Abstract
Objective
The ischemic myocardium releases multiple chemotactic factors responsible for the mobilization and recruitment of bone marrow-derived cells to injured myocardium. However, the mobilization of primitive pluripotent stem cells (PSCs) enriched in Very Small Embryonic-Like stem cells (VSELs) in various cardiac ischemic scenarios is not well understood.
Methods
Fifty four ischemic heart disease patients, including subjects with stable angina, non-ST elevation (NSTME) myocardial infarction (MI) and ST elevation myocardial infarction (STEMI), and twelve matched controls were enrolled. The absolute numbers of circulating stem/primitive cells in samples of peripheral blood (PB) were quantitated by Image Stream Analysis and conventional flow cytometry. Gene expression of PSC (Oct-4 and Nanog), early cardiomyocyte (Nkx-2.5 and GATA-4), and endothelial (vWF) markers was analyzed by real-time PCR.
Results
The absolute numbers of PSCs, stem cell populations enriched in VSELs and hematopoietic stem cells (HSCs) present in PB were significantly higher in STEMI patients at presentation and declined over time. There was a corresponding increase in pluripotent, cardiac and endothelial gene expression in unfractionated PB cells and sorted PB-derived primitive CD34+ cells. The absolute numbers of circulating VSELs and HSCs in STEMI correlated negatively with patients' age.
Conclusions
Myocardial ischemia mobilizes primitive PSCs including pluripotent VSELs into the circulation. The peak of mobilization occurs within 12 hours in patients presenting with STEMI, which may represent a therapeutic window for future clinical applications. Reduced stem cell mobilization with advancing age could explain, in part, the observation that age is associated with poor prognosis in patients with MI.
Keywords: myocardial infarction, pluripotent stem cells, Very Small Embryonic-Like stem cells, mobilization, Oct-4, CXCR4
INTRODUCTION
The bone marrow harbors a multitude of primitive and partially-committed stem cell populations that are capable of differentiating into cells of cardiac and endothelial lineages [1-5]. Acute myocardial infarction (MI) initiates a cascade of events that result in mobilization of stem/primitive cell subpopulations from the bone marrow into the peripheral blood (PB) following the gradient of chemoattractant cytokines such as SDF-1α, LIF and HGF [6-11]. Furthermore, there is a growing line of evidence that cardiomyocytes undergo continuous renewal, maintained at least in part by BM-derived stem cells (BMSCs) [12-15], accounting for up to 45% of their total number by the age of 75 [16]. Because of their known contribution to cardiomyocyte renewal and the ease with which they can be isolated and subsequently delivered, BM-derived stem cells (BMSCs) represent an attractive cell population for myocardial regenerative therapies. Promising results from animal studies [17-24] indicate that therapy with BM-derived cells can improve LV function (e.g. LV ejection fraction, LVEF), ameliorate remodeling, and improve perfusion. However, the benefit of BMSCs or G-CSF therapy in human studies remains modest and difficult to reproduce [25-26] rendering their therapeutic utilization highly controversial. A better understanding of the innate reparatory mechanisms may be required to achieve more consistent and successful BMSCs based-regenerative studies.
Zuba-Surma et al. and Wojakowski et al. demonstrated that stem cell populations enriched in very small embryonic like stem cells (VSELs) are mobilized into PB following ischemic heart injury in mice and humans [9, 27]. However, quantitative assessment of the mobilization of different pluripotent stem cells (PSCs) populations, defined based on their expression of Oct-4 and SSEA-4, and the influence of ischemic insult on their mobilization in humans is still poorly understood. In this report we examined the mobilization of PSCs and VSELs in patients with different types of myocardial ischemic insults [ST-elevation myocardial infarction (STEMI), Non ST-elevation myocardial infarction (NSTEMI), and chronic ischemic heart disease (IHD)] using a broad multidisciplinary approach consisting of conventional flow cytometry, Image Stream system, confocal microscopy, and gene expression analysis. We report the first comprehensive quantitative examination of circulating PSCs in patients with various ischemic scenarios.
MATERIALS AND METHODS
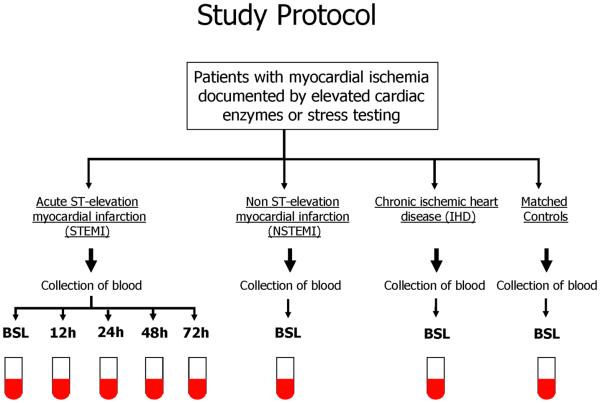
The study population consists of 54 patients; 12 patients with chronic ischemic heart disease and angina (IHD), 12 patients with non ST-elevation myocardial infarction (NSTEMI) with elevated cardiac enzymes, and 30 patients with acute ST-elevation myocardial infarction (STEMI). We enrolled 12 age- and sex-matched subjects to the study population into the control (CTRL) group. The CTRL group is asymptomatic with no history of CAD but multiple risk factors who volunteered to participate in the study. Patients with STEMI were referred within 12 h of symptom onset for primary percutaneous coronary intervention (PCI). Patients were excluded if they had a systemic inflammatory process, cancer, recent motor vehicle accident, recent surgery, active infection, history of MI or revascularization (coronary artery bypass graft, PCI), unsuccessful revascularization, or onset of the symptoms >12 h . Peripheral blood (PB) samples were obtained at presentation in all patients. In STEMI patients, PB samples were collected at presentation [(BSL) on average 4.5±3.2 hours after the onset of chest pain] and 12, 24, 48 and 72 hours after PCI. In NSTEMI patients, PB samples were obtained within 12 hours of the diagnosis. The study protocol complies with the Declaration of Helsinki and was approved by the institutional Ethics Committee. All patients provided written informed consent. Figure 1 outlines the study protocol.
