Abstract
Xanthine oxioreductase is the holoenzyme responsible for terminal purine catabolism. Under conditions of metabolic stress or heightened pro-inflammatory cytokine production this enzyme is preferentially in it’s oxidized form, xanthine oxidase, with catalytic action that generates uric acid and the free radical superoxide. As preeclampsia is characterized by heightened inflammation, oxidative stress and hyperuricemia it has been proposed that xanthine oxidase plays a pivotal role in this hypertensive disorder of pregnancy. We sought to determine whether xanthine oxidase protein content was higher in maternal tissue of preeclamptic mothers, compared to healthy pregnant controls, using immunohistochemical analysis of skin biopsies. We further compared xanthine oxidase immunoreactivity in skin biopsies from preeclamptic women and patients with several inflammatory conditions. In preeclamptic women, intense xanthine oxidase immunoreactivity was present within the epidermis. By contrast, only very faint xanthine oxidase staining was observed in skin biopsies from healthy pregnant controls. Further, a role for inflammation in the increase of xanthine oxidase was suggested by similar findings of heightened xanthine oxidase immunoreactivity in the skin biopsies from non-pregnant individuals diagnosed with conditions of systemic inflammation. The finding of increased xanthine oxidase in maternal tissue, most likely as the result of heightened maternal inflammation, suggest maternal xanthine oxidase as a source of free radical and uric acid generation in preeclampsia.
Keywords: xanthine oxidase, preeclampsia, uric acid, reactive oxygen species, epidermis
Introduction
Preeclampsia, a multi-systemic hypertensive syndrome of pregnancy, is a leading cause of maternal morbidity and mortality and increases perinatal mortality five-fold. Diagnosed by de novo gestational hypertension and proteinuria, the disorder usually becomes apparent in late pregnancy. Several pathophysiological components of preeclampsia are, however, evident much earlier in pregnancy. Markers of heightened systemic inflammation1-4 and hyperuricemia5 are evident weeks to months before clinically evident preeclampsia.
Hyperuricemia is one of the earliest and most consistent observations in preeclamptic pregnancies, first reported at the beginning of the twentieth century.6, 7 There is a direct correlation between circulating uric acid concentrations and the severity of preeclampsia, for both maternal and fetal outcomes8-10, however the clinical utility of uric acid as a predictive tool for identifying women likely to develop preeclampsia remains contested.11 Hyperuricemia in preeclampsia is commonly explained as the result of abnormal renal function; reduced glomerular filtration rates and increased re-absorption leading to decreased excretion of uric acid.12, 13 However, increased circulating concentrations of uric acid are present at less than 15 weeks gestation, prior to evident changes in renal function and the higher uric acid concentration persists with control for glomerular filtration in women destined to develop preeclampsia.5 It has therefore been postulated that in addition to alterations in renal handling, increased uric acid production is also responsible for the hyperuricemia observed in preeclampsia.7, 14, 15
Uric acid is generated by the oxidative hydroxylation of hypoxanthine and xanthine catalyzed by the holoenzyme xanthine oxioreductase (XOR). The enzyme is found primarily in endothelial and epithelial cells of the liver and gut16 but is also present in skeletal muscle, mammary gland, kidney and immune cells including monocytes and mast cells.17 Xanthine oxioreductase has two functional forms. Xanthine dehydrogenase (XDH), which couples the production of uric acid to the reduction of nicotinamide-adenine dinucleotide (NAD+), and xanthine oxidase (XO), which generates the free radical superoxide (O2−) in addition to uric acid. Xanthine oxidase is the preferentially active form under conditions of increased substrate availability, metabolic stress, hypoxia and heightened cytokine production.17-21
An increase in XO activity has been proposed as a source for oxidative stress in preeclampsia.7, 14 Xanthine oxidase expression and activity are increased in invasive cytotrophoblasts in preeclamptic pregnancies.22 Additionally, XO activity is increased in maternal and fetal blood in preeclamptic pregnancies.23 Increased XO activity would increase both reactive oxygen species and uric acid generation.
Xanthine oxidase activity is increased with heightened inflammation. In animal studies activation of the-inflammatory cascade, through several stimuli including thermal injury,24 LPS treatment,25 and carcinogen exposure,26, 27, 28 up regulates XOR with preferential XO catalytic activity. A pathogenic role for XO in preeclampsia is suggested by the heightened systemic inflammation, widespread oxidative stress and hyperuricemia characteristic of preeclampsia, all elements related to the XOR enzyme activity.
While increased XO activity has been sought and found in the placenta, the potential contributions of maternal tissue XO to the pathophysiology of preeclampsia has received little attention. We sought to determine whether XO protein content was higher in maternal tissues of preeclamptic mothers, compared to healthy pregnant controls, using immunohistochemical analysis of skin biopsies. We further compared XO immunoreactivity in skin biopsies from preeclamptic women and patients with several inflammatory conditions.
