HDAC inhibitors and amphetamine did not affect amphetamine-induced locomotor activity. However, we found that repeated co-treatment with HDAC inhibitors and amphetamine augmented the amphetamine-induced psychomotor responses during the induction of behavioral sensitization (Fig. 2 A,B). The mechanism underlying this effect is unknown. One possible explanation is that histone hyperacetylation after inhibiton of HDAC synergizes with cAMP signaling evoked by amphetamine to elicit specific gene expression and consequently altered neuronal function. We have found that VPA (175 mg/kg, i.p.) as well as d-amphetamine (2.0 mg/kg, i.p.) produced a global increase of histone H4 acetylation in the striatum (Fig. 1). However, BA failed to induce a global increase in histone H4 acetylation in the striatum. The lack of histone H4 acetylation by BA may be due to the particularly low dose used in this study (i.e. the dose not altering basal locomotor activity). Indeed, other studies used BA at much higher dose for their in vivo investigations. For example, Ferrante and colleagues used BA at the dose 1200 mg/kg (compared to ours at 630 mg/kg) daily throughout the study (postnatal day 42–125) [9]. We found that BA at the dose above 630 mg/kg would affect basal locomotor activity, precluding the use of a higher dose in our study to examine behavioral effects of HDAC inhibitors on amphetamine sensitization. Despite the lack of histone H4 acetylation, we found that BA at this dose potentiates the amphetamine behavioral sensitization. Furthermore, as is the case for amphetamine, repeated treatment with BA or VPA increased ΔFosB protein levels in the striatum (Kalda et al. unpublished data). Recently, Kumar et al. [20] demonstrated that chronic treatment with cocaine induced histone H3 hyperacetylation at the promotors for FosB, BDNF and Cdk5 in the striatum. The BA treatment (200 mg/kg i.p.) followed by cocaine (15 mg/kg i.p.) caused enhanced induction of histone H3 phosphoacetylation as compared to cocaine treatment alone. BA also enhanced the locomotor-activating effects of cocaine and this effect was more evident on the second day of cocaine exposure [20]. Our results demonstrated that co-treatment with BA (630 mg/kg i.p.) and amphetamine induced a locomotor response that peaked at day 8. These studies thus, indicate that histone hyperacetylation may enhance psychostimulant-induced locomotor responses.
Despite histone hyperacetylation in the brain, treatment with the HDAC inhibitor (VPA+SAL) did not elicit behavioral responses after a single or repeated administrations (Fig. 2 A,). Furthermore, VPA or BA did not alter amphetamine-induced locomotor activity, if HDAC inhibitors were administered 12 hours prior to amphetamine (data not shown). These results suggest that HDAC inhibitors do not have a motor effect by themselves but rather they enhance the amphetamine-induced effect during induction of sensitization.
The repeated treatment with HDAC inhibitors had an inhibitory effect on the expression of sensitized response in the apart treatment paradigm (Fig. 3 A,B). These results indicate that HDAC inhibitors may have different effects depending on the treatment paradigms. Expression profiling demonstrated that HDAC inhibitors regulated the expression only of a small number of genes (2–5%) [8,40]. The mechanisms of selective gene activation or repression by HDAC inhibitors are not well understood and might result from hyperacetylation of histones or other proteins, such as transcription factors [25]. We speculate that alterations in a specific gene expression and/or a protein acetylation may differentially modify psychomotor responses during the induction and expression of behavioral sensitization. An alternative hypothesis is that repeated treatment with VPA decreases the expression of amphetamine sensitization through GABAergic inhibition. There are several studies demonstrating that GABA plays an important role in the induction and expression of behavioral sensitization [4,16,23]. For example, Li et al. [23] demonstrated that multiple administration of VPA (37.5, 75, 150 mg/kg) dose-dependently inhibited the induction and expression of behavioral sensitization to methamphetamine in mice. Thus, it remains possible that the effect of VPA depends on the drug concentration – at lower doses the effect is mainly related with GABAergic inhibition, but at higher doses the HDAC inhibitory effect becomes evident. However, in our experiments BA also decreased the expression of amphetamine sensitization and there is no data indicating that BA affects the GABAergic system. Thus, it is likely that the shared HDAC inhibition by VPA and BA is responsible for the modulation of amphetamine-induced sensitization.
