Abstract
Endoplasmic reticulum (ER) stress regulates a wide range of cellular responses including apoptosis, proliferation, inflammation, and differentiation in mammalian cells. In this study, we observed the role of 2-deoxy-D-glucose (2DG) on inflammation of chondrocytes. 2DG is well known as an inducer of ER stress, via inhibition of glycolysis and glycosylation. Treatment of 2DG in chondrocytes considerably induced ER stress in a dose- and time-dependent manner, which was demonstrated by a reduction of glucose regulated protein of 94 kDa (grp94), an ER stress-inducible protein, as determined by a Western blot analysis. In addition, induction of ER stress by 2DG led to the expression of COX-2 protein with an apparent molecular mass of 66-70kDa as compared with the normally expressed 72-74 kDa protein. The suppression of ER stress with salubrinal (Salub), a selective inhibitor of eif2-alpha dephosphorylation, successfully prevented grp94 induction and efficiently recovered 2DG-modified COX-2 molecular mass and COX-2 activity might be associated with COX-2 N-glycosylation. Also, treatment of 2DG increased phosphorylation of Src in chondrocytes. The inhibition of the Src signaling pathway with PP2 (Src tyrosine kinase inhibitor) suppressed grp94 expression and restored COX-2 expression, N-glycosylation, and PGE2 production, as determined by a Western blot analysis and PGE2 assay. Taken together, our results indicate that the ER stress induced by 2DG results in a decrease of the transcription level, the molecular mass, and the activity of COX-2 in rabbit articular chondrocytes via a Src kinase-dependent pathway.
Keywords: cartilage, articular; chondrocytes; cyclooxygenase 2; deoxyglucose; proto-oncogene proteins pp60(c-src)
Introduction
Chondrocytes in joint cartilage are differentiated from mesenchymal cells during embryo development (Sandell and Adler, 1999; DeLise et al., 2000; Singh Khillan, 2007). They are comprised of a single-cell population responsible for the biosynthesis of matrix molecular components. Differentiated chondrocytes are characterized by the ability to synthesize cartilage-specific extracellular matrix molecules including type II collagen and sulfated proteoglycans (Archer et al., 1990; Hauselmann et al., 1994; Reginato et al., 1994; Martel-Pelletier et al., 2008). The integrity of the matrix is crucial for the unique biosynthetic properties of cartilage and depends on conservation of the quality of the matrix constituents (Chandrasekhar and Phadke, 1988; Jennings et al., 2001; L'Hermette et al., 2006).
Balance of anabolic and catabolic processes within the tissue is important for homeostasis maintenance of cartilage. This homeostasis is destroyed in degenerative diseases, such as rheumatoid arthritis. Arthritis is characterized by loss and degeneration of matrix cartilage (Brandt, 1998; Tatari, 2007; Hashimoto et al., 2008). Prostaglandins are synthesized from arachidonic acid (AA) by mediation of the cyclooxygenase (COX) enzymes, either constitutively or in response to cell specific stress, stimuli, or signaling molecules (Smith et al., 1996; Vane et al., 1998). COXs isoforms, cyclooxygenase-1 (COX-1), and cyclooxygenase-2 (COX-2), encoded by distinct genes, have been identified in eukaryotic cells. COX-1 is constitutively expressed in most cell types and acts to maintain physiological functions. In contrast, COX-2 is largely an inducible enzyme, by pro-inflammatory cytokines, tumor promoters, oncogenes, and growth factors, and is involved mainly in the regulation of inflammation responses (Smith et al., 1994; Langenbach et al., 1999; O'Banion, 1999; Crofford, 2000; Crofford et al., 2000) in numerous types of cells such as monocytes, fibroblasts, and endothelial cells (Rodriguez et al., 1993; Perkins and Kniss, 1997; Rich et al., 1998; Tanabe and Tohnai, 2002; Zaric and Ruegg, 2005; Mbonye et al., 2006). Previous evidence suggested that inflammatory mediators such as cyclooxygenases (COXs) and prostaglandins (PGs) impact the matrix homeostasis of articular chondrocytes by altering their metabolism (Lee et al., 2008). Also, inflammatory response mediators are involved with osteoarthritis (OA) (Hardy et al., 2002; Yoon et al., 2002; Gosset et al., 2006, 2008).
Cox-2 expression is regulated at transcription, post-transcription, and translation. COX-2 transcription is induced by various exogenous stimuli that regulate the intracellular signaling pathway, which in turn modulates the activity of transcription factors (Herschman et al., 1997). Stabilization and nuclear export of COX-2 mRNA at post-transcriptional levels are also necessary for maximal COX-2 induction (Ristimaki et al., 1994; Srivastava et al., 1994; Jang et al., 2003). In addition, activities of MAPKs, including ERKs, p38MAPK, and JNKs, were reported to be important for COX-2 expression (Chen et al., 2001; Hunot et al., 2004). COX-2 is an N-glycoprotein with four glycosylation sites (Hla and Neilson, 1992; Nemeth et al., 2001). It has been previously shown that inhibition of COX-2 N-glycosylation by site-directed mutagenesis or tunicamycin (TN), a protein N-glycosylation inhibitor, results in expression of COX-2 with reduced molecular mass and activity (Otto et al., 1993), indicating the importance of this cotranslational modification in COX-2 enzyme catalysis.