Materials and Methods
Study subjects
The study was approved by the institutional review board and all subjects provided informed consent. Skin biopsies from pregnant women were collected at Magee-Womens Hospital (Pittsburgh, PA), at the time of cesarean section. Preeclampsia was diagnosed as new onset gestational hypertension, proteinuria, hyperuricemia, and reversal of hypertension and proteinuria by twelve weeks postpartum. Hypertension was defined as an increase of 30 mmHg systolic or 15 mmHg diastolic blood pressure compared to values obtained before 20 weeks gestation and an absolute blood pressure >140/90 mmHg. Proteinuria was defined as >300 mg/24 hour collection or >2+ on voided or 1+ on catheterized random urine sample or a protein creatinine ratio > 0.3. Hyperuricemia was defined as >1 SD above normal values for the time of gestation 29. Women with chronic hypertension or those with additional medical complications were excluded. Pregnant control samples were collected from women at term undergoing elective cesarean section. Women in this group had normal blood pressure and no medical complications.
Skin biopsies from non-pregnant controls and subjects with chronic inflammatory conditions were collected at the Veterans Affairs Medical Center (Pittsburgh, PA), for immunopathological examination and diagnosis. The diagnosis of lupus erythematous was based upon characteristic dermatopathological findings, clinical presentation and serological profiles such as antinuclear antibody. Dermatitis diagnosis was based upon clinical presentation and nonspecific spongiotic pathologic change. Lichen simplex chronicus was characterized by typical clinical appearance of scaly thickened plaques and epidermis. Diagnosis of mixed connective tissue disease was by the presence of antinuclear antibody on direct skin immunofluorescence and characteristic clinical presentation and presence of high concentrations of circulating autoantibody such as antinuclear antibody and antinuclear ribonucleoprotein (nRNP) antibody. Bullous pemphigoid was diagnosed by the presence of skin blisters on erythematous skin and presence of immunoreactants along skin basement membrane zone.
Tissue collection and processing
Skin samples were collected in non-pregnant individuals using punch biopsies. In the pregnant women biopsies were obtained from the cesarean section skin incision. In the non-pregnant subjects with chronic inflammatory disorders the biopsies were collected from lesional skin at various sites including abdomen, trunk, thigh and forearm. In the non-pregnant control population the skin biopsies were collected from benign skin lesions located on the trunk, back and forearm. Skin biopsies were immediately collected in OCT compound, flash frozen and stored at − 80’C until further processing.
Immunohistochemical Fluorescent Staining
The frozen biopsies were cryosectioned to a thickness of 6 μm, fixed in ice-cold acetone for 10 minutes and allowed to air dry. Non-specific binding was blocked with 10% normal goat serum (NGS) in phosphate buffered saline (PBS) for 20 min. Endogenous biotin was blocked using the streptavidin-biotin blocking kit (Vector Laboratories; Burlingame, CA). The skin biopsies were incubated with a primary antibody directed against XO (rabbit polyclonal IgG, 1:3000 dilution in 10% NGS, Rockland, Gilbertville, PA) for 60 minutes at room temperature and washed in PBS. Amplification and visualization of antibody-antigen binding was accomplished using the biotin-streptavidin-FITC labeling technique in which the sections were incubated with biotinylated goat-anti-rabbit IgG (1:750 dilution in 10% NGS, Vector Laboratories) for 45 minutes, washed extensively with PBS and subsequently incubated with FITC-conjugated streptavidin (1:500 dilution in PBS, Vector Laboratories) for 15 minutes in the dark at room temperature. Following several PBS rinses the tissue was counter-stained with 0.5% pontamine sky blue (Sigma) to eliminate auto-fluorescence of connective tissue. The slides were again rinsed with PBS and mounted with Vectasheild mounting media (Vector Laboratories). Negative controls were obtained by omission of primary antibody. Sections were viewed with a Zeiss Axiophot Epiflourescence microscope equipped with filters to selectively view the rhodamine (Pontamine sky blue fluoresces in the rhodamine spectrum) and fluorescein images without cross contamination.
Immunohistochemical Fluorescent Quantification and Statistical Analysis
Immunohistochemical fluorescent quantification was performed by calculating the mean green fluorescent intensity within the squamous portion of the epithelial layer, including the stratum granulosum and stratum corneum layers of the epidermis. The squamous portion of the epidermis was outlined on blinded digital images for each skin biopsy and mean green fluorescent intensity was measured within the outlined segment using Image J software (National Institute of Health, http://rsbweb.nih.gov/ij/). Due to a relatively small sample size the data were not found to be normally distributed and as such data are presented as median +/− interquartile ranges. Differences in XO immunofluorescent intensity between the healthy non-pregnant, healthy pregnant and preeclamptic subject groups were examined using a Kruskal-Wallis non-parametric one-way analysis of variance with Dunn’s post-hoc analysis. Statistical analysis was set at p<0.05.
Results
Sample Population
Skin biopsies were collected from five healthy pregnant controls and five preeclamptic women. The demographic data for these two groups of women is outlined in Table I. There were no differences in maternal age, pre-pregnancy body mass index (BMI) and early pregnancy blood pressures between the two groups. As expected both systolic and diastolic pressures were higher in the preeclamptic group at the time of delivery, along with serum uric acid values. Additionally proteinuria was undetectable in all pregnant control patients. The mean gestational age at delivery was also significantly lower in the preeclamptic group. All women in both groups were non-smokers.
Table I.
