Abstract
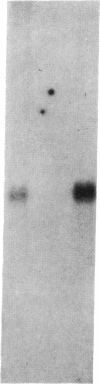
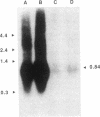
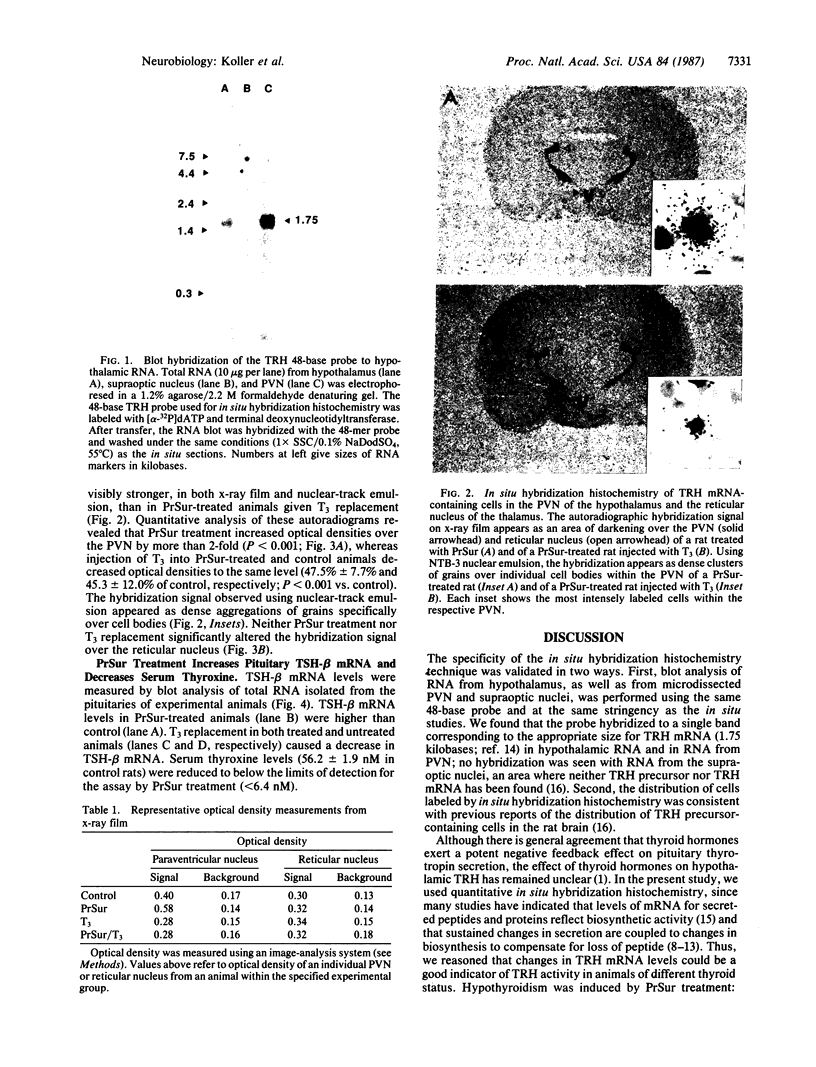
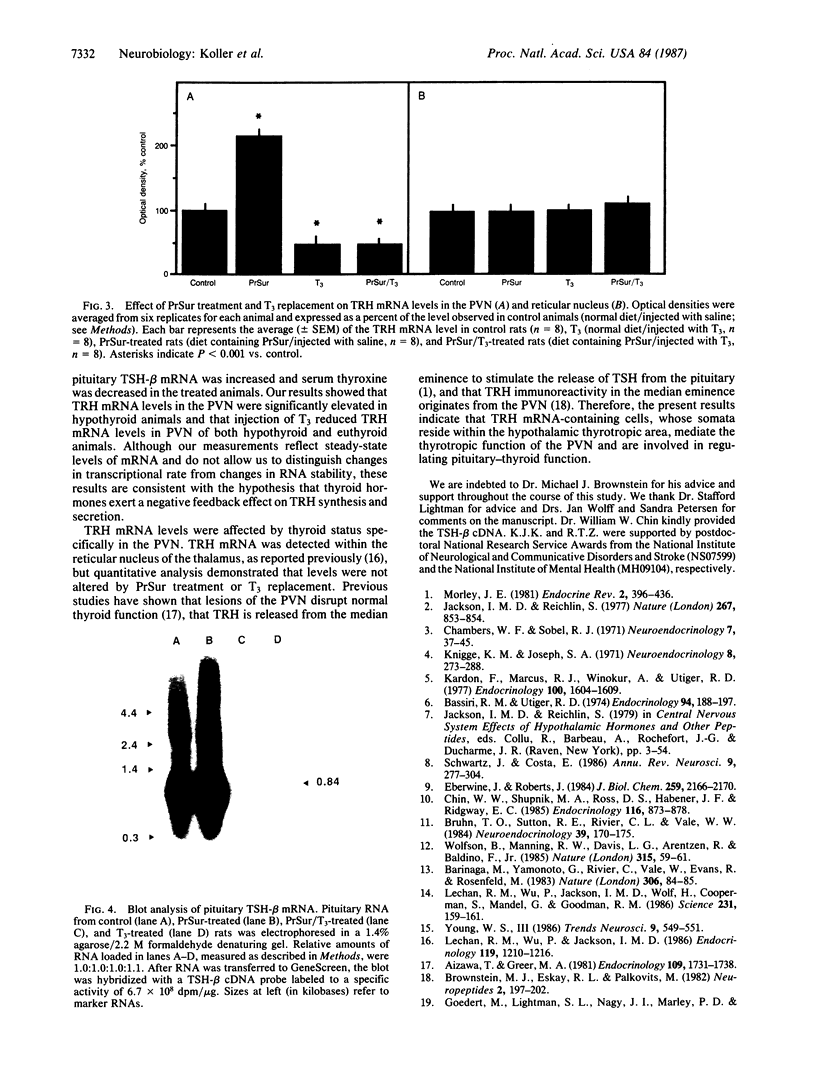
Cellular levels of messenger RNA encoding thyrotropin-releasing hormone (TRH) were measured in the paraventricular nucleus of the hypothalamus and the reticular nucleus of the thalamus in male rats after chemical thyroidectomy and thyroid hormone replacement. TRH mRNA levels were measured by quantitative in situ hybridization histochemistry using a 35S-labeled synthetic 48-base oligodeoxynucleotide probe and quantitative autoradiography. Chemical thyroidectomy, produced by the administration of 6-(n-propyl)-2-thiouracil (PrSur), reduced plasma thyroxine below detection limits and significantly increased TRH mRNA in the paraventricular nucleus. Treatment with exogenous L-triiodothyronine (T3) reduced TRH mRNA to the same level in both hypothyroid and euthyroid animals. Neither PrSur treatment nor T3 replacement influenced TRH mRNA levels in the reticular nucleus of the thalamus. Blot hybridization analysis of electrophoretically fractionated total RNA from pituitaries of these animals indicated that thyrotropin-beta mRNA levels were elevated after thyroidectomy and reduced by T3 treatment, showing that the pituitary-thyroid axis was indeed stimulated by PrSur treatment. These results suggest that thyroid hormones are involved, either directly or indirectly, in regulating the biosynthesis of TRH in the thyrotropic center of the hypothalamus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa T., Greer M. A. Delineation of the hypothalamic area controlling thyrotropin secretion in the rat. Endocrinology. 1981 Nov;109(5):1731–1738. doi: 10.1210/endo-109-5-1731. [DOI] [PubMed] [Google Scholar]
- Barinaga M., Yamonoto G., Rivier C., Vale W., Evans R., Rosenfeld M. G. Transcriptional regulation of growth hormone gene expression by growth hormone-releasing factor. Nature. 1983 Nov 3;306(5938):84–85. doi: 10.1038/306084a0. [DOI] [PubMed] [Google Scholar]
