Abstract
Several hemoglobins were explored by UV-Vis and resonance Raman spectroscopy to define sulfheme complex formation. Evaluation of these proteins upon the reaction with H2O2 or O2 in the presence of H2S suggest: (a) the formation of the sulfheme derivate requires a HisE7 residue in the heme distal site with an adequate orientation to form an active ternary complex; (b) that the ternary complex intermediate involves the HisE7, the peroxo or ferryl species, and the H2S molecule. This moiety precedes and triggers the sulfheme formation.
Keywords: sulfhemoglobin, sulfmyoglobin, hemoglobin I (HbI), hydrogen sulfide (H2S), hydrogen peroxide (H2O2), histidine (His), ferryl species
Introduction
Historically, hydrogen sulfide (H2S) has been regarded as a poisonous gas with a wide spectrum of cytotoxic effects [1]. However, a new controversial role is emerging for H2S in the chemistry of biological systems. It has been found that H2S is synthesized endogenously in mammalian tissues and that it functions as a neuromodulator, and a smooth muscle relaxant [2,3]. Furthermore, it has been suggested that the interaction of H2S with cytochrome c oxidase, hemoglobin (Hb) and/or myoglobin (Mb), can decrease cellular ATP, activating the KATP channels, and causing in turn hyperpolarization (neurons) or smooth muscle relaxation [4,5].
In this respect, the reaction of H2S with Hb and Mb, in the presence of H2O2 or O2, results in covalent modification of the heme pyrrole ring bearing the 4-vinyl group, generating the so-called sulfmyoglobin and sulfhemoglobin derivatives [6,9]. The suggested structure has been described as a chlorin with H2S added only across the β-β double bond of the pyrrole “B” [7–9]. These sulfheme derivatives have lower O2 affinity, which can in principle activate the KATP channels [5]. Increased levels of sulfhemoglobin in humans can lead to rare cases of the blood disease sulfhemoglobinemia, treatments for which remain unreliable [10–12] since the mechanism underlying sulfheme complex formation has not been elucidated. The sulfheme complex has a characteristic absorption band at 620 nm that is probably due to the distortion of heme group planarity and alterations within of neighboring side-chains relative to the native protein [7–9]. It has been suggested though, that the interaction of Hb (or Mb) with H2O2 produces ferryl Compound I, (FeIV=O Por•+) and ferryl Compound II (FeIV=O Por) [13], and that H2S may interact with these species to produce the sulfheme derivative [7,8]. However, other hemeproteins, including the hemoglobins from the clam Lucina pectinata (HbI, HbII and HbIII), also form these ferryl species in the presence of H2S without generating the sulfheme derivatives [13–14].
The clam Lucina pectinata has three peculiar hemoglobins with distinctive chemical-physical properties [14]. The monomeric HbI binds and transports H2S while HbII and HbIII, are O2 reactive. The proposed mechanism suggests that HbI reacts with H2S in the presence of O2 to form a hemeFe(III)-H2S low spin complex. It is noteworthy to mention that in the presence of H2O2 and H2S, HbI forms stable Compound I species without generating the sulfheme derivative [13]. It has also been reported that neither HbII nor HbIII, which produce stable Compund II, form the sulfheme product. Interestingly, HbI, HbII, and HbIII contain a distinctive distal structural organization involving a GlnE7 residue instead of the typical His found in Hb and Mb [15,16].
Therefore, it is plausible to hypothesize that the distal site environment may play a crucial role in sulfheme production since the reactivity of H2S with hemeproteins depends on the H2S concentration and the polarity of the distal heme cavity [17]. To confirm the role of the distal residues on sulheme formation, we have evaluated the interaction of Mb, human Hb, HbI, HbII/III, several HbI mutants and the hemoglobins from the Macrobdella and Lumbricus, with H2O2 and H2S. Specifically, single point mutations were introduced into the Lucina HbI heme pocket to mimic the Mb distal site and to identify the residues involved in sulfheme formation. Here, we report unprecedented results of a new role for HisE7, in the presence of H2S and H2O2 or O2, essential for the formation of the sulfhemeprotein derivative.
Material and Methods
Sperm whale Mb and human Hb were purchased from Sigma-Aldrich. Native HbI and HbII/HbIII proteins, as well as the HbI mutants were prepared and purified as previously reported [14,18–19]. The purified hemoglobins from Macrobdella and Lumbricus, were provided by Dr. Serge Vinogradov. The sulfheme samples were prepared by mixing solutions of the ferric hemoglobins with solutions of H2O2 and H2S [17] in a ratio of 1:2:20 (Mb:H2O2:H2S). The samples were then equilibrated and the optical spectra were recorded using an Agilent 8453 spectrophotometer. The resonance Raman (RR) spectra were obtained as described before [17].