Clinical characteristics of pregnant patients
| Clinical characteristics |
Healthy Pregnant Controls (n = 5) |
Preeclampsia (n =5) |
Mann-Whitney p- value |
|---|---|---|---|
| Maternal Age (yrs) | 28.4 ± 4.9 | 29.6 ± 9.1 | 0.80 |
| Maternal Smoking (%) |
0 | 0 | 1.0 |
| Maternal race | 4 Caucasian 1 Black |
4 Caucasian 1 Asian |
N/A |
| Maternal Pre- pregnancy BMI |
26.8 ± 6.8 | 30.5 ± 5.4‡ | 0.43 |
| Systolic pressure prior to 20 weeks gestation (mmHg) |
114.2 ±13.3 | 120.0 ± 8.8 | 0.48 |
| Diastolic pressure prior to 20 weeks gestation (mmHg) |
75.0 ± 7.5 | 74.5 ± 7.3 | 0.92 |
| Systolic pressure at term (mmHg) |
115.6 ±14.3 | 167.8 ± 17.2 | < 0.01* |
| Diastolic pressure at term (mmHg) |
72.4 ±12.1 | 94.2 ± 11.1 | 0.018* |
| Proteinurea | 0/5 | 5/5 | N/A |
| Weeks gestation at delivery |
39.0 ± 0.6 | 32.7 ± 3.8 | 0.01* |
| Serum uric acid at delivery (mg/dL) |
3.98 ± 1.0 | 5.98 ± 1.3 | 0.02* |
| Infant birth weight (g) |
3470.6 ± 587.3 | 2399.8 ± 1060.3 | 0.08 |
| Infant birth weight centile |
62.0 ± 37.1 | 62.7 ± 41.9 | 0.98 |
| Number of primiparas |
5/5 | 3/5 | N/A |
| Laboring prior to c- section |
2/5 | 1/5 | N/A |
statistically significant differences between healthy pregnant controls and preeclamptic women
In the normal non-pregnant population skin biopsies were collected from six non-pregnant controls, three males and three females. Additionally, biopsies were collected from five patients with systemic lupus erythematosis, three dermatitis patients, one patient with lichen simplex, one patient with mixed connective tissue disease and five bullous pemphigoid patients, of whom two had remittent disease. The majority of the patients were female and the ages of patients ranged from 34 to 73 years. The demographic data for these patients is outlined in Table II.
Table II.
Clinical characteristics of non-pregnant patients
| Clinical characteristics |
Healthy Controls (n = 6) |
Systemic Lupus Erythematosis (n =5) |
Dermatitis (n =3) |
Lichen Simplex (n=l) |
Mixed Connective Tissue Disease (n=1) |
Bullous pemphigod (n=5) |
|---|---|---|---|---|---|---|
| Age | 34.8 ± 8.7 | 37.4 ± 14.4 | 50 | 42 | 45 | 73 ± 6.4 |
|
Female: Male
Ratio |
3:3 | 5:0 | 2:0 | 0:1 | 1:0 | 0:5 |
|
Site of skin
biopsy |
3 trunk 2 back 1 forearm |
3 forearm 1 trunk 1 chest |
2 forearm | 1 forearm | 1 forearm | 2 abdomen 1 thigh 2 forearm |
|
Other noted
medical conditions |
---- |
---- |
---- |
---- |
---- |
Hypertension in 2 patients * both on anti- hypertensive medication |
Increased XO Immunoreactivity in Epidermis of Preeclamptic Women
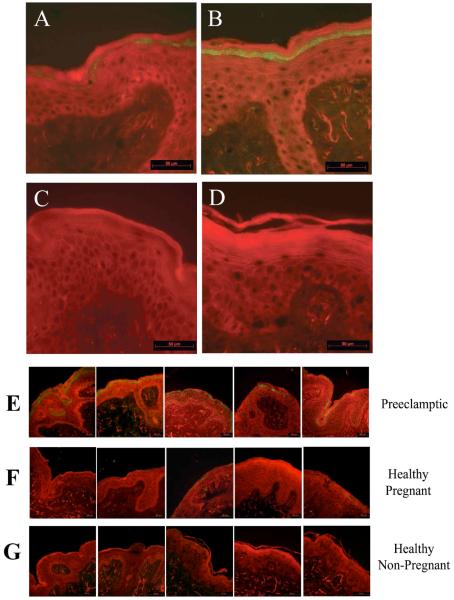
All 5 skin biopsies collected from preeclamptic patients demonstrated distinct and intense XO immunoreactivity within the stratum granulosum layer of the epidermis (Figure 1, Panel B and E; Figure 2). Faint and sporadic XO positive staining was observed in the same layer of the epidermis in all the healthy pregnant control biopsies (Figure 1, Panel A and F; Figure 2). Omission of primary antibody resulted in no visible staining (Figure 1, Panel D).
Figure 1.
Xanthine oxidase immunoreactivty (green staining) is present in stratum granulosum layer of epidermis in preeclamptic women (panel B) compared to faint XO immunoreactivity in the same epidermal layer of healthy pregnant controls (panel A). Healthy non-pregnant controls demonstrate minimal epidermal XO immunoreactivity (panel C). These patterns of immunohistochemical staining were observed in all 5 preeclamptic skin biopsies (panel E), all 5 healthy pregnant controls (panel F) and all 5 healthy non-pregnant controls (panel G). Omission of the primary antibody directed against XO results in no observable immunoreactivity (panel D). Scalebar = 50μm.