Figure 1.
Study protocol detailing the patient populations and timing of blood samples. Patients with ST-elevation myocardial infarction (STEMI) were enrolled upon their admission. Samples were drawn at presentation (prior to revascularization) and 12, 24, 48 and 72 hours following revascularization. Patients with non-ST-elevation myocardial infarction (NSTEMI) with elevated cardiac enzymes or angina without elevated cardiac enzymes (IHD) were enrolled upon admission and a single blood sample was obtained prior to revascularization.
Flow cytometric analysis and FACS sorting of circulating primitive stem cells from PB
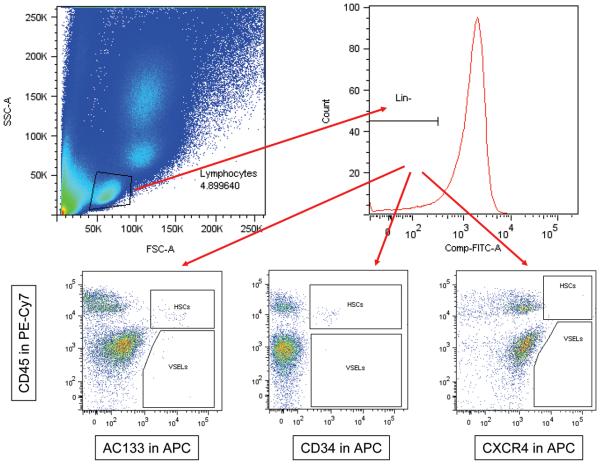
PB samples were collected at the indicated times (Figure 1). Erythrocytes were lysed twice using BD PharmLyse lysing buffer (BD Biosciences, San Jose, CA) at room temperature for 10 min and subsequently washed in phosphate-buffered saline (PBS) to yield total nucleated cells (TNCs). TNCs were subsequently stained for hematopoietic lineages markers (Lin) using the following fluorescein isothiocyanate (FITC) conjugated antibodies (Abs) against human: CD2 (clone RPA-2.10); CD3 (clone UCHT1); CD14 (clone M5E2); CD16 (clone 3G8); CD19 (clone HIB19); CD24 (clone ML5); CD56 (clone NCAM16.2); CD66b (clone G10F5) and CD235a (clone GA-R2). These Abs were purchased from BD Biosciences. The cells were simultaneously stained for the panleukocytic marker - CD45 (PE-Cy7 conjugated Abs, clone HI30; BD Biosciences) and one of the following antigens CXCR4 (APC conjugated Abs, clone 12G5, BD Biosciences), CD34 (APC conjugated Abs, clone 581, BD Biosciences), CD133 (CD133/1; APC conjugated Abs, Miltenyi Biotec, Auburn, CA) and SSEA-4 (PE conjugated Abs, clone E025016, eBioscience, San Diego, CA). Staining was performed in PBS with 2% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), on ice for 30 min. Cells were subsequently washed, resuspended and analyzed using an LSR II (BD Biosciences). At least 106 events were acquired from each sample. The absolute numbers of PSCs and VSELs were calculated (individually for each patient) per 1μl of PB based on the percentage content of these cells detected by flow cytometry and the absolute number of white blood cells (WBCs) per 1 μl of PB. Figure 2 outlines the flow cytometry analysis protocol. Flowjo software was used for analysis (Tree Star, Ashland, OR).
Figure 2.
Strategy for flow cytometric analysis of Very Small Embryonic-Like stem cells and hematopoietic stem cells. Representative dot-plots showing lymphocyte gating on the side scatter (SSC) and forward scatter (FSC). Lymphocytes negative for the lineage markers were further divided based on the expression of CD45, AC133, CD34, and CXCR4 markers. VSELs, very small embryonic like stem cells; HSCs, hematopoietic stem cells.
Following lysis of erythrocytes, the populations of PB cells enriched in VSELs (Lin-/CD45−/CD133+, Lin-/CD45−/CD34+ and Lin-/CD45−/CXCR4+) were sorted using a multiparameter fluorescence-activated cell sorting (FACS) with a MoFlo cell sorter (Beckman Coulter, Fullerton, CA) according to previously published protocol [3] and used for immunocytofluorescence analyses. Similarly, total fractions of CD34+ cells were sorted for real-time RT-PCR (RQ-PCR) analysis of gene expression.