Results and Discussion
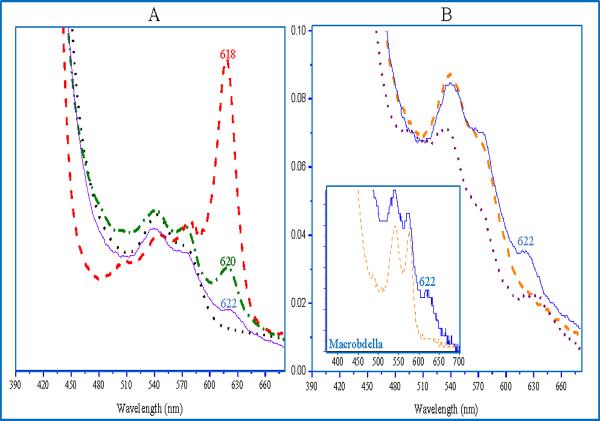
Myoglobin was used as the control since the properties of the sulfmyoglobin have been described in several reports [6–9]. Figure 1A shows the UV-Vis spectra obtained upon reacting metMb, metHb, metHbI and metHbI GlnE7His with H2O2 and H2S. Formations of the sulfmyoglobin and sulfhemoglobin derivates are clearly demonstrated by the presence of the characteristic sulfheme Q band at 618 and 620nm, respectively. Remarkably, HbI did not form the sulfheme derivate, as judged by the absence of a defined band near 620nm. On the other hand, when Gln is replaced by His in HbI GlnE7His mutant, a distinctive band at 622nm was observed that noticeably indicates the existence of the sulfheme complex. Indeed, Figure 1B shows that this band is due the formation of sulfheme derivative and not to a modification of the heme induced by H2O2 itself. It is well known that the heme may become oxidatively modified when it interacts with H2O2 [20]. However, modification of the heme by H2O2 can be discarded since the alteration of the heme, observed by the formation of the characteristic 620 sulfheme band, occurs only after the addition of H2S. Therefore, the modification of the heme in all the proteins studied here can be attributed to the interaction of H2S. Similar reactions were observed for the analogous oxyheme complexes and H2S [7].
Figure 1.
UV-Vis spectra of Mb, human Hb, HbI, the HbI GlnE7His mutant and Macrobdella Hb. Figure 1A. Optical spectra of metMb (dash line), met human Hb (dotted-dashed line), metHbI (dotted line), and HbI GlnE7His (solid line) in the presence of H2O2 and H2S. Figure 1B. Optical spectra of the reaction sequence of metHbI GlnE7His (dotted line) with H2O2 (dashed line) and H2S (solid line). The inserted optical spectra of Macrobdella Hb with H2O2 (dashed line) and H2S (solid line).
In general, Table 1 summarizes the spectrophotometric results obtained with the proteins evaluated for sulfheme formation, together with their distal site amino acid residues. The only proteins that exhibit the band near 620nm, were the ones that have His in the E7 position, as in horse heart Mb, human Hb, and the HbI GlnE7His mutant. This common feature indicates that the distal His is crucial for sulfheme complex formation. Moreover, the fact that no sulfheme complex was observed for the HbI PheB10His mutant, which has a His residue in its active site, albeit not in the same orientation towards the heme group as the distal His, strongly suggests that the orientation of the His is also fundamental for sulfheme formation. The sulfheme formation was also observed in the giant hemoglobins from Macrobdella which bear His at position E7 (see Figure 1B insert). The variety of hemeproteins that produce the sulfheme complex, ranging from the giant, multi-chain, extracellular hemoglobins from Macrobdella and Lumbricus [21,22] to the monomeric Mb and the Lucina HbIGlnE7His mutant strongly indicates that the formation of the sulfheme derivative requires an intermediate ternary complex involving His E7, a peroxo or ferryl species and H2S. Other protein changes, although present and necessary, are not unique for the formation of the sulf heme derivative, suggesting that the ternary complex intermediate is the pathway for the formation of the sulfhemeprotein species. It is appropriate to mention that no sulfheme formation was observed in a recent study of sulfide binding by group 2 2/2 bacterial Hbs, in line with the absence of distal E7His residues [23] and therefore, lacking the ternary complex intermediate necessary for the sulfheme formation.
Table 1.