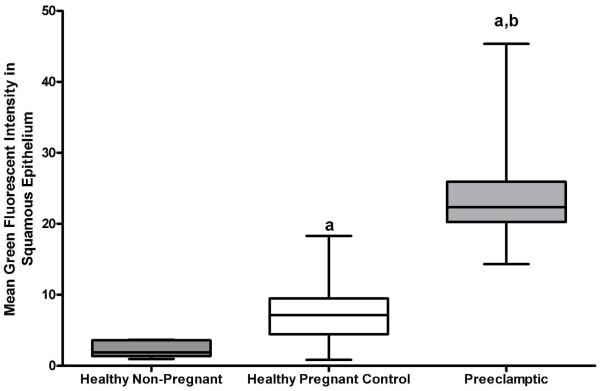
Figure 2.
Quantitative analysis of XO immunohistochemical fluorescent intensity within the squamous epithelial layers of skin biopsies collected from healthy non-pregnant (n=5), healthy pregnant (n=5) and preeclamptic (n=5) study subjects. Data is presented as median ± IQR. P<0.05, Kruskal-Wallis one-way analysis of variance; (a) P<0.05, Dunn’s post hoc analysis compared to healthy non-pregnant subjects; (b) P<0.05, Dunn’s post hoc analysis compared to healthy pregnant subjects.
Increased XO Immunoreactivity in Epidermis of Patients with Inflammatory Conditions
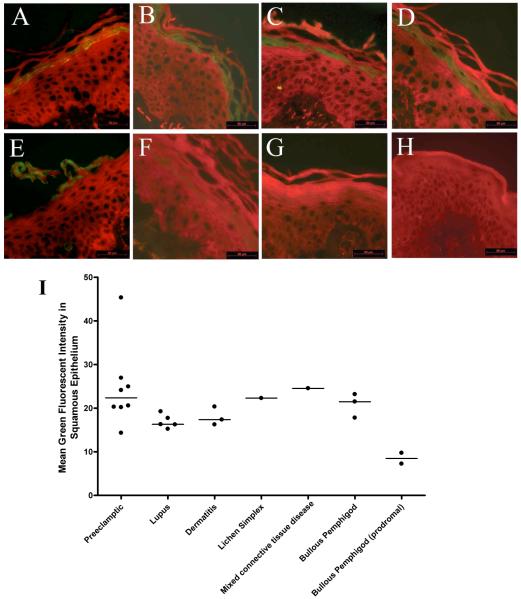
All skin biopsies collected from non-pregnant patients with inflammatory conditions, including lupus, dermatitis, lichen simplex, mixed connective tissue disease and bullous phemphigod demonstrated immunoreactivity to XO within the stratum granulosum layer of the epidermis (Figure 3, Panel A-E, I). In contrast, skin biopsies collected from healthy non-pregnant individuals, both males and females, demonstrated very little to no XO immunoreactivity (Figure 1, Panel C and G; Figure 3, Panel G). Similarly in individuals with the inflammatory skin condition bullous pemphigoid that was in remission, XO staining was minimally present when the disease was not active (Figure 3, Panel F).
Figure 3.
Xanthine oxidase immunoreactivity (green staining) is present in the stratum granulosum layer of the epidermis from patients with conditions of chronic inflammation including systemic lupus erythematosis (panel A), dermatitis (panel B), lichen simplex (panel C), mixed connective tissue disease (panel D) and bullous phemphigod (panel E). Patients with bullous phemphigod in which the disease is in remission demonstrate little epidermal XO immunoreactivity (panel F). Similarly, minimal XO immunoreactivity is observed in epidermis of healthy non-pregnant controls (panel G). Omission of the primary antibody directed against XO results in no observable immunoreactivity (panel H). Representative images are shown, along with quantitative analysis of XO immunofluorescent intensity within the squamous epithelial layers (panel I) Scalebar = 50μm.
Discussion
Xanthine oxidase generates uric acid and superoxide, and has been postulated to contribute to the pathogenesis of preeclampsia. Increased XOR and XO activity are present in the placentas of preeclamptic pregnancies.22 Our findings suggest maternal XO as a further source of free radical and uric acid generation. In preeclamptic women, there was intense XO immunoreactivity within the epidermis. By contrast, only very faint XO staining was observed in skin biopsies from healthy pregnant controls. Further, a role for inflammation in the increase of XO was suggested by similar findings in non-pregnant individuals diagnosed with conditions of systemic inflammation.