The lumen of the endoplasmic reticulum (ER) is a specialized organelle for the production of secretory and membrane proteins, and provides an environment for this synthesis. In addition, the ER provides a cellular site to assist protein folding and to assure that the accurately folded protein is delivered to the correct secretory pathway (Chevet et al., 2001; Kaufman, 2002; Ron, 2002). If confusion such as redox status and perturbation of calcium homeostasis occurs in the endoplasmic reticulum, misfolded and/or unfolded proteins will be accumulated in the endoplasmic reticulum (Zhang and Kaufman, 2006a, 2006b; Malhotra and Kaufman, 2007). This perturbation in the endoplasmic reticulum results in destruction of the homeostasis for principle cellular responses, including production of properly folded proteins by reducing endoplasmic reticulum efficiency, resulting in activation of the unfolded protein response (UPR) pathway. To maintain cellular homeostasis against any ER dysfunction, the UPR pathway increases the expression of molecular chaperones including glucose regulated protein (grp) 78 and grp94, a molecular chaperone that acts within the ER, to accumulate the protein folding activity and thus prevent the aggregation of unfolded proteins in the ER (Kozutsumi et al., 1988).
The damage of these ER functions has been implicated in several human pathologies including viral infection, ischemic injury, neurodegenerative disorders, and metabolic diseases. In addition, inhibition of N-glycosylation has been reported to be associated with inflammation induced by endoplasmic reticulum stress (Aridor and Balch, 1999; Hung et al., 2004; Schroder and Kaufman, 2005). 2-deoxy-D-glucose (2DG) is a synthetic glucose wherein the hydroxyl group at the second position of the glucose molecule is replaced with a hydrogen group (Sols and Crane, 1954; Tower, 1958). 2DG is known as a potent inducer of endoplasmic reticulum stress (ER stress) by inhibiting glycolysis and N-glycosylation of proteins (Harding et al., 2002; Zhang and Kaufman, 2004; Schroder and Kaufman, 2005). Treatment of 2DG in cells induces ER stress and activates the UPR in the endoplasmic reticulum in rabbit articular chondrocytes. In a recent study we reported that the Src kinase pathway is a key modulator in the regulation of cellular responses such as inflammation and differentiation in chondrocytes (Yu et al., 2006). Here, we show that the treatment of chondrocytes with 2DG induced ER stress and promoted expression of inflammation molecules such as COX-2 and PGE2. Furthermore, the COX-2 expression and PGE2 production induced by 2DG occurred through a Src kinase pathway.
Results
Treatment of 2DG induced ER stress and reduced COX-2 expression/N-glycosylation in chondrocytes
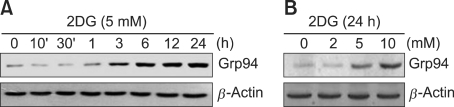
2-deoxy-D-glucose (2DG) is known as an ER stress inducer, acting via prevention of N-glycosylation of proteins, and this compound may regulate cellular responses. To confirm the effects of 2DG, chondrocytes were treated with 5 mM 2DG for the indicated time periods or treated with the specific concentrations of 2DG for 24 h (Figure 1). We determined the expression level of glucose-regulated protein of 94 kDa (Grp94), an indicator for ER stress, in 2DG treated condrocytes (Figure 1). As shown in Figure 1, the expression of grp94 was dramatically enhanced in response to 2DG in a dose- and time-dependent manner, as determined by a Western blot analysis, respectively. The increase in grp94 was apparent ~3 h after treatment of 2DG (Figure 1A). These data indicated that 2DG induces ER stress in rabbit articular chondrocytes (Figure 1). Actin was not affected by 2DG-induced ER stress in chondrocytes. Accordingly, we used actin as a loading control (Figure 1).
Figure 1.
Treatment of 2DG led to ER stress in primary rabbit chondrocytes. (A) Chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods in rabbit articular chondrocytes. (B) Chondrocytes were untreated or treated with specific concentrations of 2DG for 24 h in rabbit articular chondrocytes. (A, B) Expressions of glucose-regulated protein of 94 kDa (grp94) were detected by a Western blot analysis. Expressions of β-actin were used as a loading control. The data represent a typical experiment, and similar results were obtained from four independent experiments.
