Abstract
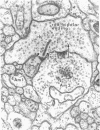
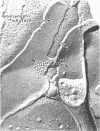
In the inner plexiform layer of the rabbit retina, the synaptic endings of bipolar cells contact a pair of postsynaptic processes at an unusual type of specialized junction, the dyad synapse. One of the members of the postsynaptic dyad may return conventional feedback synapses onto the bipolar endings. Freeze-fracturing demonstrates that, opposite the presynaptic active zone, both postsynaptic membranes contain an aggregate of intramembrane particles that remain associated with the outer leaflet (E face) of the fractured plasmalemma; this is a feature typical of excitatory synapses in the central nervous system. Intracellular recordings followed by injection of horseradish peroxidase showed that at the dyad synapse the endings of rod bipolar cells are usually presynaptic to the dendrites of two amacrine cells, one narrow-field and bistratified (AII) and the other wide-field (A17). Only the A17 rod amacrine cell returns feedback synapses onto the bipolar endings. Both amacrine cells respond to illumination with transient-sustained depolarizations, dominated by rods; thus, the polarity of their light responses is the same as that of rod bipolar cells. We conclude that the dyad synapses established by rod bipolar cells with the two types of amacrine cells are excitatory.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunken W. J., Daw N. W. 5-HT2 antagonists reduce ON responses in the rabbit retina. Brain Res. 1986 Oct 1;384(1):161–165. doi: 10.1016/0006-8993(86)91232-1. [DOI] [PubMed] [Google Scholar]
- Dacheux R. F., Frumkes T. E., Miller R. F. Pathways and polarities of synaptic interactions in the inner retina of the mudpuppy: I. Synaptic blocking studies. Brain Res. 1979 Jan 26;161(1):1–12. doi: 10.1016/0006-8993(79)90191-4. [DOI] [PubMed] [Google Scholar]
- Dacheux R. F., Raviola E. Horizontal cells in the retina of the rabbit. J Neurosci. 1982 Oct;2(10):1486–1493. doi: 10.1523/JNEUROSCI.02-10-01486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux R. F., Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986 Feb;6(2):331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Holmgren I. Electron microscopy of the indoleamine-accumulating neurons in the retina of the rabbit. Cell Tissue Res. 1979 Mar 19;197(2):175–194. doi: 10.1007/BF00233913. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kolb H. A bistratified amacrine cell and synaptic cirucitry in the inner plexiform layer of the retina. Brain Res. 1975 Feb 7;84(2):293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Freed M. A., Nakamura Y., Sterling P. Four types of amacrine in the cat retina that accumulate GABA. J Comp Neurol. 1983 Sep 20;219(3):295–304. doi: 10.1002/cne.902190305. [DOI] [PubMed] [Google Scholar]
- Kolb H., Nelson R., Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21(7):1081–1114. doi: 10.1016/0042-6989(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Kolb H., Nelson R. Rod pathways in the retina of the cat. Vision Res. 1983;23(4):301–312. doi: 10.1016/0042-6989(83)90078-0. [DOI] [PubMed] [Google Scholar]
- Landis D. M., Reese T. S. Differences in membrane structure between excitatory and inhibitory synapses in the cerebellar cortex. J Comp Neurol. 1974 May 1;155(1):93–125. doi: 10.1002/cne.901550107. [DOI] [PubMed] [Google Scholar]
- Landis D. M., Reese T. S., Raviola E. Differences in membrane structure between excitatory and inhibitory components of the reciprocal synapse in the olfactory bulb. J Comp Neurol. 1974 May 1;155(1):67–91. doi: 10.1002/cne.901550106. [DOI] [PubMed] [Google Scholar]
- McGuire B. A., Stevens J. K., Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984 Dec;4(12):2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol. 1982 May;47(5):928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
- Nelson R., Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol. 1985 Sep;54(3):592–614. doi: 10.1152/jn.1985.54.3.592. [DOI] [PubMed] [Google Scholar]
- Nelson R., Kolb H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res. 1983;23(10):1183–1195. doi: 10.1016/0042-6989(83)90032-9. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G., Goebel D. J. Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983 Sep 1;219(1):25–35. doi: 10.1002/cne.902190104. [DOI] [PubMed] [Google Scholar]
- Raviola E., Raviola G. Structure of the synaptic membranes in the inner plexiform layer of the retina: a freeze-fracture study in monkeys and rabbits. J Comp Neurol. 1982 Aug 10;209(3):233–248. doi: 10.1002/cne.902090303. [DOI] [PubMed] [Google Scholar]
- Raviola G., Raviola E. Light and electron microscopic observations on the inner plexiform layer of the rabbit retina. Am J Anat. 1967 May;120(3):403–425. doi: 10.1002/aja.1001200303. [DOI] [PubMed] [Google Scholar]
- Sandell J. H., Masland R. H. A system of indoleamine-accumulating neurons in the rabbit retina. J Neurosci. 1986 Nov;6(11):3331–3347. doi: 10.1523/JNEUROSCI.06-11-03331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]