Xanthine Oxidase in Skin
The epidermis is composed of keratinized stratified squamous epithelium arranged in four distinct layers: the stratum basalis, stratum spinosum, stratum granulosum and stratum corneum. In both the preeclamptic and inflammatory conditions, XO staining was localized within the stratum granulosum layer of the epidermis. This highly keratinized layer consists of flattened cells containing keratohyalin granules, and is thought to play an important barrier role towards the external environment. Xanthine oxidase activity has previously been reported in human hair roots and several catabolites of nucleic acids, including hypoxanthine, xanthine and uric acid have been described in keratinized structures,30, 31 however, we could find no further studies directly demonstrating either the presence or activity of the enzyme within healthy human epidermis. The presence of enzymatically active XO has, however, been documented within this barrier layer of the epidermis in rats, mice and guinea pigs.32 It is speculated that the spatial localization of XO within the stratum granulosum may be linked to terminal differentiation of keratinocytes.33 It is proposed that the activity may be a component of the innate immunity of the integumentary system, protecting the skin against microbial attacks.32, 34
Xanthine Oxidase and Inflammation
Our data demonstrate increased XO protein content in skin with increased systemic inflammation. Small amounts of XO were observed in the skin of healthy non-pregnant women, more with the heightened inflammation characteristic of pregnancy35 and much more in women with preeclampsia in which the inflammatory response of normal pregnancy is further increased. Increased XO immunoreactivity was also present in subjects with several inflammatory skin diseases or with the systemic inflammation of lupus erythematosis but not in patients with the inflammatory condition bullous pemphigoid when the disease was not active. The correlation we have demonstrated between XO enzyme in skin and inflammation in humans is complemented by prior studies demonstrating a dramatic increase in circulating XO activity in patients with autoimmune conditions that was reduced by glucocorticoid treatment.36 Specific to skin, XO activity is increased in dermal tissue homogenates from psoriatic plaques,37 thought in part to explain the hyperuricemia often observed in individuals with psoriasis.38 In vitro stimulation of inflammatory cascades in human keratinocytes through UV irradiation also stimulates an up-regulation of XO expression with subsequent increases in superoxide production by these cells.39
The role of inflammation to increase skin XO content is supported by animal experiments. Thermal skin injury in rats results in amplified catalytic activity of xanthine oxidase with increased circulating uric acid and reactive oxygen species.24 Direct stimulation of pro-inflammatory cascades by sub-dermal injections of lipopolysaccharide increases superoxide and peroxynitrite formation within the epidermis of mice, subsequently abolished by the xanthine oxidase inhibitor allopurinol.25 Additionally, skin tumor promotion in mice activates the immune system with a parallel increase in epidermal XO protein content and activity.26, 27, 28 Notable in many of these studies is the demonstration of XO enzymatic activity in the skin, which we did not determine in our study.
The association between inflammation and increased tissue XO content is consistent with pro-inflammatory response elements in the 5′ flanking region of the human XDH/XO gene.40 Further, activated leukocytes preferentially convert XDH into XO in endothelial cells.41-43 Importantly, cytokine induced increases in XO protein content translates into measurable increases in catalytic activity, resulting in increased generation of uric acid and superoxide. In human mammary epithelial cell TNF-alpha, IL-1β and interferon-gamma respectively elicited a 2, 2.5 and 8-fold increase in enzymatic activity.19
Limitations of Study
There are limitations of this study to consider when interpreting this data. First is the limited number of skin biopsies available for examination from the study groups. While the results of this study warrant further collection of samples and examination of the XO enzyme system within maternal epidermis we feel that the observation of heightened immunoreactivity in all five skin biopsy collected from preeclamptic women with limited imunoreactivity in all five skin biopsy collected from our healthy pregnant controls (Figure 1, panels E and F) is a finding worth expedited dissemination.
Secondly, all skin biopsies collected from pregnant women were collected from one anatomical site on the lower abdomen, at the site of cesarean section incision. Therefore we cannot conclusively determine whether this heightened XO immunoreactivity would be observed in the epidermis from other locations on the body. Since the skin samples were taken at the conclusion of the cesarean section procedure it is possible that this may have resulted in localized inflammation within the skin at the incision site. However, since this same insult would be present in both the healthy pregnant controls as well as the preeclamptic women it is most likely not a contributing factor to the differential XO expression observed in the skin biopsies from this area. Further, biopsy sites from non-pregnant inflammatory condition patients were located at numerous locations on the body including abdomen, trunk, thigh and forearm and no differences in XO immunoreactivity was observed based on biopsy site alone.
It is also important to note that all preeclamptic skin biopsies were collected from women demonstrating overt signs and symptoms of the disorder. Therefore it cannot be determined whether these women entered pregnancy with a heightened immune response and constitutive elevations in tissue XO expression, or whether this increase developed during pregnancy prior to clinically evident disease, and/or whether this observed increase in XO protein was the result of the disease process. Thus, we cannot state that this increase in tissue XO in preeclamptic women contributes to the initiation of this syndrome. We would, however, argue that this potentially very large increase of XO observed in the third trimester skin, in association with an increased source of substrate from the enhanced placental aponecrosis characteristic of preeclampsia,4 could help drive forward the pathological processes of preeclampsia. These observations suggest an interesting link between the maternal epidermis and the pathological process of preeclampsia. It is intriguing to consider that the elevations in circulating uric acid observed in our preeclamptic patients may possibly be in part the result of increased production in the skin in a fashion similar to that observed in patients with psoriasis in whom increased XO expression in the epidermal psoratic plaques is paralleled with an increase in circulating uric acid.38 However it should be stressed that the epidermal tissue bed is most likely only one of many potential sources of both uric acid and superoxide. It is likely that numerous maternal, and possibly fetal, tissue beds demonstrate a parallel increase in XO.