Sulfheme formation in the heme proteins used in this study and their distal active site residues. Note that each Macrobdella and Lumbricus Hbs have four different globin chains [21,22]. (−), not observed
| Protein | Residues Position | Sulfheme Q band (nm) | ||
|---|---|---|---|---|
| E7 | B10 | E11 | ||
| Horse heart wt-Mb | His | Leu | Val | 618 |
| Human wt-Hb | His | Leu | Val | 620 |
| Lucina wt-HbII/III | Gln | Phe | Phe | - |
| Lucina wt-HbI | Gln | Phe | Phe | - |
| Lucina PheE11Val | Gln | Phe | Val | - |
| Lucina PheB10Leu | Gln | Leu | Phe | - |
| Lucina GlnE7His | His | Phe | Phe | 622 |
| Lucina PheB10His | Gln | His | Phe | - |
| Macrobdella Hb | His | YFFL | LLLF | 622 |
| Lumbricus Hb | His | FLWW | VIVV | 622 |
The intensity of the 620 nm band (Figure 1A and 1B) is different in each of the sulfheme proteins evaluated. This band arises from the forbidden electronic transition in the visible or Q region of the spectra, which becomes partially allowed due to configuration interactions between the heme chlorin orbitals. Distortion of the heme can increase or decrease the energy of heme orbitals [24], thus affecting the intensity of the band associated with the forbidden transition. In this regard, heme planarity distortion increases these interactions, increasing in turn the intensity of the 620 nm band. It is likely that the observed variation in the intensities of the 620 nm band in the sulfheme proteins studied here can be accounted for by variation in the distortion of heme planarity.
To further demonstrate that the presence and orientation of His is vital for sulfheme formation, an analysis of the porphyrin distortion and alteration in the conformation of the vinyl groups was undertaken using. The first RR evidence indicating sulfheme complex formation in Mb and the Lucina HbI GlnE7His mutant, was the observation of an increase in the number and intensity of the RR bands (Figure not shown), which was not observed in HbI. In the sulfheme complex, alteration of the porphyrin macrocycle from D4h symmetry to a less symmetric chlorin C2 heme leads to a distortion of the macrocycle planarity and changes in the heme electronic properties, resulting in the activation of several out-of-plane heme modes [25].
Moreover, as Figure 2A indicates that the resonance Raman spectra of Mb and the HbI GlnE7His mutant, in the presence of H2O2 and H2S, show a substantial increase in the intensity of the modes at 1353 and 1390cm−1, which are absent in their metaquo-heme complexes and in the HbI protein. The development of these bands in the Mb and HbI GlnE7His spectra, and not in the HbI spectrum, indicates a distortion of the heme group due to the incorporation of the sulfur ring [25]. This suggestion is supported by the fact that the 1353 and 1390cm−1, peaks have been related to the B1g pyrrole deformation of chlorin hemes [25,27].
Figure 2.
Resonance Raman spectra of Mb, HbI, and the HbI GlnE7His mutant. Figure 2A shows the adjacent modes to ν4 of metMb (dashed line), metHbI (dotted line), and HbI GlnE7His (solid line), in the presence of H2O2 and H2S. Figure 2B illustrates the decomposition of the vinyl bands. The left panel shows the met complexes while the right panel demonstrates heme proteins in the presence of H2O2 and H2S. The filled squared lines are the fit after deconvolution.
Sulfheme formation in Mb and the HbI GlnE7His mutant was also confirmed by evaluating the vinyl bands (vC=C) at ~1620 cm−1 [26,27], in the RR spectra of Mb, HbI and HbI GlnE7His, with and without H2O2 and H2S, as shown in Figure 2B. The Figure clearly shows that in the absence of H2O2 and H2S, the vinyl modes can be deconvoluted into two distinct bands at 1620 and 1626 cm−1. Upon the exposure of the hemeprotein samples were exposed to H2O2 and H2S, a substantial reduction of the 1626 cm−1 vinyl band was observed for Mb and the HbI GlnE7His mutant, while no change was observed for the HbI protein. The reduction of the 1626 cm−1 band is associated with a decrease in conjugation of the system induced by the incorporation of the characteristic sulf ring, as shown in the insert of Figure 2A [25]. In this regard, the chlorin heme is a less conjugated system than the porphyrin macrocycle, which agrees with the observed decrease in the 1626 cm−1 band intensity. Curiously, the absence of these bands in the Lucina Hbs with the distal GlnE7 is in agreement with the conclusion that the decrease in conjugation and the formation of new bands are strongly linked to the formation of the chlorin derivative characteristic of the sulfheme complex. Formation of the latter requires the presence of the distal HisE7 in the heme active site.