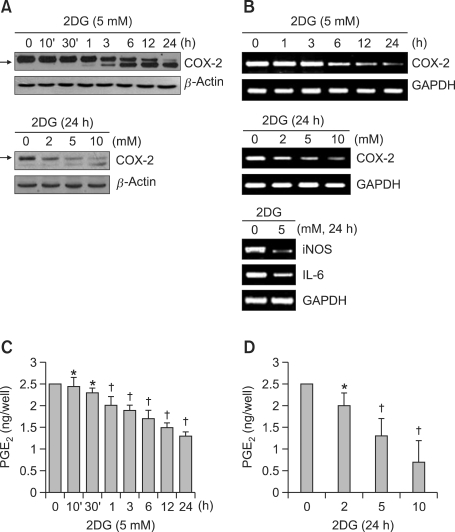
To determine the role of ER stress on COX-2 expression of chondrocytes, we investigated COX-2 expression in 2DG treated cells. Treatment of cells with 2DG led to a dramatic reduction of COX-2 expression and a shift in the molecular mass of COX-2 from 72-74 to 66-70 kDa (marked with arrow) in a time- and dose-dependent manner, determined via a Western blot analysis (Figure 2A). Remarkably, the 2DG-mediated decrease in COX-2 molecular mass is associated with inhibition of COX-2 N-glycosylation during translation. The reduction in COX-2 protein levels appears to be due to a time- and dose-dependent decrease in transcription upon 2DG treatment (Figure 2B). 2DG did not affect COX-1 protein expression (data not shown). Next, we tested whether there are other inflammatory-related genes such as inducible nitric oxide synthase (iNOS) and interleukin (IL)-6 influenced by 2DG in condrocytes. As expected, 2DG led to decrease in iNOS and IL-6, as shown by RT-PCR. These results provided that 2DG regulates not only COX-2 expression but also other inflammatory-related genes in rabbit articular chondrocytes (Figure 2B, lower pannel). We subsequently evaluated whether 2DG-modified COX-2 is associated with (non) proteolytic mechanisms. However, inhibition of COX-2 glycosylation by 2DG at 10 mM did not interfere with MG132 (data not shown). To assess whether 2DG-modified COX-2 is functional, the effect of 2DG on the production of PGE2, a major and stable COX-2 metabolite, in articular chondrocytes was next investigated. As shown in Figures 2C and 2D, production of PGE2 was significantly decreased in cells treated with 2DG, which was considerably detectable ~1 h after 2DG treatment. A maximum of ~40% inhibition of PGE2 production by 2DG treatment during 24 h was observed. These results suggest that 2DG inhibits COX-2 activity by preventing COX-2 N-glycosylation. Taken together, these data indicate that 2DG-modified COX-2 molecular mass and activity might be associated with COX-2 N-glycosylation.
Figure 2.
Treatment of 2DG decreased the expression and activity of COX-2 in chondrocytes. (A) Primary chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods (upper panel) or with the specified concentrations of 2DG for 24 h (lower panel). Expression of COX-2 was detected by a Western blot analysis and expression of β-actin was used as a loading control. (B) Articular chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods (upper panel) or with the specificied concentrations of 2DG for 24 h (middle and lower pannels). Expressions of COX-2, iNOS and IL-6 were detected by RT-PCR, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. (C, D) Chondrocytes were untreated or treated with 5 mM 2DG for the indicated time periods (C) or with the specificied concentrations of 2DG for 24 h (D). Production of PGE2 was performed by a PGE2 assay kit. Data are presented as results of a typical experiment (A-B) and as mean values with standard deviation (C, D) (n = 4). *, P < 0.05, †, P < 0.01 compared with untreated cells. The arrow indicates expression of the reduced molecular mass of COX-2 (~66 kDa) following 2DG treatment.
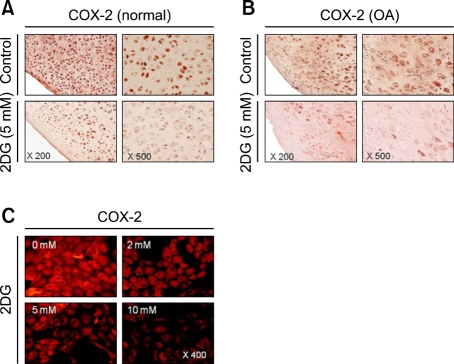
We next examined whether ER stress by 2DG causes reduction of COX-2 in cartilage explants and chondrocytes. Cartilage explants were treated with 5 mM 2DG for 24 h (Figure 3A). 2DG caused a reduction of COX-2, as demonstrated by immunocytochemical staining in cartilage (Figure 3A). We also investigated the expression of COX-2 by 2DG in human osteoarthritic cartilage obtained from patients. The decrease in COX-2 levels by 2DG was also detected in human osteoarthritic cartilage (Figure 3B). Similar to the effects on cartilage, immunofluorescence staining also revealed that 2DG caused a reduction of COX-2 in chondrocytes (Figure 3C). The above results clearly indicate that 2DG regulated reduction of COX-2 expression in both cartilage explants and chondrocytes (Figure 3).
Figure 3.
Treatment of 2DG caused reduction of COX-2 in both cartilage explants and chondrocytes. (A) Cartilage explants from undamaged part of rabbit cartilage (normal) were untreated or treated with 5 mM 2DG for 48 h. Expressions of COX-2 were detected by immunohistochemical staining. (B) Cartilage explants from osteoarthritis-affected human joint cartilage (OA) were untreated or treated with 5 mM 2DG for 48 h. Expressions of COX-2 were detected by immunohistochemical staining. (C) Primary rabbit articular chondrocytes were untreated or treated with 5 mM 2DG for 24 h. Expressions of COX-2 were detected by immunofluorescence staining. The data are typical results from four independent experiments with similar results.
ER stress effect by 2DG modulated COX-2 expression and activity in primary chondrocytes
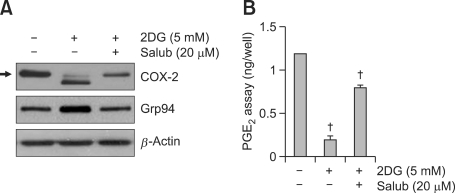
The above results show that 2DG regulated reduction of COX-2 by the ER stress pathway in rabbit articular chondrocytes. We further investigated the relationship between ER stress and COX-2 expression by the treatment of 2DG in rabbit articular chondrocytes. We examined the effects of salubrinal (inhibitor of ER stress, Salub) on the reduction of COX-2 expression by 2DG-induced ER stress in chondrocytes (Figure 4). As expected, treatment of cells with 2DG in the presence of salubrinal abrogated the 2DG-induced increase in grp94 protein level (Figure 4A). Furthermore, the expression level and N-glycosylation of COX-2 were recovered by inhibition of ER stress with salubrinal in cells (Figure 4A). Consistent with the effect of COX-2 expression, the production of PGE2 was rescued by salubrinal, detected by a PGE2 assay (Figure 4B). These findings suggest that the reduction of COX-2 by 2DG is caused via an ER stress related pathway (Figure 4).