This study demonstrated an increase in XO protein content in the epidermis of preeclamptic women. Increased XO mass may not necessarily reflect increased XO enzyme activity. While this does limit our conclusions regarding increased maternal tissue XO activity, animal studies of epidermal XO generated in response to inflammatory activators demonstrate significant increases in XO in a similar epidermal location identified in our study.26-28, 32 Furthermore in these studies increased epidermal XO expression and protein concentrations were paralleled by an increase in enzyme activity, as measured by increases in circulating uric acid and reactive oxygen species generation. 24-26,28
Finally, it is worth considering that maternal BMI may play a role in altered xanthine oxidase expression in preeclamptic women. XOR has been characterized as a novel regulator of adipogenesis. In vitro knockdown of XOR expression results in inhibition of adipocyte generation.44 In vivo, XOR knockout mice demonstrate a 50% reduction in adipose mass while conversely a mouse strain with a spontaneously obese phenotype (ob/ob) exhibit increased concentrations of XOR mRNA and urate in adipose tissue.44 While there were significant differences in maternal BMI between our control and preeclamptic group (Table 1) there was significant overlap between the BMI values in the two groups and we found no obvious increases in XO immunoreactivity with increasing maternal BMI in our preeclamptic group. However, the role of maternal obesity in the pathophysiology of preeclampsia is a research priority within our group and we hope to further investigate the impact of maternal BMI on xanthine oxidase expression and activity in future investigations.
Conclusion
The relevance of these findings to the understanding of the pathophysiology of preeclampsia, is two-fold. First they emphasize the importance of increased inflammation within the syndrome, demonstrating the far-reaching effects of an activated maternal immune system. Secondly, skin is an enormous organ and if what we have demonstrated in abdominal skin is representative of the entire surface area of skin of a preeclamptic woman this could potentially be an enormous contributor to the high concentrations of uric acid and increased reactive oxygen species in preeclamptic women.
The increased production of superoxide would contribute significantly to the widespread oxidative stress observed in preeclamptic mothers, directly and indirectly impacting the health and functioning of vascular endothelium. Although the implications of increased XO activity rest largely with the increased generation of superoxide it is possible that uric acid may also contribute to the pathophysiology of preeclampsia. While moderate amounts of uric acid have a protective anti-oxidant effect elevated concentrations of uric acid, particularly with compromised antioxidant availability, manifest free radical activity.45, 46 There is clinical and experimental evidence that uric acid can directly contribute to the pathological processes leading to hypertension, cardiovascular and renal disease.47, 48, 49 Similar studies in preeclampsia are warranted as well as further examination of the effects of uric acid on other components of the pathogenesis of preeclampsia such as anti-angiogenic factors. These findings, highlighting the pathogenic potential of uric acid, have led to speculation by our group15, and others14, 50, that hyperuricemia observed in preeclamptic women may be more than simply a marker of disease severity and may in fact directly contribute to the pathology of the disorder.
The finding of increased XO in maternal tissue, most likely as the result of heightened maternal inflammation, provides another potential factor contributing to the pathological processes involved in the progression of preeclampsia. The safety during pregnancy of directly inhibiting XO with allopurinal remains to be determined before tests of therapy would be appropriate. However if uric acid itself is a pathogen there is likely enough experience with the use of the uricosuric probenecid in pregnancy to allow clinical trials.
Acknowledgments
This work was supported in part by NIH grant P01 HD303967 and the Magee-Womens Research Institute Postdoctoral Fellowship.
Sources of Support: National Institute of Health (grant # P01 HD303967), Magee-Womens Research Institute Postdoctoral Fellowship.
List of Abbreviations
- BMI
body mass index
- FITC
Fluorescein isothiocyanate
- IL-1B
interleukin-1-beta
- mmHg
millimeters of mercury
- NAD+
nicotinamide-adenine dinucleotide
- NGS
normal goat serum
- nRNP
nuclear ribonucleoprotein
- O2−
superoxide
- OCT
optimal cutting temperature
- PBS
phosphate buffered saline
- TNF-alpha
tumor necrosis factor-alpha
- UV
ultra violet
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
- XOR
xanthine oxioreductase
Footnotes
Conflict of Interest/Disclosure
None.