Furthermore, we propose here that the peroxo or the ferryl species involved in sulfheme formation are the ones with which His has a direct interaction, and that the H2S proton interaction with the His-ferryl (or peroxo)-heme ternary complex triggers the formation of the sulfheme complex. This proposal is supported by the fact that formation of sulfheme was only observed with proton donor thiols such as methanethiol (CH3SH), hydrogen sulfide (HSH) and cysteine (R-CH2SH), while no sulfheme was detected with the dimethyl sulfide (CH3SCH3) molecule (Figure not shown). Thus, these results suggest that the proton form the HSR group plays also a significant role in the formation with heme ferryl ternary complex trigging the formation of the sulfheme derivatives.
Conclusion
In summary, we propose that the generation of the sulfheme derivative requires the presence of peroxo or ferryl species and a distal HisE7 residue properly oriented to form the active ternary complex. Our results clearly indicate the direction to be taken in further investigations of the mechanism for sulfheme protein complex formation.
Research Highlights: “Structural determinants for the formation of sulfhemeprotein complexes”.
-
■
HisE7 is crucial for the sulfheme formation
-
■
HisE7 needs an appropriate orientation for an adequate interaction
-
■
the ternary intermediate involves HisE7, peroxo or ferryl species, and H2S molecule
-
■
this moiety precedes and triggers the sulfheme formation
ACKNOWLEDGMENT
This work was supported in part by funds from the National Science Foundation, (Grant 0843608) and NIH-NIGMS/MBRS-SCORE 5 S06GM008103-36. We thank Dr. Syun-Ru Yeh for the resonance Raman facilities and Dr. Carmen Cadilla for the construction of the HbI mutants. Special thank to Dr. Jack Peisach for his motivation and his inspiring work on sulfheme proteins. We also thank graduate and undergraduate students Cacimar Ramos, Laura Granell, Tatiana Quiñones, Darya Marchany and Frances Marie Pietri for their assistance during the work
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- [2].Kimura H. Hydrogen sulfide: from brain to gut. Antioxid. Redox Signal. 2010;9:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- [3].Leslie M. Nothing rotten about hydrogen sulfide's medical promise. Science. 2008;5880:1155–1157. doi: 10.1126/science.320.5880.1155. [DOI] [PubMed] [Google Scholar]
- [4].Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- [5].Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- [6].Johnson EA. The reversion to haemoglobin of sulphhaemoglobin and its coordination derivatives. Biochim. Biophys. Acta. 1970;207:30–40. doi: 10.1016/0005-2795(70)90134-0. [DOI] [PubMed] [Google Scholar]
- [7].Berzofsky JA, Peisach J, Blumberg WE. Sulfheme proteins. I. Optical and magnetic properties of sulfmyoglobin and its derivatives. J. Biol. Chem. 1971;246:3367–3377. [PubMed] [Google Scholar]
- [8].Chatfield MJ, La Mar GN, Kauten RJ. Proton NMR characterization of isomeric sulfmyoglobins: preparation, interconversion, reactivity patterns, and structural features. Biochemistry. 1987;26:6939–6950. doi: 10.1021/bi00396a013. [DOI] [PubMed] [Google Scholar]
- [9].Evans SV, Sishta BP, Mauk AG, Brayer GD. Three-dimensional structure of cyanomet-sulfmyoglobin C. Proc. Natl. Acad. Sci. 1994;91:4723–4726. doi: 10.1073/pnas.91.11.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guidotti TL. Occupational exposure to H2S in the sour gas industry: some unresolved issues. Int. Arch. Occup. Environ. Health. 1994;66:153–160. doi: 10.1007/BF00380773. [DOI] [PubMed] [Google Scholar]