Figure 4.
ER stress by treatment of 2DG reduced COX-2 expression and PGE2 production in rabbit articular chondrocytes. (A, B) Chondrocytes were treated with 5 mM 2DG for 24 h in the presence of 20 uM salubrinal (salub). (A) Expression levels of COX-2 and grp94 were detected by a Western blot analysis. (B) Production levels of PGE2 were determined using a PGE2 assay kit. These data are results of a typical experiment (A) and given as mean values with standard deviation (B) (n = 4). *, P < 0.05, †, P < 0.01 compared with untreated cells. The arrow indicates expression of the reduced molecular mass of COX-2 (~66 kDa) following 2DG treatment.
COX-2 expression by 2DG-induced ER stress was regulated through the SRC pathway
Next, we investigated the molecular mechanisms of COX-2 modulation by regulating ER stress via 2DG. We examined the possible signaling pathways, that is, ERK-1/-2, p38 kinase, and the SRC pathway, because these pathways, identified in previous reports, are related with COX-2 expression of chondrocytes (Yu et al., 2006). Among these signaling pathways, phosphorylation of SRC was dominantly increased by 2DG treatment. Activity of ERK-1/-2 and p38 kinase, respectively, was also increased by 2DG treatment alone (data not shown). However, inhibition of ERK-1/-2 and p38 kinase with PD98059 or SB203580 slightly inhibited grp94 expression and COX-2 expression was vaguely recovered by 2DG (data not shown).
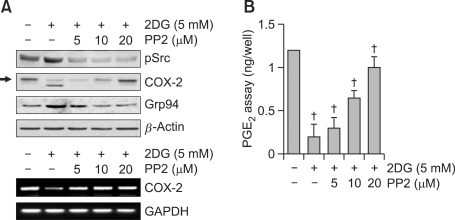
To examine the effects of the SRC pathway on 2DG modulated COX-2 expression, cells were precisely examined with various concentrations of PP2 (a potent inhibitor of Src kinase) for 1 h before 2DG treatment (Figure 5). The inhibition of Src with PP2 significantly blocked grp94 expression in a dose-dependent manner. Also, 2DG treatment dose-dependently inhibited COX-2 glycosylation, resulting in expression of the low molecular mass of COX-2 (mostly ~66 kDa), as demonstrated by a Western blot analysis (Figure 5A, upper panel). As expected, PP2 did not affect 2DG-mediated expression of steady-state COX-2 mRNA 2 (Figure 5B, lower panel). Also, production of PGE2 was recovered via inhibition of pSrc with PP2 treatment, detected by a PGE2 assay (Figure 5B). These results collectively demonstrate that the Src pathway is necessary for 2DG induced reduction of COX-2 expression/N-glycosylation and a loss of COX-2 activity in rabbit articular chondrocytes (Figure 5).
Figure 5.
COX-2 expression by 2DG-induced ER stress was regulated via the Src pathway in primary chondrocytes. (A, B) Articular chondrocytes were treated with 2DG for 24 h in the presence of PP2 (a potent inhibitor of Src kinase). (A) Expression levels of pSrc, COX-2, and grp94 were detected by a Western blot analysis (A, upper panel). Transcriptional level of COX-2 was determined by RT-PCR (A, lower panel). GAPDH was used as a loading control (A, lower panel). (B) Production of PGE2 was determined using a PGE2 assay kit. The data are typical results from four independent experiments with similar results and are given as mean values with standard deviation (B) (n = 4). *, P < 0.05, †, P < 0.01 compared with untreated cells. The arrow indicates expression of the reduced molecular mass of COX-2 (~66 kDa) following 2DG treatment.