Imaging flow cytometric analysis with Image Stream system
PB-derived TNCs were isolated as detailed above. TNCs were fixed with 4% paraformaldehyde (Sigma Aldrich, St. Louis, MO) for 20 min, permeabilized with 0.1% Triton X-100 solution (Sigma Aldrich, St. Louis, MO) for 10 min and washed twice with PBS. TNCs were subsequently stained for intranuclear transcription factor Oct-4 using anti-mouse/human Oct-4 antibody (purified, clone 9E3, Millipore, Billerica, MA) for 2h at 37°C, followed by washing the incubation with the secondary anti-mouse IgG antibody conjugated with PE (BioLegend, San Diego, CA) for 2h at 37°C. Cells were further washed and stained for CD45 (FITC conjugated Abs; clone HI30, BD Biosciences), hematopoietic lineages markers (Lin, as detailed above) and CD34 (PE-Cy5 conjugated Abs; clone 581, BD Biosciences) or CD133 (biotin conjugated Abs, clone CD133/1, Miltenyi Biotec). Staining with biotinylated antibodies was followed with staining with streptavidin conjugated with PE-Cy5 (BD Pharmingen, San Jose, CA) to visualize the CD133 or CD34 expression. 7-aminoactinomycin D (7-AAD) was added for 10 minutes before analysis (BD Pharmingen; 40μM) to visualize nucleated objects. Samples were run directly on the Image Stream System (ISS) 100 (Amnis Corporation, Seattle, WA). Signals from FITC, APC, 7-AAD and PE-Cy5 were detected by channels 3, 4, 5 and 6, respectively, while side scatter and brightfield images were collected in channels 1 and 2, respectively.
Immunohistochemistry
Immunofluorescence identification of pluripotent specific transcription factors and intracellular proteins was performed on sorted stem cell populations enriched in VSELs. Briefly, cells were fixed with 4% paraformaldehyde for 10 minutes (Sigma Aldrich) then washed with PBS (Sigma Aldrich). Cells were permeabilized following fixation with 0.1% Triton-X 100 (Sigma Aldrich) for 10 minutes, blocked with 2% donkey serum (Jackson Immunoresearch laboratories, West Grove, PA) for 30 min and then stained with primary antibodies against Oct-4 (clone 9E3, Millipore) for 16 hours at 4° C. Primary antibodies were washed three consecutive times with PBS before secondary antibodies were added at a concentration of 1:100. Staining with secondary anti- mouse IgG antibodies conjugated with TRITC (Jackson Immunoresearch laboratories) was performed at 37° C for 2 hours and then cells were washed three times with PBS. Cells were additionally stained for SSEA-4 (FITC conjugated Abs, clone MC-813-70; BioLegend) and CD45 (biotin conjugated Abs, clone HI30, BD Biosciences) followed by incubation with streptavidin conjugated with Cy5 (BioLegend) and finally nuclei were stained with DAPI (Molecular Probes, Carlsbad, CA). All immunofluorescence photomicrographs were acquired using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY).
Real-Time RT-PCR
To study mRNA levels for PSCs antigens PSCs (Oct-4, Nanog) as well as early myocardial (Nkx2.5/Csx, GATA4) and endothelial (vWF) markers, total mRNA was isolated using RNeasy Mini Kit (Qiagen Inc., Valencia, CA) and reverse-transcribed using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Measurements of mRNA levels of PSC, cardiac, and endothelial markers and β2-microglobulin were performed by RQ-PCR using an ABI PRISM 7000 Sequence Detection System (ABI, Foster City, CA). The 25 ul of reaction mixture contained SYBR Green PCR Master Mix, forward and reverse primers for specific gene. Primers were designed with Primer Express software. All of the primer sequences are provided in Supplemental Table 1. The threshold cycle (Ct), i.e., the cycle number at which the amount of amplified gene of interest reached a fixed threshold, was subsequently determined. The relative quantification of Oct-4, Nonog, Nkx2.5/Csx, GATA4, and vWF mRNA expression was performed with the comparative Ct method. Briefly, the relative quantification value of target gene, normalized to an endogenous control (β2-microglobulin gene) and relative to a calibrator, was expressed as 2-ΔΔCt (fold difference), where Δc = Ct of target genes - Ct of endogenous control gene (β2-microglobulin), and ΔΔCt = ΔCt of samples for target gene - ΔCt of calibrator for the target gene. To avoid the possibility of amplifying contaminating DNA: 1) all of the primers for RQ-PCR were designed with an intron sequence inside cDNA to be amplified, 2) reactions were performed with appropriate negative controls (template-free controls), 3) uniform amplification of the products was rechecked by analyzing the melting curves of the amplified products (dissociation graphs), 4) the melting temperature (Tm) was 57°C to 60°C, the probe Tm was at least 10°C higher than primer Tm.
Measurement of Blood Cytokine Levels
Blood samples were collected in the above mentioned time points both in STEMI patients and controls in EDTA tubes. Tubes were centrifuged for 2000 RPM for 15 minutes. Plasma was divided into aliquots and stored at −80°C. Plasma levels of stroma-derived-factor 1 (SDF-1α), granulocyte-colony-stimulating factor (G-CSF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and stem-cell factor (SCF) were quantified using the Luminex platform system: Milliplex Human Cytokine Kit (Millipore, Billerica, MA, USA) according to the manufacturer's protocols.