4.2. Learning processes governing amphetamine sensitization
Several recent studies have demonstrated that chromatin modification, specifically histone hyperacetylation, plays an important role in the molecular mechanism of learning and formation of a long-term memory in Aplysia and mice [1,12,19,21]. Since associative learning is an integrated component of psychostimulant-induced sensitization [5,37], HDAC inhibitors may potentiate amphetamine-induced behavioral sensitization by enhancing associative learning and memory consolidation. To study the effects of HDAC inhibitors on amphetamine-induced associative learning, we used a context-specific model - mice given repeated treatments (SAL+SAL, SAL+AMPH, VPA+SAL or BA+SAL, VPA+AMPH or BA+AMPH) in the locomotor test environment (Paired groups) or in a non-test environment (Third world) (Unpaired groups). The Paired and Unpaired mice were treated identically with amphetamine for 8 days and there were no procedural differences between these two groups. Therefore the only diference is in the context-association of drug administration, while they are expected to undergo the same process of neural sensitization.
Behavioral sensitization is the manifestation of neuronal plasticity that occurs as a consequence of repeated exposure to drug of abuse. However, under some circumstances, a contextual stimulus can gain powerful control over the ability of the sensitized neural substrate to influence behavior [3,37]. It has been proposed that a neural sensitization is modulated by two associative mechanisms. One of these is a conditioned facilitation, resulting from the Pavlovian pairing of the context and drug, which produces a conditional response to drug-associated cues. This can add to the sensitized response in the drug-paired context [38,39]. The other is an occasion-setting mechanism that prevents the expression of sensitisation in the environment where the drug is not expected but enhances the expression of sensitisation in the context where the drug is expected [2,37].
4.2.1. The effect of HDAC inhibitors on excitatory Pavlovian associations
There are considerable evidences that drug-paired context can elicit facilitatory conditional responses that resemble the drug unconditional response [3,26,38,39]. In the present experiments, the facilitatory conditional response was clearly observed in the Paired SAL+AMPH mice, when they were given saline and placed in the drug-associated environment (Fig. 4 A,B). Whereas the Unpaired SAL+AMPH mice, which had the same treatment but were placed in the Third World environment, did not exhibit the facilitatory conditional response. Our data are in good agreement with the findings by Anagnostaras et al. [3] that the conditional response, produced by the facilitatory drug environment conditioning, contributes primarily to the early phase of the sensitized response and it accounts for only a small proportion of the overall response. Surprisingly, we found that the Paired VPA+AMPH mice did not show the facilitatory conditional response (Fig. 4 A). In contrast, there was statistical difference between the Unpaired and the Paired BA+AMPH groups. It is unclear why the Paired VPA+AMPH mice failed to demonstrate the facilitatory conditioning. We speculate that there may be at least two mechanisms underlying this effect. i) Lack of excitatory drug environment conditioning in the Paired VPA+AMPH mice, may be caused by the GABAergic effect of VPA. ii) Repeated treatment with HDAC inhibitors decreases the development of Pavlovian excitatory drug-environment conditioning. Future studies are needed to clarify this issue.