Discussion
COX-2 has four glycosylation sites and N-glycosylation of COX-2 is prevented by glycosylation inhibitors such as 2-deoxy-D-glucose and tunicamycin (Hla and Neilson, 1992; Otto et al., 1993; Nemeth et al., 2001). Interestingly, inhibition of N-glycosylation leads to the expression of COX-2 with declined molecular mass and activity, which is important for COX-2 enzyme catalysis. Endoplasmic reticulum (ER) stress can regulate several cellular responses including apoptosis, proliferation, inflammation, and differentiation of numerous cells (Hotamisligil, 2005; Pallet et al., 2008; Ulianich et al., 2008; Hsu et al., 2009). Glucosamine (GS) has been well established as a substitutable medical drug for rheumatoid arthritis or osteoarthritis (Reginster et al., 2001; Hua et al., 2005). Previous studies have demonstrated that GS regulates expression of COX-2 and production of PGE2 via IL-1β treatment in A549 human lung epithelial cells (Jang et al., 2007). Also, GS reduced IL-1β induced COX-2 expression at both transcriptional and translational levels in chondrocytes and synoviocytes (Nakamura et al., 2004). GS also has been implicated in ER stress in skeletal muscle cells, carydiomyocytes, and astrogilal cells (Matthews et al., 2007; Ngoh et al., 2009; Raciti et al., 2010). ER-stress can be induced by N-glycosylation inhibition of COX-2 via treatment of 2DG. The mechanism by which ER stress regulates COX-2 expression in articular chondrocytes is presently unknown. The present findings obtained from GS treated cells closely coincide with the observed effect on COX-2 expression by 2DG. Based on this finding, therefore, we demonstrated that the induction of ER stress by 2DG reduced COX-2 expression/N-glycosylation and activity, and this effect was regulated by the Src signaling pathway in rabbit articular chondrocytes. As shown in Figure 1, treatment of 2DG induced GRP94 expression in a time- and dose-dependent manner, as determined by a Western blot analysis, in rabbit articular chondrocytes. We also confirmed that this ER stress by 2DG regulated inflammation of chondrocytes. COX-2 is mainly an inducible enzyme, by stimulating cellular stresses and inflammatory cytokines, and is involved in the modulation of inflammation (Smith et al., 1996; Vane et al., 1998). As shown in Figure 2, similar to the expression levels of GRP protein, the expression of COX-2 by 2DG mediated ER stress was increased in a dose- and time-dependent manner in chondrocytes. It is of particular note that the expression pattern of COX-2 by 2DG treatment is different compared with that of normal COX-2. There are two apparent patterns of COX-2, ~66-70 kDa protein and ~72-74 kDa protein, in 2DG treated cells. The lower molecule protein of ~66-70 KDa is an unglycosylated COX-2 due to the effects of 2DG and the higher molecule protein of ~72-74 KDa is a normally glycosylated COX-2 in chondrocytes. In chondrocytes treated with 2DG, the unglycosylated COX-2 protein increased in quantity whereas the glycosylated COX-2 protein decreased (Figure 2A). Consistent with the COX-2 expression level, a PGE2 assay also revealed that 2DG reduced production of PGE2 in a dose- and time-dependent manner in chondrocytes (Figure 2C, D). The production of PGE2 decreased within 1 h after 2DG treatment (Figure 2C). Furthermore, immunohistochemical staining and immunofluorescence staining reflected a reduction of COX-2 expression in articular cartilage and chondrocytes (Figure 3). Treatment of 2DG in the presence of salubrinal reduced GRP94 expression while COX-2 expression/N-glycosylation and PGE2 production were recovered by salubrinal in rabbit articular chondrocytes (Figure 4). These effects accurately showed that 2DG decreased expression and the molecular mass of COX-2, and the loss of COX-2 activity was regulated by ER stress.
To elucidate the mechanism regulating the 2DG reduced expression of COX-2, we evaluated several signaling pathways under consideration of the findings of previous reports. Since MAP kinase and SRC pathways are the major signaling pathways involved in COX-2 expression of chondrocytes, we tested whether the above results were attributable to regulation of these pathways. Interestingly, MAP kinase, ERK-1/2, and p38 kinase were activated, at least in part, by treatment of 2DG; however, the inhibition of ERK-1/2 and p38 kinase with PD98059 or SB203580 failed to completely recover COX-2 expression (data not shown). Furthermore, the phosphorylation of SRC occurred by the addition of 2DG in chondrocytes. The inhibition of phosphorylated SRC with PP2 treatment also recovered COX-2 expression/N-glycosylation and PGE2 production while GRP94 was decreased via the addition of PP2 in chondrocytes. RT-PCR revealed the same results as the above results in chondrocytes. In conclusion, 2DG-induced ER stress reduced COX-2 expression/N-glycosylation, and PGE2 production was associated with the SRC pathway in rabbit articular chondrocytes.
Methods
Culture of primary chondrocytes and experimental conditions
Rabbit articular chondrocytes from joint cartilage slices of 2-week-old New Zealand White rabbits were isolated with 0.2% collagenase type II (381 units/ml of solid, Sigma, MO), as described previously (Ryu et al., 2002). To summarize, cartilage slices were dissociated enzymatically for 6 h in 0.2% collagenase type II (381 units/ml of solid, Sigma, St.Louis, MO) in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, CA). Individual cells were obtained by brief centrifugation. The cells were suspended in DMEM supplemented with 10% (v/v) bovine calf serum, 50 µg/ml streptomycin, and 50 units/ml penicillin, and were then plated on culture dishes at a density of 5 × 104 cells/cm2. The medium was replaced every 2 days after seeding. The 3 day cell cultures were treated with various reagents with a glucose-free medium before treatment with the reagent.
Western blot analysis
Whole cell lysates were prepared as described previously. Proteins from chondrocytes were extracted using a buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Nonidet P-40, and 0.1% SDS supplemented with protease inhibitors and phosphatase inhibitors. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane, and the nitrocellulose membrane was blocked with 5% non-fat dry milk in Tris-buffered saline. The following antibodies were employed to detect proteins: anti-COX-2 (Cayman Chemical, Ann Arbor, MI), anti-grp94 (Santa Cruz, CA), anti-phospho-Src (Santa Cruz, CA), and anti-β-actin (Santacruz, CA). The blots were developed using a peroxidase-conjugated secondary antibody and an ECL system.