- Bassiri R. M., Utiger R. D. Thyrotropin-releasing hormone in the hypothalamus of the rat. Endocrinology. 1974 Jan;94(1):188–197. doi: 10.1210/endo-94-1-188. [DOI] [PubMed] [Google Scholar]
- Bruhn T. O., Sutton R. E., Rivier C. L., Vale W. W. Corticotropin-releasing factor regulates proopiomelanocortin messenger ribonucleic acid levels in vivo. Neuroendocrinology. 1984 Aug;39(2):170–175. doi: 10.1159/000123974. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers W. F., Sobel R. J. Effect of thyroxine-agar tube application to the rat hypothalamus. Neuroendocrinology. 1971;7(1):37–45. doi: 10.1159/000121953. [DOI] [PubMed] [Google Scholar]
- Chin W. W., Muccini J. A., Jr, Shin L. Evidence for a single rat thyrotropin-beta-subunit gene: thyroidectomy increases its mRNA. Biochem Biophys Res Commun. 1985 May 16;128(3):1152–1158. doi: 10.1016/0006-291x(85)91061-7. [DOI] [PubMed] [Google Scholar]
- Chin W. W., Shupnik M. A., Ross D. S., Habener J. F., Ridgway E. C. Regulation of the alpha and thyrotropin beta-subunit messenger ribonucleic acids by thyroid hormones. Endocrinology. 1985 Mar;116(3):873–878. doi: 10.1210/endo-116-3-873. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eberwine J. H., Roberts J. L. Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J Biol Chem. 1984 Feb 25;259(4):2166–2170. [PubMed] [Google Scholar]
- Eiden L. E., Hotchkiss A. J. Cyclic adenosine monophosphate regulates vasoactive intestinal polypeptide and enkephalin biosynthesis in cultured bovine chromaffin cells. Neuropeptides. 1983 Dec;4(1):1–9. doi: 10.1016/0143-4179(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Goedert M., Lightman S. L., Nagy J. I., Marley P. D., Emson P. C. Neurotensin in the rat anterior pituitary gland. Nature. 1982 Jul 8;298(5870):163–165. doi: 10.1038/298163a0. [DOI] [PubMed] [Google Scholar]
- Jackson I. M., Reichlin S. Brain thyrotrophin-releasing hormone is independent of the hypothalamus. Nature. 1977 Jun 30;267(5614):853–854. doi: 10.1038/267853a0. [DOI] [PubMed] [Google Scholar]