Statistical Analysis
Data are expressed as mean ± SEM. Differences were analyzed using the unpaired Student t-test or ANOVA (one way or multiple comparisons) as appropriate. Post hoc multiple comparison procedures (MCP) were performed using 2 sided Dunnett or Dunn tests as appropriate with control samples as the control category. The significance level throughout the analyses was chosen to be 0.05. All statistical analyses were performed using the SPSS (version 16.0) statistical software (SPSS Inc., Chicago, IL). All authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree with the manuscript as written.
RESULTS
Mobilization of pluripotent Oct-4+, SSEA4+ cells and stem cell populations enriched in VSELs in patients with myocardial ischemia
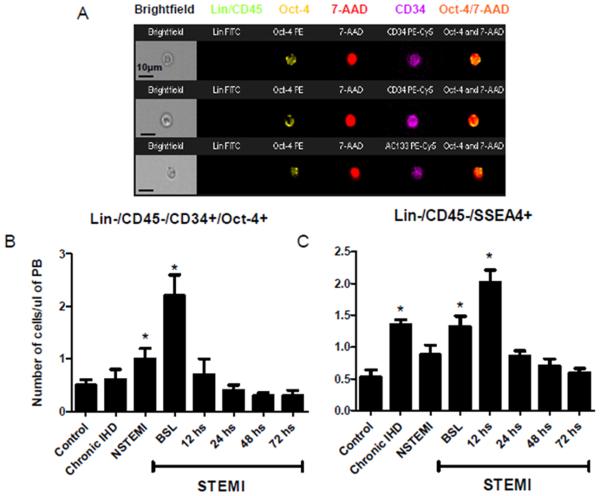
In myocardial ischemia, the absolute circulating number of Oct4+ VSELs, as analyzed by Image Stream system (ISS), was significantly higher than controls. Representative images of Oct-4+ primitive cells obtained by ISS are shown in Figure 3A. In healthy subjects, the number of circulating VSELs was low (0.5 ± 0.1 cells/μl of PB). The absolute number of Lin-/CD45−/CD34+/Oct4+ cells was higher among patients with myocardial ischemia with significantly higher levels in STEMI patients (0.5 ± 0.1 vs. 0.6 ± 0.2 vs. 1.0 ± 0.2 vs. 2.2 ± 0.4 cells/μl of PB in controls vs. IHD vs. NSTEMI vs. peak STEMI patients respectively, P < 0.05 Control vs. peak STEMI numbers). However, the difference between controls and patients with chronic IHD and NSTEMI was not statistically significant (Figure 3B). We did not observe differences in the absolute numbers of circulating stem cells in different time points in NSTEMI patients (Data not shown). In acute STEMI the number of Oct4+ VSELs reached peak at baseline (2.2 ± 0.4 cells/μl of PB) and decreased afterwards reaching a nadir of 0.3±0.1 cells/μl of PB at 72 hours (Figure 3B). Based on the unique capabilities of ISS technology, we were able to quantify PSCs accurately by distinguishing real intranuclear Oct-4 expression from false positives events.
Figure 3.
Mobilizations of Oct-4 positive pluripotent VSELs in ischemic heart disease patients and controls. Panel A. Representative Image Stream pictures of circulating Oct-4 positive VSELs lacking the expression of hematopoietic lineages (Lin) and CD45 markers (Green) and positive for Oct-4 (yellow) and CD34 or CD133 (magenta). Nuclei are stained with 7-AAD (red). The combined image in the far right demonstrates the co-localization of Oct-4 in the nucleus. Panel B. Bar graphs showing the absolute numbers of circulating Lin-/CD45−/CD34+/Oct-4+ cells in the peripheral blood of ischemic heart disease patients and controls; showing a peak mobilization early in STEMI patients. Panel C. Bar graphs showing the absolute numbers of circulating Lin-/CD45−/SSEA-4+ cells in the peripheral blood of ischemic heart disease patients and controls; showing a peak mobilization early in STEMI patients. (* P < 0.05 as compared to controls). NSTEMI, non-ST-elevation myocardial infarction; PB, peripheral blood; STEMI, ST-elevation myocardial infarction.
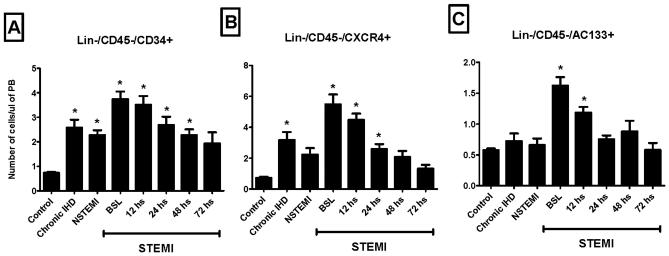
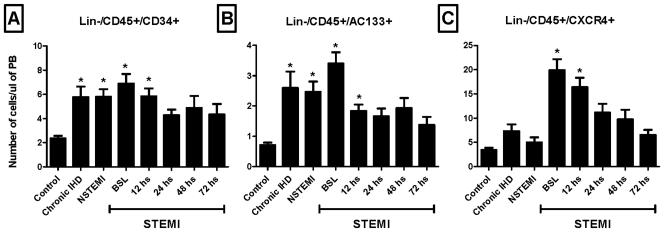
A similar pattern of mobilization was noted in the absolute numbers of circulating Lin-/CD45−/SSEA-4+ nonhematopoietic PSCs assessed by conventional flow cytometry. Their numbers peaked in the circulation of STEMI patients 12 hours after presentation (Figure 3C). Mobilization of Lin-/CD45−/AC133+, Lin-/CD45−/CD34+, and Lin-/CD45−/CXCR4+ cells enriched in VSELs was highest at presentation (within 12 hours of symptom onset) in STEMI patients (Figure 4). The absolute numbers of all three populations were significantly higher among STEMI patients at the time of presentation (BSL) as compared to controls, IHD, and NSTEMI patients (3-8 fold increase as compared to controls; P <0.01).