References
- 1.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007 Apr;28(2):192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Walther T, Wallukat G, Jank A, et al. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005 Dec;46(6):1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 3.Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007 Aug;50(2):269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu C, Luthy DA, Zhang C, Walsh SW, Leisenring WM, Williams MA. A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. Am J Hypertens. 2004 Feb;17(2):154–160. doi: 10.1016/j.amjhyper.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Powers RW, Bodnar LM, Ness RB, et al. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006 Jan;194(1):160. doi: 10.1016/j.ajog.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Slemmons JM, B. LJ. The uric acid content of maternal and fetal blood. J Biol Chem. 1917;32:63–69. [Google Scholar]
- 7.Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996 Jan;174(1 Pt 1):288–291. doi: 10.1016/s0002-9378(96)70410-6. [DOI] [PubMed] [Google Scholar]
- 8.Redman CW, Beilin LJ, Bonnar J, Wilkinson RH. Plasma-urate measurements in predicting fetal death in hypertensive pregnancy. Lancet. 1976 Jun 26;1(7974):1370–1373. doi: 10.1016/s0140-6736(76)93024-5. [DOI] [PubMed] [Google Scholar]
- 9.Voto LS, Illia R, Darbon-Grosso HA, Imaz FU, Margulies M. Uric acid levels: a useful index of the severity of preeclampsia and perinatal prognosis. J Perinat Med. 1988;16(2):123–126. doi: 10.1515/jpme.1988.16.2.123. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Bodnar LM, Lain KY, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005 Dec;46(6):1263–1269. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- 11.Cnossen JS, de Ruyter-Hanhijarvi H, van der Post JA, Mol BW, Khan KS, ter Riet G. Accuracy of serum uric acid determination in predicting pre-eclampsia: a systematic review. Acta Obstet Gynecol Scand. 2006;85(5):519–525. doi: 10.1080/00016340500342037. [DOI] [PubMed] [Google Scholar]
- 12.Chesley LC, Williams LO. Renal glomerular and tubular function in relation to the hyperuricemia of pre-eclampsia and eclampsia. Am J Obstet Gynecol. 1945;50:367–375. [Google Scholar]
- 13.Yoshimura A, Ideura T, Iwasaki S, Koshikawa S. Significance of uric acid clearance in preeclampsia. Am J Obstet Gynecol. 1990 Jun;162(6):1639–1640. doi: 10.1016/0002-9378(90)90953-5. [DOI] [PubMed] [Google Scholar]
- 14.Kang DH, Finch J, Nakagawa T, et al. Uric acid, endothelial dysfunction and preeclampsia: searching for a pathogenetic link. J Hypertens. 2004 Feb;22(2):229–235. doi: 10.1097/00004872-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge SA, Roberts JM. Uric Acid as a Pathogenic Factor in Preeclmapsia. Placenta, Trophoblast Research. 2008;22(Supplement A):S67–S72. doi: 10.1016/j.placenta.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin HM, Hancock JT, Salisbury V, Harrison R. Role of xanthine oxidoreductase as an antimicrobial agent. Infect Immun. 2004 Sep;72(9):4933–4939. doi: 10.1128/IAI.72.9.4933-4939.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med. 2002 Sep 15;33(6):774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 18.Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988 May;254(5 Pt 1):G768–774. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- 19.Page S, Powell D, Benboubetra M, et al. Xanthine oxidoreductase in human mammary epithelial cells: activation in response to inflammatory cytokines. Biochim Biophys Acta. 1998 Jul 23;1381(2):191–202. doi: 10.1016/s0304-4165(98)00028-2. [DOI] [PubMed] [Google Scholar]
- 20.Kurose I, Granger DN. Evidence implicating xanthine oxidase and neutrophils in reperfusion-induced microvascular dysfunction. Ann N Y Acad Sci. 1994 Jun 17;723:158–179. [PubMed] [Google Scholar]
- 21.Wiezorek JS, Brown DH, Kupperman DE, Brass CA. Rapid conversion to high xanthine oxidase activity in viable Kupffer cells during hypoxia. J Clin Invest. 1994 Dec;94(6):2224–2230. doi: 10.1172/JCI117584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol. 2000 Jan;156(1):321–331. doi: 10.1016/S0002-9440(10)64733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karabulut AB, Kafkasli A, Burak F, Gozukara EM. Maternal and fetal plasma adenosine deaminase, xanthine oxidase and malondialdehyde levels in pre-eclampsia. Cell Biochem Funct. 2005 Jul-Aug;23(4):279–283. doi: 10.1002/cbf.1152. [DOI] [PubMed] [Google Scholar]
- 24.Friedl HP, Till GO, Trentz O, Ward PA. Roles of histamine, complement and xanthine oxidase in thermal injury of skin. Am J Pathol. 1989 Jul;135(1):203–217. [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai K, Kadiiska MB, Jiang JJ, Stadler K, Mason RP. Free radical production requires both inducible nitric oxide synthase and xanthine oxidase in LPS-treated skin. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4616–4621. doi: 10.1073/pnas.0510352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiners JJ, Jr., Thai G, Rupp T, Cantu AR. Assessment of the antioxidant/prooxidant status of murine skin following topical treatment with 12-O-tetradecanoylphorbol-13-acetate and throughout the ontogeny of skin cancer. Part I: Quantitation of superoxide dismutase, catalase, glutathione peroxidase and xanthine oxidase. Carcinogenesis. 1991 Dec;12(12):2337–2343. doi: 10.1093/carcin/12.12.2337. [DOI] [PubMed] [Google Scholar]