PGE2 assay
PGE2 production was determined by measuring the levels of cellular and secreted PGE2 using a PGE2 linked immunosorbent assay kit purchased from Amersham Biosciences. Chondrocytes were seeded in standard 96-well microtiter plates at 2 × 104 cells/well. After treatment with reagents, total cell lysates were used to quantify the amount of PGE2 according to the manufacturer's protocol. PGE2 levels were calculated against a standard curve of PGE2.
Immunocytochemical staining and immunofluorescence staining
Rabbit joint cartilage explants or human osteoarthritic cartilage were fixed in 4% paraformaldehyde in PBS for 24 h at 4℃, washed with PBS, dehydrated with graded ethanol, embedded in paraffin, and sectioned at 4 µM thickness. The sections were stained by standard procedures using antibodies against COX-2 (Cayman Chemical, Ann Arbor, MI) and visualized by developing with a kit purchased from DAKO (Carpinteria, CA), following the procedure recommended by the manufacturer. Expression of COX-2 in rabbit articular chondrocytes was determined by indirect immunofluorescence microscopy, as described previously (Yoon et al., 2002). Briefly, chondrocytes were fixed with 3.5% paraformaldehyde in PBS for 10 min at room temperature. The cells were permeabilized and blocked in PBS containing 0.1% Triton X-100 and 5% fetal calf serum for 30 min. The fixed chondrocytes were washed with PBS and incubated for 1 h with the antibody (10 µg/ml) against COX-2. The cells were washed for 30 min, and observed under a fluorescence microscope.
RNA extraction and reverse transcriptation-polymerase chain reaction (RT-PCR)
Total RNA was isolated using TRIZOL (Life Technology. MD) according to the manufacturer's protocol. Total RNA was reverse-transcribed using a Maxime RT Premix kit (Intron Biotechnology, Seoul, Korea) in accordance with the manufacturer's instructions with a PCR thermal cycler. RNA PCR conditions were 94℃ for 30 s, 62℃ for 30 s, and 72℃ for 30 s for a total of 25 cycles. The PCR primers used were COX-2 (298-bp product) sense, 5-TCAGCCACGCAGCAAATCCT-3, and antisense, 5-GTGATCTGGATGTCACG-3, and glyceraldehyde-3-phosphate dehydrogenase (363-bp product) sense, 5-CATCATCCCTGCCTCTACTGG-3, and antisense, 5-TCCACCACCCTGTTGCTGTA-3. The amplified products were analyzed on a 1.5% agarose gel and visualized by ethidium bromide staining.
Data analysis and statistics
The results are expressed as mean values with standard deviation. Values calculated from the specified number of determinations. An Anova test was used to compare individual treatments with their respective control values. Significance was defined at the P < 0.05 or 0.01 level.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (2010-0003239 & 2009-0084569).
Abbreviations
- 2DG
2-deoxy-D-glucose
References
- 1.Archer CW, McDowell J, Bayliss MT, Stephens MD, Bentley G. Phenotypic modulation in sub-populations of human articular chondrocytes in vitro. J Cell Sci. 1990;97(Pt 2):361–371. doi: 10.1242/jcs.97.2.361. [DOI] [PubMed] [Google Scholar]
- 2.Aridor M, Balch WE. Integration of endoplasmic reticulum signaling in health and disease. Nat Med. 1999;5:745–751. doi: 10.1038/10466. [DOI] [PubMed] [Google Scholar]
- 3.Brandt KD. The importance of nonpharmacologic approaches in management of osteoarthritis. Am J Med. 1998;105:39S–44S. doi: 10.1016/s0002-9343(98)00075-8. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekhar S, Phadke K. Interleukin-1-induced alterations in proteoglycan metabolism and matrix assembly. Arch Biochem Biophys. 1988;265:294–301. doi: 10.1016/0003-9861(88)90131-2. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Tang Q, Gonzales MS, Bowden GT. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. 2001;20:3921–3926. doi: 10.1038/sj.onc.1204530. [DOI] [PubMed] [Google Scholar]
- 6.Chevet E, Cameron PH, Pelletier MF, Thomas DY, Bergeron JJ. The endoplasmic reticulum: integration of protein folding, quality control, signaling and degradation. Curr Opin Struct Biol. 2001;11:120–124. doi: 10.1016/s0959-440x(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 7.Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, van de Putte LB. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000;43:4–13. doi: 10.1002/1529-0131(200001)43:1<4::AID-ANR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Crofford LJ. Clinical experience with specific COX-2 inhibitors in arthritis. Curr Pharm Des. 2000;6:1725–1736. doi: 10.2174/1381612003398753. [DOI] [PubMed] [Google Scholar]
- 9.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 10.Gosset M, Berenbaum F, Levy A, Pigenet A, Thirion S, Saffar JL, Jacques C. Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis Res Ther. 2006;8:R135. doi: 10.1186/ar2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosset M, Berenbaum F, Levy A, Pigenet A, Thirion S, Cavadias S, Jacques C. Mechanical stress and prostaglandin E2 synthesis in cartilage. Biorheology. 2008;45:301–320. [PubMed] [Google Scholar]