Figure 4.
Bar graphs showing the absolute numbers of circulating VSELs in the peripheral blood of ischemic heart disease patients and controls; showing a peak mobilization early in STEMI patients. (* P < 0.05 as compared to controls). NSTEMI, non-ST-elevation myocardial infarction; PB, peripheral blood; STEMI, ST-elevation myocardial infarction.
Mobilization of hematopoietic stem cells (HSCs) in patients with myocardial ischemia
Our flow cytometry analyses detected significant mobilization of Lin-/CD45+/AC133+, Lin-/CD45+/CD34+, and Lin-/CD45+/CXCR4+ HSCs in patients with myocardial ischemia when compared to controls (Figure 5). Lin-/CD45+/CXCR4+ but not Lin-/CD45+/AC133+ and Lin-/CD45+/CD34+ cells were significantly higher in STEMI patients as compared to other ischemic heart patients (3-10 fold increase; P <0.05). The higher numbers of mobilized Lin-/CD45+/CXCR4+ cells early in STEMI patients can potentially be a reflection of the active recruitment by the infarcted myocardium via the SDF-1/CXCR4 axis.
Figure 5.
Bar graphs showing the absolute numbers of circulating HSCs in the peripheral blood of ischemic heart disease patients and controls; showing a peak mobilization early in STEMI patients (* P < 0.05 as compared to controls). NSTEMI, nonST-elevation myocardial infarction; PB, peripheral blood; STEMI, ST-elevation myocardial infarction.
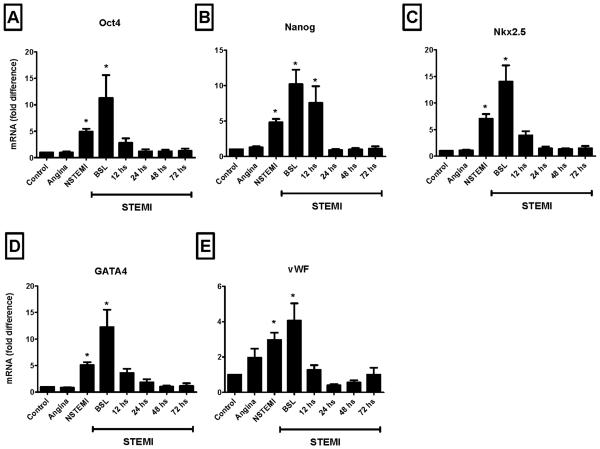
Expression of pluripotent, cardiac and endothelial markers in circulating cells by RT-PCR
The expression of pluripotent, cardiac and endothelial markers by PB TNCs was significantly higher in NSTEMI and STEMI patients when compared to controls or chronic IHD patients (Figure 6). The mRNA level of these genes peaked in STEMI patients at the time of presentation (BSL) and paralleled the peak mobilization of pluripotent stem cells.
Figure 6.
Bar graphs showing the mRNA expression of pluripotent markers - Oct-4 and Nanog (Panel A and Panel B, respectively), cardiac markers - Nkx2.5/Csx and GATA4 (Panel C and Panel D, respectively) and endothelial antigen - vWF (Panel E) in PB TNCs isolated from ischemic heart disease patients and controls. The expression of primitive, cardiac and endothelial genes was consistently higher in STEMI patients early after the acute event (* P < 0.05 as compared to controls). NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
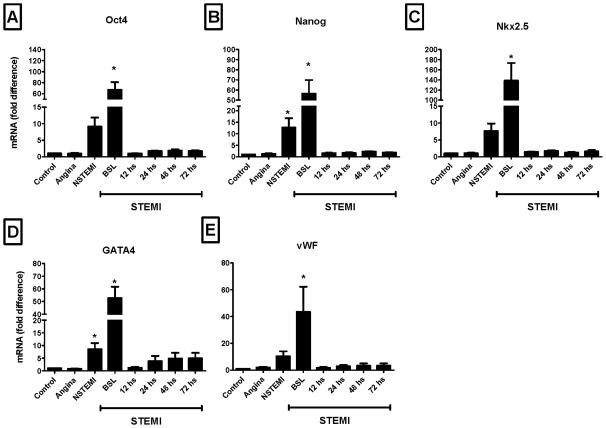
Recently, Wojakowski et al demonstrated significant expression of these markers in sorted VSELs [9]. In agreement with Wojakowski's data, the expression of pluripotent, cardiac and endothelial markers was significantly higher in sorted PB-derived CD34+ cells as compared to unfractionated PB-derived TNCs (Figure 7). This enrichment was confined to CD34+ cells sorted early after the acute injury. This data indicates that the fraction of CD34+ cells is enriched in PSCs and VSELs. Because of the limitations in multi-parameter/multi-antigen isolation of stem cells for clinical purposes by FACS, these findings indicate a potentially useful enriched population for clinical applications in ischemic heart disease patients.