4.2.2. The effect of HDAC inhibitors on an occasion-setting mechanism
It has been proposed that an occasion-setting mechanism, acting via conditional facilitation or inhibition, can gate the expression of the sensitized neural substrate [2]. Occasion-setters are a class of conditional stimuli that do not themselves elicit a conditional response, but they modulate the ability of other stimuli to elicit responses [15]. The present results suggest that amphetamine produced robust behavioral sensitization in all environments, but when an amphetamine challenge was given in the test environment that was novel for the Unpaired groups (but not for the Paired groups), the expression of sensitization was the context specific. The Unpaired SAL+AMPH mice did not express the locomotor sensitization in an environment that had never been paired with amphetamine administration, since amphetamine-sensitized responses did not differ significantly from the Unpaired SAL+SAL group (Fig. 5 A,B).
To evaluate whether HDAC inhibitors affect the occasion-setting mechanism, we compared the Paired and Unpaired mice after amphetamine challenge There were no differences between the Unpaired and Paired SAL+AMPH groups. However, there was significant difference in amphetamine-sensitized response between the Unpaired and Paired VPA (or BA)+AMPH mice after amphetamine challenge. Thus, the conditional amphetamine-sensitized responses manifested significantly only in the group co-treated with HDAC inhibitors and amphetamine. The data suggest a complex interaction between environment and VPA or BA, as these drugs seemed to enhance the context-specificity of sensitization expression (i.e. increased the difference between the Paired and Unpaired groups), rather than augmenting expression of sensitization per se. It has been speculated that if animals receive amphetamine only in one distinctive environment they form an expectation that they would receive the drug only in that environment. This expectation can serve as a facilitatory occasion-setter in the treatment-associated context and as an inhibitory occasion-setter in different context [3]. Although, there was a trend of reduced expression of amphetamine sensitization in the Unpaired VPA+AMPH group (compared to the SAL+AMPH group, Fig. 5 A), there were no significant difference between the Unpaired BA+AMPH and SAL+AMPH mice (Fig. 5 B). Therefore, we conclude that the facilitatory occasion-setting mechanism in the Paired VPA (or BA)+AMPH mice is involved in the enhanced expression of amphetamine psychomotor sensitization.
In conclusion, the result of HDAC inhibitors and amphetamine co-treatment experiments suggest that chromatin modification (specifically histone hyperacetylation) may affect both the non-associative changes (via altering neuronal plasticity) and an associative learning (via the facilitatory occasion-setting mechanism). The apart treatment paradigm demonstrated that repeated treatment with HDAC inhibitors inhibited the expression of amphetamine sensitization, indicating that HDAC may play a critical role in the modulation of the expression of amphetamine sensitization depending on the treatment paradigms.
Acknowledgements
This study was supported by NIH grants NS37403 and NS41083 (JFC) and by European Union's FP6 grant LSHM-CT-2005-512012 (AZ). We thank Abbie Tillman for correcting the English language.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- [2].Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- [3].Anagnostaras SG, Schallert T, Robinson TE. Memory processes govering amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26:703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- [4].Bartoletti M, Gubellini C, Ricci F, Gaiardi M. Baclofen blocks the development of sensitization to the locomotor stimulant effect of amphetamine. Behav Pharmacol. 2005;16:553–558. doi: 10.1097/01.fbp.0000179279.98029.e9. [DOI] [PubMed] [Google Scholar]
- [5].Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- [6].Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, Simon AJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mito- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen JF, Moratalla R, Yu L, Martin AB, Xu K, Bastia E, Hackett E, Alberti I, Schwarzschild MA. Inactivation of adenosine A2A receptors selectively attenuates amphetamine-induced behavioral sensitization. Neuropsychopharmacology. 2003;28:1086–1095. doi: 10.1038/sj.npp.1300152. [DOI] [PubMed] [Google Scholar]