- 12.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 13.Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, Koki A, Tripp CS. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46:1789–1803. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Nakasa T, Hikata T, Asahara H. Molecular network of cartilage homeostasis and osteoarthritis. Med Res Rev. 2008;28:464–481. doi: 10.1002/med.20113. [DOI] [PubMed] [Google Scholar]
- 15.Hauselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, Kuettner KE, Thonar EJ. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107(Pt 1):17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Herschman HR, Reddy ST, Xie W. Function and regulation of prostaglandin synthase-2. Adv Exp Med Biol. 1997;407:61–66. doi: 10.1007/978-1-4899-1813-0_9. [DOI] [PubMed] [Google Scholar]
- 17.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl 2):S73–S78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 19.Hsu JL, Chiang PC, Guh JH. Tunicamycin induces resistance to camptothecin and etoposide in human hepatocellular carcinoma cells: role of cell-cycle arrest and GRP78. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:373–382. doi: 10.1007/s00210-009-0453-5. [DOI] [PubMed] [Google Scholar]
- 20.Hua J, Suguro S, Hirano S, Sakamoto K, Nagaoka I. Preventive actions of a high dose of glucosamine on adjuvant arthritis in rats. Inflamm Res. 2005;54:127–132. doi: 10.1007/s00011-004-1333-6. [DOI] [PubMed] [Google Scholar]
- 21.Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC, Lai MD. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004;279:46384–46392. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- 22.Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc Natl Acad Sci USA. 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang BC, Munoz-Najar U, Paik JH, Claffey K, Yoshida M, Hla T. Leptomycin B, an inhibitor of the nuclear export receptor CRM1, inhibits COX-2 expression. J Biol Chem. 2003;278:2773–2776. doi: 10.1074/jbc.C200620200. [DOI] [PubMed] [Google Scholar]
- 24.Jang BC, Sung SH, Park JG, Park JW, Bae JH, Shin DH, Park GY, Han SB, Suh SI. Glucosamine hydrochloride specifically inhibits COX-2 by preventing COX-2 N-glycosylation and by increasing COX-2 protein turnover in a proteasome-dependent manner. J Biol Chem. 2007;282:27622–27632. doi: 10.1074/jbc.M610778200. [DOI] [PubMed] [Google Scholar]
- 25.Jennings L, Wu L, King KB, Hammerle H, Cs-Szabo G, Mollenhauer J. The effects of collagen fragments on the extracellular matrix metabolism of bovine and human chondrocytes. Connect Tissue Res. 2001;42:71–86. doi: 10.3109/03008200109014250. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 28.L'Hermette MF, Tourny-Chollet C, Polle G, Dujardin FH. Articular cartilage, degenerative process, and repair: current progress. Int J Sports Med. 2006;27:738–744. doi: 10.1055/s-2005-872824. [DOI] [PubMed] [Google Scholar]
- 29.Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–1246. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 30.Lee WK, Yu SM, Cheong SW, Sonn JK, Kim SJ. Ectopic expression of cyclooxygenase-2-induced dedifferentiation in articular chondrocytes. Exp Mol Med. 2008;40:721–727. doi: 10.3858/emm.2008.40.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22:351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Matthews JA, Belof JL, Acevedo-Duncan M, Potter RL. Glucosamine-induced increase in Akt phosphorylation corresponds to increased endoplasmic reticulum stress in astroglial cells. Mol Cell Biochem. 2007;298:109–123. doi: 10.1007/s11010-006-9358-5. [DOI] [PubMed] [Google Scholar]
- 34.Mbonye UR, Wada M, Rieke CJ, Tang HY, Dewitt DL, Smith WL. The 19-amino acid cassette of cyclooxygenase-2 mediates entry of the protein into the endoplasmic reticulum-associated degradation system. J Biol Chem. 2006;281:35770–35778. doi: 10.1074/jbc.M608281200. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura H, Shibakawa A, Tanaka M, Kato T, Nishioka K. Effects of glucosamine hydrochloride on the production of prostaglandin E2, nitric oxide and metalloproteases by chondrocytes and synoviocytes in osteoarthritis. Clin Exp Rheumatol. 2004;22:293–299. [PubMed] [Google Scholar]
- 36.Nemeth JF, Hochgesang GP, Jr, Marnett LJ, Caprioli RM. Characterization of the glycosylation sites in cyclooxygenase-2 using mass spectrometry. Biochemistry. 2001;40:3109–3116. doi: 10.1021/bi002313c. [DOI] [PubMed] [Google Scholar]
- 37.Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol. 2009;297:H1711–H1719. doi: 10.1152/ajpheart.00553.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- 39.Otto JC, DeWitt DL, Smith WL. N-glycosylation of prostaglandin endoperoxide synthases-1 and -2 and their orientations in the endoplasmic reticulum. J Biol Chem. 1993;268:18234–18242. [PubMed] [Google Scholar]
- 40.Pallet N, Bouvier N, Bendjallabah A, Rabant M, Flinois JP, Hertig A, Legendre C, Beaune P, Thervet E, Anglicheau D. Cyclosporine-induced endoplasmic reticulum stress triggers tubular phenotypic changes and death. Am J Transplant. 2008;8:2283–2296. doi: 10.1111/j.1600-6143.2008.02396.x. [DOI] [PubMed] [Google Scholar]