Figure 7.
Bar graphs showing the mRNA expression of pluripotent markers - Oct-4 and Nanog (Panel A and Panel B, respectively), cardiac markers - Nkx2.5/Csx and GATA4 (Panel C and Panel D, respectively) and endothelial antigen - vWF (Panel E) in sorted CD34+ cells isolated from ischemic heart disease patients and controls. The expression of primitive, cardiac and endothelial genes was consistently higher in STEMI patients early after the acute event (* P < 0.05 as compared to controls). NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Cellular size and immunophenotype of mobilized PSCs
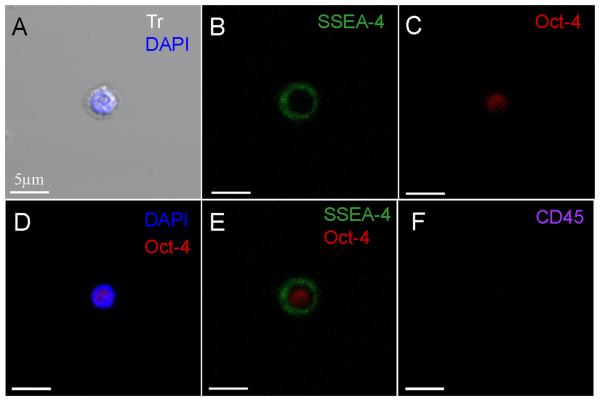
Sorted stem cell subpopulations enriched in VSELs were analyzed with ISS which confirmed their pluripotent phenotypic features such as their small size (7-8 μm on average) as well as their higher nuclear to cytoplasm ratio (Data not shown). Similarly, when assessed by confocal microscopy, VSELs appeared small in size with a large nucleus staining positive for Oct-4 (Figures 3A and 8) surrounded by a small rim of cytoplasm and staining positive for SSEA-4 on the surface. However, these characteristics are shared with multiple pluripotent stem cells as well as other stem cells and cannot be used solely to distinguish PSCs [28]. Therefore, these features should be used in conjunction with other distinguishing characteristics such as surface markers and differentiation capacity.
Figure 8.
Representative confocal microscopic images documenting the expression of primitive markers in circulating very small embryonic like stem cells. The pluripotent nature of circulating VSELs is evidenced by the positivity for the primitive surface marker SSEA-4 (FITC, green) and the nuclear marker Oct-4 (TRITC, red). Circulating VSELs are negative for the expression of CD45 (Cy5, Magenta). Nuclei are stained with DAPI (blue). The merged image demonstrates the co-localization of Oct-4 in the nucleus and SSEA-4 on the surface. The scale indicates 5μm.
Plasma cytokine levels
The levels of G-CSF, VEGF, HGF, and SCF were higher in STEMI patients than in controls with the highest level observed in the first 24 hours after the acute event. On the other hand, the level of SDF-1α was lower early after the acute event and gradually increased to levels similar to those in controls by 48 hours after the acute event (Table 2). Thus, there appears to be a correlation between the early mobilization of BM-derived stem cells and changes in serum cytokine levels.
Table 2.
Plasma Cytokine Levels (pg/ml) in STEMI patients and Controls.
| Control | STEMI-BSL | STEMI-24 hours |
STEMI-48 hours |
STEMI-72 hours |
|
|---|---|---|---|---|---|
| SDF-1α | 5344±1226 | 2198±458 | 2514±577 | 2837±592 | 3190±824 |
| G-CSF | 118±28 | 95±18 | 166±38 | 78±15 | 60±23 |
| VEGF | 102±23 | 232±45 | 337±77 | 199±39 | 178±54 |
| HGF | 1719±992 | 3866±1166 | 2327±776 | 2739±826 | 2486±939 |
| SCF | 23±8 | 35±7 | 33±8 | 28±7 | 21±6 |
Demographic correlations of VSELs and HSCs mobilization in STEMI patients
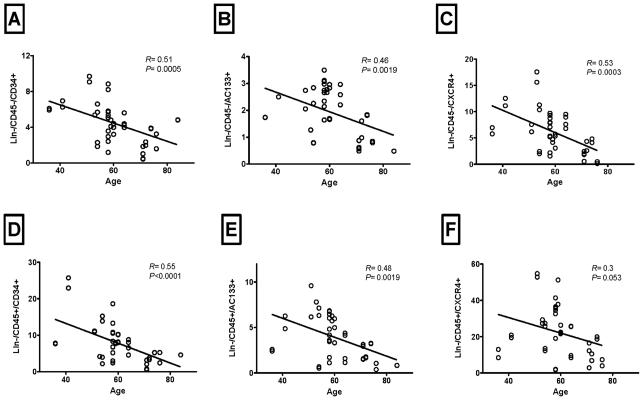
Our data suggest that all three populations enriched in VSELs (Lin-/CD45−/AC133+, Lin-/CD45−/CD34+, and Lin-/CD45−/CXCR4+) and HSCs (Lin-/CD45+/AC133+, Lin-/CD45+/CD34+, and Lin-/CD45+/CXCR4+) in STEMI patients correlated significantly negatively with patients age (Figure 9). However, we did not observe significant correlation with serum markers of myonecrosis or left ventricular function at baseline.
Figure 9.