- 27.Rahman S, Bhatia K, Khan AQ, et al. Topically applied vitamin E prevents massive cutaneous inflammatory and oxidative stress responses induced by double application of 12-O-tetradecanoylphorbol-13-acetate (TPA) in mice. Chem Biol Interact. 2008 Apr 15;172(3):195–205. doi: 10.1016/j.cbi.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Pence BC, Reiners JJ., Jr. Murine epidermal xanthine oxidase activity: correlation with degree of hyperplasia induced by tumor promoters. Cancer Res. 1987 Dec 1;47(23):6388–6392. [PubMed] [Google Scholar]
- 29.Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol. 1984 Feb;91(2):128–132. doi: 10.1111/j.1471-0528.1984.tb05895.x. [DOI] [PubMed] [Google Scholar]
- 30.Santoianni P, Ayala F. Nucleic acids enzymes in human hair roots. Acta Derm Venereol. 1973;53(6):449–452. [PubMed] [Google Scholar]
- 31.Ogura R, Kumano S. Free nucleosides and bases in the hyperkeratotic epidermis. Curr Probl Dermatol. 1983;11:145–158. doi: 10.1159/000408671. [DOI] [PubMed] [Google Scholar]
- 32.Gossrau R, Frederiks WM, van Noorden CJ. Histochemistry of reactive oxygen-species (ROS)-generating oxidases in cutaneous and mucous epithelia of laboratory rodents with special reference to xanthine oxidase. Histochemistry. 1990;94(5):539–544. doi: 10.1007/BF00272619. [DOI] [PubMed] [Google Scholar]
- 33.Reiners JJ, Jr., Rupp T. Conversion of xanthine dehydrogenase to xanthine oxidase occurs during keratinocyte differentiation: modulation by 12-O-tetradecanoylphorbol-13-acetate. J Invest Dermatol. 1989 Jul;93(1):132–135. doi: 10.1111/1523-1747.ep12277382. [DOI] [PubMed] [Google Scholar]
- 34.Vorbach C, Harrison R, Capecchi MR. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003 Sep;24(9):512–517. doi: 10.1016/s1471-4906(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 35.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999 Feb;180(2 Pt 1):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 36.Miesel R, Zuber M. Elevated levels of xanthine oxidase in serum of patients with inflammatory and autoimmune rheumatic diseases. Inflammation. 1993 Oct;17(5):551–561. doi: 10.1007/BF00914193. [DOI] [PubMed] [Google Scholar]
- 37.Kizaki H, Matsuo I, Sakurada T. Xanthine oxidase and guanase activities in normal and psoriatic epidermis. Clin Chim Acta. 1977 Feb 15;75(1):1–4. doi: 10.1016/0009-8981(77)90492-2. [DOI] [PubMed] [Google Scholar]
- 38.Eisen AZ, Seegmiller JE. Uric acid metabolism in psoriasis. J Clin Invest. 1961 Aug;40:1486–1494. doi: 10.1172/JCI104379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deliconstantinos G, Villiotou V, Stavrides JC. Alterations of nitric oxide synthase and xanthine oxidase activities of human keratinocytes by ultraviolet B radiation. Potential role for peroxynitrite in skin inflammation. Biochem Pharmacol. 1996 Jun 28;51(12):1727–1738. doi: 10.1016/0006-2952(96)00110-4. [DOI] [PubMed] [Google Scholar]
- 40.Xu P, Huecksteadt TP, Hoidal JR. Molecular cloning and characterization of the human xanthine dehydrogenase gene (XDH) Genomics. 1996 Jun 1;34(2):173–180. doi: 10.1006/geno.1996.0262. [DOI] [PubMed] [Google Scholar]
- 41.Phan SH, Gannon DE, Varani J, Ryan US, Ward PA. Xanthine oxidase activity in rat pulmonary artery endothelial cells and its alteration by activated neutrophils. Am J Pathol. 1989 Jun;134(6):1201–1211. [PMC free article] [PubMed] [Google Scholar]
- 42.Phan SH, Gannon DE, Ward PA, Karmiol S. Mechanism of neutrophil-induced xanthine dehydrogenase to xanthine oxidase conversion in endothelial cells: evidence of a role for elastase. Am J Respir Cell Mol Biol. 1992 Mar;6(3):270–278. doi: 10.1165/ajrcmb/6.3.270. [DOI] [PubMed] [Google Scholar]
- 43.Wakabayashi Y, Fujita H, Morita I, Kawaguchi H, Murota S. Conversion of xanthine dehydrogenase to xanthine oxidase in bovine carotid artery endothelial cells induced by activated neutrophils: involvement of adhesion molecules. Biochim Biophys Acta. 1995 Mar 16;1265(2-3):103–109. doi: 10.1016/0167-4889(94)00202-p. [DOI] [PubMed] [Google Scholar]
- 44.Cheung KJ, Tzameli I, Pissios P, et al. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab. 2007 Feb;5(2):115–128. doi: 10.1016/j.cmet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Maples KR, Mason RP. Free radical metabolite of uric acid. J Biol Chem. 1988 Feb 5;263(4):1709–1712. [PubMed] [Google Scholar]
- 46.Abuja PM. Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Lett. 1999 Mar 12;446(2-3):305–308. doi: 10.1016/s0014-5793(99)00231-8. [DOI] [PubMed] [Google Scholar]
- 47.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003 Jun;41(6):1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 48.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002 Dec;13(12):2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 49.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001 Nov;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 50.Schackis RC. Hyperuricaemia and preeclampsia: is there a pathogenic link? Med Hypotheses. 2004;63(2):239–244. doi: 10.1016/j.mehy.2004.02.018. [DOI] [PubMed] [Google Scholar]