- [11].Tangerman A, Bongaerts G, Agbeko R, Semmekrot B, Severijnen R. The origin of hydrogen sulfide in a newborn with sulfhaemoglobin induced cyanosis. J. Clin. Pathol. 2002;55:631–633. doi: 10.1136/jcp.55.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dupouy J, Petureau F, Montastruc JL, Oustric S, Degano B. A rare cause of cyanosis: Sulphaemoglobinaemia related to thiocolchicoside. Rev. Mal. Respir. 2010;27:80–83. doi: 10.1016/j.rmr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- [13].De Jesus W, Cortes J, López-Garriga J. Formation of Compounds I and Compound II Ferryl Species in the Reactions of Hemoglobin I from Lucina pectinata with Hydrogen Peroxide. Arch. Biochem. Biophys. 2001;390:304–308. doi: 10.1006/abbi.2001.2392. [DOI] [PubMed] [Google Scholar]
- [14].Kraus DW, Wittenberg JB. Hemoglobins of the Lucina pectinata/bacteria symbiosis I. J. Biol. Chem. 1990;265:16043–16053. [PubMed] [Google Scholar]
- [15].Rizzi M, Wittenberg JB, Coda A, Ascenzi P, Bolognesi M. Structural bases for sulfide recognition in Lucina pectinata hemoglobin I. J. Mol. Biol. 1996;258:1–5. doi: 10.1006/jmbi.1996.0228. [DOI] [PubMed] [Google Scholar]
- [16].Gavira JA, Camara-Artigas A, De Jesús-Bonilla W, López-Garriga J, Lewis A, Pietri R, Yeh S-R, Cadilla CL, García-Ruiz JM. Structure and Ligand Selection of Hemoglobin II from Lucina pectinata. J. Biol. Chem. 2008;283:9414–9423. doi: 10.1074/jbc.M705026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pietri R, Lewis A, León RG, Casabona G, Kiger L, Yeh SR, Fernandez-Alberti S, Marden MC, Cadilla CL, López-Garriga J. Factors controlling the reactivity of hydrogen sulfide with hemeproteins. Biochemistry. 2009;48:4881–4894. doi: 10.1021/bi801738j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].León RG, Munier-Lehmann H, Barzu O, Baudin-Creuza V, Pietri R, López-Garriga J, Cadilla CL. High-level production of recombinant sulfide-reactive hemoglobin I from Lucina pectinata in Escherichia coli. High yields of fully functional holoprotein synthesis in the BLi5 E. coli strain. Protein Expr. Purif. 2004;38:184–195. doi: 10.1016/j.pep.2004.08.014. [DOI] [PubMed] [Google Scholar]
- [19].Ramos C, Pietri R, Lorenzo W, Roman E, Granell L, Cadilla C, López-Garriga J. Recombinant hemoglobin II from Lucina pectinata: A large-scale method for hemeprotein expression in E. coli. Protein J. 2010;2:143–51. doi: 10.1007/s10930-010-9234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reeder BJ. Antioxid. Redox Signal. 2010 doi: 10.1089/ars.2009.2974. In press. [DOI] [PubMed] [Google Scholar]
- [21].Lamy JN, Green BN, Toulmond A, Wall JS, Weber RE, Vinogradov SN. The giant hexagonal bilayer hemoglobins. Chem. Revs. 1996;96:3113–3124. doi: 10.1021/cr9600058. [DOI] [PubMed] [Google Scholar]
- [22].Suzuki T, Vinogradov SN. Globin and Linker Sequences of the Giant Extracellular Hemoglobin from the Leech Macrobdella decora. J. Protein Chem. 2003;22:231–242. doi: 10.1023/a:1025064318790. [DOI] [PubMed] [Google Scholar]
- [23].Nicoletti FP, Comandini A, Bonamore A, Boechi L, Boubeta FM, Feis A, Smulevich G, Boffi A. Sulfide binding properties of truncated hemoglobins. Biochemistry. 2010;49:2269–2278. doi: 10.1021/bi901671d. [DOI] [PubMed] [Google Scholar]
- [24].Li D, Stuehr DJ, Yeh SR, Rousseau DL. Heme Distortion Modulated by Ligand-Protein Interactions in Inducible Nitric-oxide Synthase. J. Biol. Chem. 2004;279:26489–26499. doi: 10.1074/jbc.M400968200. [DOI] [PubMed] [Google Scholar]
- [25].Andersson LA, Loehr TM, Lim AR, Mauk AG. Sulfmyoglobin. Resonance Raman spectroscopic evidence for an iron-chlorin prosthetic group. J. Biol. Chem. 1984;24:15340–15349. [PubMed] [Google Scholar]
- [26].Hu S, Smith KM, Spiro TG. Assignment of Protoheme Resonance Raman Spectrum by Heme Labeling in Myoglobin. J. Am. Chem. Soc. 1996;50:12638–12646. [Google Scholar]
- [27].Silfa E, Almeida M, Cerda J, Wu S, Lopez-Garriga J. Orientation of the Heme Vinyl Groups in the Hydrogen Sulfide-Binding Hemoglobin I from Lucina pectinata. Biospectroscopy. 1998;4:311–326. doi: 10.1002/(sici)1520-6343(1998)4:5<311::aid-bspy3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]