The negative correlation between the peak mobilization of different VSELs' subpopulations (Panels A-C) and HSCs (Panels D-F) in STEMI patients and patients' age. All three VSELs' subpopulations correlated negatively with age (P < 0.05). HSCs showed statistically significant or strong trends towards negative correlation with patients' age.
DISCUSSION
The infracted myocardium releases a multitude of chemokines, growth factors and cytokines responsible for the dynamic mobilization, homing, and incorporation of BM-derived stem cells to the infarction zone [11, 29]. In this study, we present for the first time a quantitative analysis of mobilized pluripotent Oct-4+ and SSEA-4+ cells including Very Small Embryonic-Like stem cells (VSELs) in the peripheral blood of patients with various scenarios of myocardial ischemia. This is an extension of our previous reports demonstrating the mobilization of VSELs in patients with acute myocardial infarction [30] and the most comprehensive evaluation of the circulating number of pluripotent, Very Small Embryonic-Like, and hematopoietic stem cells performed to date in patients with different degrees of myocardial ischemia. Circulating stem cells were elevated in patients with IHD and non ST-elevation myocardial infarction although to a lesser extent than in patients with ST-elevation myocardial infarction. We noticed that the absolute number of PSCs, stem cell populations enriched in VSELs and HSCs were significantly higher in patients with ST-elevation myocardial infarction and peaks in the early phase following the acute event. These findings suggest that the degree of ischemia, the amount of territory involved, and/or the presence of myocardial necrosis may influence mobilization. Mobilization in STEMI patients correlated negatively with age in all subpopulations examined. Bone marrow-derived stem cell populations enriched in VSELs have been shown to differentiate into cardiomyocytes in vitro [4, 31] and regenerate the myocardium in vivo [30, 32], highlighting the clinical relevance of the presented data.
Adult bone marrow contains a multitude of non-committed, partially-committed, and committed stem cell populations that contribute to the regeneration of nonhematopoietic tissues [33]. The most primitive of these stem cells in adults are the pluripotent non-hematopoietic stem cells such as VSELs that express various pluripotent markers including Oct-4, Nanog and SSEA-1/4 [34-35]. VSELs isolated from adult BM exhibit morphological features characteristic of embryonic stem cells such as small size, large nucleus with open euchromatin and high nuclear to cytoplasm ratio when compared with HSCs and differentiated PB cells [28]. Upon appropriate stimulation, VSELs are capable of differentiating in vitro into cells of all three germ layers (ecto-, endo- and mesoderm) including cardiac cells [4, 31]. When transplanted in infarcted myocardium, BM-derived stem cell populations enriched in VSELs give rise to cardiomyocytes and improve left ventricular function [30, 32].
The exclusive expression of Oct-4 in pluripotent and embryonic stem cells has been recently challenged by reports demonstrating its presence in differentiated PB cells [36]. Therefore, in our studies, we examined the expression of Oct-4 and SSEA-4 with dual fluorescent immunostaining with the stem cell marker, CD34. We also excluded differentiated PB cells by excluding from our analyses cells staining positive for the differentiated lineage antibodies. The influx of Lin-/CD45−/CD34+/Oct4+ and Lin-/CD45−/SSEA4+ cells in PB was significantly higher among STEMI patients as compared to controls and other ischemic heart disease patients. However, the peak mobilization of Oct-4+ cells preceded that of SSEA-4+ cells and the explanation for this is not readily apparent. It is possible that cytokines responsible for SSEA-4+ and Oct-4+ cells' mobilization are different and hence the differences in the timing of their peak mobilization. It may also be the case that the cells with a different expression of these two markers represent cells with different level of maturation/primitivity which may explain the response of these cells to mobilizing chemokines. Our experience with human umbilical cord blood (UCB) indicates that SSEA-4+ VSELs are scarcer and exhibit more primitive morphology than Oct-4+ cells [37]. This may indicate that primitive/pluripotent SSEA-4+ stem cells are anchored to their niches and/or more resistant to mobilization stimuli. Furthermore, we confirmed the pluripotent nature of isolated PB-derived VSELs on the morphological and phenotypic levels through our Image Stream and confocal microscopy analyses. PB VSELs isolated, based on their surface expression markers, from patients with myocardial ischemia are small in size (7-8 μm in diameter) and have characteristically large nucleus surrounded by a narrow rim of cytoplasm similar to their BM and cord blood counterparts (Figures 3 and 8) [4, 38].
Several reports have confirmed the mobilization of partially committed and committed stem cells originating from the BM in response to myocardial ischemic injury [7-10, 39-40]. Upon appropriate stimuli, BM-derived cells are mobilized in the circulation and migrate to the injured myocardium in a dynamic fashion following a cytokine gradient of SDF-1, LIF and HGF [6, 41]. However, the BM response to chronic IHD and NSTEMI has not been thoroughly investigated. In this study, we demonstrate consistent mobilization of stem cell populations enriched in VSELs in peripheral blood of patients with ischemia with or without infarction, although the mobilization is less robust in chronic IHD and NSTEMI than in STEMI (Figure 4). Mobilized PB-derived TNCs strongly exhibit markers of pluripotency, cardiac and endothelial lineages (Figure 6) and hence can potentially contribute to the repair of the injured myocardium. Indeed, our previous studies in animals and humans demonstrate the commitment of BM-derived stem cell populations enriched in VSELs and PSCs for myocardial regeneration [30, 32]. Moreover, we observed the expression of these markers to be further up-regulated in sorted CD34+ cells only in the very early phase after the acute injury (Figure 7). The reduction in the expression of these markers can be related to “back homing” of CD34+ and other selected subpopulations not incorporated in the myocardium. Our data indicate the enrichment of CD34+ fraction of PB-derived cells in pluripotent markers suggesting the presence of pluripotent stem cells in this fraction. Of note, multiple human studies have successfully utilized BM-derived CD34+ cells in myocardial regeneration [42-43].