- Kardon F., Marcus R. J., Winokur A., Utiger R. D. Thyrotropin-releasing hormone content of rat brain and hypothalamus: results of endocrine and pharmacologic treatments. Endocrinology. 1977 Jun;100(6):1604–1609. doi: 10.1210/endo-100-6-1604. [DOI] [PubMed] [Google Scholar]
- Kelsey J. E., Watson S. J., Burke S., Akil H., Roberts J. L. Characterization of proopiomelanocortin mRNA detected by in situ hybridization. J Neurosci. 1986 Jan;6(1):38–42. doi: 10.1523/JNEUROSCI.06-01-00038.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knigge K. M., Joseph S. A. Neural regulation of TSH secretion: sites of thyroxine feedback. Neuroendocrinology. 1971;8(5):273–288. doi: 10.1159/000122014. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lechan R. M., Wu P., Jackson I. M. Immunolocalization of the thyrotropin-releasing hormone prohormone in the rat central nervous system. Endocrinology. 1986 Sep;119(3):1210–1216. doi: 10.1210/endo-119-3-1210. [DOI] [PubMed] [Google Scholar]
- Lechan R. M., Wu P., Jackson I. M., Wolf H., Cooperman S., Mandel G., Goodman R. H. Thyrotropin-releasing hormone precursor: characterization in rat brain. Science. 1986 Jan 10;231(4734):159–161. doi: 10.1126/science.3079917. [DOI] [PubMed] [Google Scholar]
- Morley J. E. Neuroendocrine control of thyrotropin secretion. Endocr Rev. 1981 Fall;2(4):396–436. doi: 10.1210/edrv-2-4-396. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Costa E. Hybridization approaches to the study of neuropeptides. Annu Rev Neurosci. 1986;9:277–304. doi: 10.1146/annurev.ne.09.030186.001425. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Reppert S. M. Suprachiasmatic nucleus vasopressin messenger RNA: circadian variation in normal and Brattleboro rats. Science. 1986 Apr 18;232(4748):390–393. doi: 10.1126/science.3961487. [DOI] [PubMed] [Google Scholar]
- Wolfson B., Manning R. W., Davis L. G., Arentzen R., Baldino F., Jr Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature. 1985 May 2;315(6014):59–61. doi: 10.1038/315059a0. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Bonner T. I., Brann M. R. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]