- [8].Della Ragione F, Criniti V, Della Pietra V, Borriello A, Oliva A, Indaco S, Yamamoto T, Zappia V. Genes modulated by histone acetylation as new effectors of butyrate activity. FEBS Lett. 2001;499:199–204. doi: 10.1016/s0014-5793(01)02539-x. [DOI] [PubMed] [Google Scholar]
- [9].Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch S. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fischle W, Wang Y, Allis D. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- [11].Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- [12].Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- [13].Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–1089. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- [14].Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- [15].Holland PC. Occasion setting in Pavlovian conditioning. In: Medin DL, editor. The Physiology of Learning and Motivation. vol. 28. Academic Press; San Diego: 1992. pp. 69–125. [Google Scholar]
- [16].Itzhak Y, Martin JL. Effect of riluzole and gabapentin on cocaine and methamphetamine-induced behavioral sensitization in mice. Psychopharmacology. 2000;151:226–233. doi: 10.1007/s002130000394. [DOI] [PubMed] [Google Scholar]
- [17].Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet Suppl. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- [18].Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- [19].Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kumar A, Choi KW, Renthal W, Tsankova N, Theobald DE, Turong HT, Russo SJ, LaPlant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nesteler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- [21].Levenson JM, O'Riordan KJ, Brown KJ, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- [22].Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci USA. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li JX, Han R, Deng YP, Chen SQ, Liang JH. Different effects of valproate on methaphetamine- and cocaine-induced behavioral sensitization in mice. Behav Brain Res. 2005;61:125–132. doi: 10.1016/j.bbr.2005.01.015. [DOI] [PubMed] [Google Scholar]
- [24].Loscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- [25].Marks PA, Miller T, Richon VM. Histone deacetylases. Curr Opin Pharmacol. 2003;3:344–351. doi: 10.1016/s1471-4892(03)00084-5. [DOI] [PubMed] [Google Scholar]
- [26].Martin-Iverson MT, Fawcett SL. Pavlovian conditioning of psychomotor stimulant-induced behaviors: has convenience led us astray? Behav Pharmacol. 1996;7:24–41. [PubMed] [Google Scholar]
- [27].Mattson MP. Methylation and acetylation in nervous system development and neurodegenerative disorders. Ageing Res Rev. 2003;2:329–342. doi: 10.1016/s1568-1637(03)00013-8. [DOI] [PubMed] [Google Scholar]
- [28].Nestler EJ. From neurobiology to treatment: progress against addiction. Nat Rev Neurosci. 2001;2:119–128. [Google Scholar]
- [29].Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- [30].Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull 37 Suppl. 2003;2:17–24. [PubMed] [Google Scholar]
- [31].Perrine SP, Miller BA, Faller DV, Cohen RA, Vichinsky EP, Hurst D, Lubin BA, Papayannopoulou T. Sodium butyrate enhances fetal globin gene expression in erythroid progenitors of patients with Hb SS and beta thalassemia. Blood. 1989;74:454–459. [PubMed] [Google Scholar]
- [32].Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- [33].Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- [34].Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- [35].Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- [36].Stewart J. Neurobiology of conditioning to drugs of abuse. Ann NY Acad Sci. 1992;654:335–346. doi: 10.1111/j.1749-6632.1992.tb25979.x. [DOI] [PubMed] [Google Scholar]
- [37].Stewart J, Vezina P. Conditioning and behavioral sensitization. In: Kalivas PW, Barnes CD, editors. Sensitization in the Nervous System. Telford Press; Cadwell, NJ: 1988. pp. 207–224. [Google Scholar]
- [38].Tilson HA, Rech RA. Conditioned drug effects and absence of tolerance to d-amphetamine induced motor activity. Pharmacol Biochem Behav. 1973;1:149–153. [Google Scholar]
- [39].Tirelli E, Terry P. Amphetamine-induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes. Behav Pharmacol. 1998;9:409–419. doi: 10.1097/00008877-199809000-00004. [DOI] [PubMed] [Google Scholar]
- [40].Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 1996;5:245–253. [PMC free article] [PubMed] [Google Scholar]
- [41].Yasmineh WG, Yunis SJ. Isolation of mammalian heterochromatin and euchromatin. Methods Cell Biol. 1974;8:151–177. doi: 10.1016/s0091-679x(08)60450-1. [DOI] [PubMed] [Google Scholar]