- 41.Perkins DJ, Kniss DA. Rapid and transient induction of cyclo-oxygenase 2 by epidermal growth factor in human amnion-derived WISH cells. Biochem J. 1997;321(Pt 3):677–681. doi: 10.1042/bj3210677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raciti GA, Iadicicco C, Ulianich L, Vind BF, Gaster M, Andreozzi F, Longo M, Teperino R, Ungaro P, Di Jeso B, Formisano P, Beguinot F, Miele C. Glucosamine-induced endoplasmic reticulum stress affects GLUT4 expression via activating transcription factor 6 in rat and human skeletal muscle cells. Diabetologia. 2010;53:955–965. doi: 10.1007/s00125-010-1676-1. [DOI] [PubMed] [Google Scholar]
- 43.Reginato AM, Iozzo RV, Jimenez SA. Formation of nodular structures resembling mature articular cartilage in long-term primary cultures of human fetal epiphyseal chondrocytes on a hydrogel substrate. Arthritis Rheum. 1994;37:1338–1349. doi: 10.1002/art.1780370912. [DOI] [PubMed] [Google Scholar]
- 44.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE, Gossett C. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 45.Rich G, Yoder EJ, Moore SA. Regulation of prostaglandin H synthase-2 expression in cerebromicrovascular smooth muscle by serum and epidermal growth factor. J Cell Physiol. 1998;176:495–505. doi: 10.1002/(SICI)1097-4652(199809)176:3<495::AID-JCP6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 46.Ristimaki A, Garfinkel S, Wessendorf J, Maciag T, Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J Biol Chem. 1994;269:11769–11775. [PubMed] [Google Scholar]
- 47.Rodriguez AM, Aquilino SA, Lund PS, Ryther JS, Southard TE. Evaluation of strain at the terminal abutment site of a fixed mandibular implant prosthesis during cantilever loading. J Prosthodont. 1993;2:93–102. doi: 10.1111/j.1532-849x.1993.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 48.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu JH, Kim SJ, Kim SH, Oh CD, Hwang SG, Chun CH, Oh SH, Seong JK, Huh TL, Chun JS. Regulation of the chondrocyte phenotype by beta-catenin. Development. 2002;129:5541–5550. doi: 10.1242/dev.129.23.5541. [DOI] [PubMed] [Google Scholar]
- 50.Sandell LJ, Adler P. Developmental patterns of cartilage. Front Biosci. 1999;4:D731–D742. doi: 10.2741/sandell. [DOI] [PubMed] [Google Scholar]
- 51.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 52.Singh Khillan J. Differentiation of embryonic stem cells into cartilage cells. Curr Protoc Stem Cell Biol. 2007;Chapter 1:Unit 1F.1. doi: 10.1002/9780470151808.sc01f01s2. [DOI] [PubMed] [Google Scholar]
- 53.Smith WL, Meade EA, DeWitt DL. Interactions of PGH synthase isozymes-1 and -2 with NSAIDs. Ann N Y Acad Sci. 1994;744:50–57. doi: 10.1111/j.1749-6632.1994.tb52723.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 55.Sols A, Crane RK. The inhibition of brain hexokinase by adenosinediphosphate and sulfhydryl reagents. J Biol Chem. 1954;206:925–936. [PubMed] [Google Scholar]
- 56.Srivastava SK, Tetsuka T, Daphna-Iken D, Morrison AR. IL-1 beta stabilizes COX II mRNA in renal mesangial cells: role of 3'-untranslated region. Am J Physiol. 1994;267:F504–F508. doi: 10.1152/ajprenal.1994.267.3.F504. [DOI] [PubMed] [Google Scholar]
- 57.Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68-69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 58.Tatari H. [The structure, physiology, and biomechanics of articular cartilage: injury and repair] Acta Orthop Traumatol Turc. 2007;41(Suppl 2):1–5. [PubMed] [Google Scholar]
- 59.Tower DB. The effects of 2-deoxy-D-glucose on metabolism of slices of cerebral cortex incubated in vitro. J Neurochem. 1958;3:185–205. doi: 10.1111/j.1471-4159.1958.tb12625.x. [DOI] [PubMed] [Google Scholar]
- 60.Ulianich L, Garbi C, Treglia AS, Punzi D, Miele C, Raciti GA, Beguinot F, Consiglio E, Di Jeso B. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci. 2008;121:477–486. doi: 10.1242/jcs.017202. [DOI] [PubMed] [Google Scholar]
- 61.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 62.Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK, Yoo YJ, Huh TL, Chun JS. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J Biol Chem. 2002;277:8412–8420. doi: 10.1074/jbc.M110608200. [DOI] [PubMed] [Google Scholar]
- 63.Yu SM, Lee WK, Yoon EK, Lee JH, Lee SR, Kim SJ. Src Kinase Regulates Nitric Oxide-induced Dedifferentiation and Cyclooxygenase-2 Expression in Articular Chondrocytes via p38 Kinase-dependent Pathway. Immune Netw. 2006;6:204–210. [Google Scholar]
- 64.Zaric J, Ruegg C. Integrin-mediated adhesion and soluble ligand binding stabilize COX-2 protein levels in endothelial cells by inducing expression and preventing degradation. J Biol Chem. 2005;280:1077–1085. doi: 10.1074/jbc.M410006200. [DOI] [PubMed] [Google Scholar]
- 65.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 66.Zhang K, Kaufman RJ. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb Exp Pharmacol. 2006a:69–91. doi: 10.1007/3-540-29717-0_3. [DOI] [PubMed] [Google Scholar]
- 67.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006b;66:S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]