Studies in sex-mismatched heart- and bone marrow-transplantation demonstrate the role of BM-derived cells in the chimerism of cardiomyocytes reaching 50% of the total cardiomyocyte count during the normal human life span [14-16]. In a seminal paper, Deb et al. demonstrated that donor's BM-derived cells contribute to the chimerism of the recipient's myocardium as well as other organs such as the liver [13]. Understanding the underpinnings of these reparatory mechanisms especially in the setting of acute myocardial infarction can provide helpful clues for future therapeutic studies utilizing BM-derived VSELs in myocardial regeneration. Mobilized BM-derived cells can potentially be contributing to the reparatory mechanisms by reducing apoptosis and stimulating the resident cardiac stem cells rather than differentiating into cardiomyocytes [44].
Our data is consistent with previous reports from our group and others demonstrating the mobilization of stem cell subpopulations in acute myocardial infarctionAlthough there are discrepancies in the reported literature about changes and absolute numbers of circulating stem cells in AMI patients, the findings can be explained by the differences in patient characteristics, flow cytometry protocols used, or timing of blood sampling. Nonetheless, the majority of the literature supports significant and consistent stem cell mobilization in the early phase of myocardial infarction. This report extends these observations to Oct-4+ and SSEA-4+ pluripotent stem cells. It is however important to mention that the surface markers outlined in our study are not unique to any given population of stem or pluripotent cells. CD34+ and CD133+ cells isolated from the peripheral blood have been shown to differentiate into endothelial cells in vitro and home to ischemic limb in animal in vivo models indicating that they include endothelial progenitor cells (EPCs) [45-46]. Thus, these lineage negative cells represent multiple overlapping subpopulations, including EPCs among others, that are capable of repopulating the injured heart and aid in its regeneration. On the other hand, utilizing the co-expression of SSEA4 and Oct4 allowed us to further examine the pluripotent portion of these populations.
In conclusion, we present for the first time quantitative evidence of circulating Oct-4+ and SSEA-4+ cells in patients with various degrees of myocardial ischemia. The patients' capacity to mobilize these pluripotent stem/primitive cells is hampered in the elder patients with STEMI. Understanding the significance and underpinnings of this mobilization will be crucial in planning future studies examining the role of VSELs and other primitive cell populations in myocardial regeneration and may reveal the optimal therapeutic window suitable for pluripotent cellular therapies for myocardial regeneration.
Supplementary Material
Table 1.
Demographic, clinical and laboratory characteristics of study population and controls.
| Controls (n = 12) |
Chronic IHD (n = 12) |
NSTEMI (n = 12) |
STEMI (n = 30) |
|
|---|---|---|---|---|
| Age (years) | 47±9 | 58±10 | 51±10 | 61±11 |
| Female | 50% | 42% | 33% | 23% |
| HTN | 25% | 83% | 83% | 72% |
| DM | 8% | 50% | 33% | 19% |
| Hyperlipidemia | 25% | 83% | 83% | 93% |
| Smoking | 8% | 50% | 50% | 60% |
| Peak Troponin | NA | 0±0.1 | 3±5.3 | 62±39 |
| Peak CK | NA | 76±47 | 206±190 | 2877±3240 |
| Peak CK-MB | NA | 2±0.9 | 19±32 | 210±114 |
ACKNOWLEDGMENTS
Funding Sources:
Drs. Abdel-Latif and Ziada are supported by the University of Kentucky Clinical and Translational Science Pilot Award.
Dr. Zuba-Surma is supported by the “Polish Foundation of Science” homing program grant number 2008/15.
Dr. Ratajczak is supported by NIH grant R01 CA106281, NIH R01 DK074720, EU structural funds, Innovative Economy Operational Program (POIG.01.01.02-00-109/09-00), and Stella and Henry Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure: None.
REFERENCES
- 1.D'Ippolito G, Diabira S, Howard G, Menei P, Roos B, Schiller P. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y, Jahagirdar B, Reinhardt R, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 3.Kucia M, Halasa M, Wysoczynski M, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 4.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 5.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 6.Kucia M, Dawn B, Hunt G, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood following myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massa M, Rosti V, Ferrario M, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 8.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 9.Wojakowski W, Tendera M, Kucia M, et al. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wojakowski W, Tendera M, Zebzda A, et al. Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006;27:283–289. doi: 10.1093/eurheartj/ehi628. [DOI] [PubMed] [Google Scholar]
- 11.Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39:363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.de Weger RA, Verbrugge I, Bruggink AH, et al. Stem cell-derived cardiomyocytes after bone marrow and heart transplantation. Bone Marrow Transplant. 2008;41:563–569. doi: 10.1038/sj.bmt.1705939. [DOI] [PubMed] [Google Scholar]
- 13.Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 14.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 15.Rupp S, Koyanagi M, Iwasaki M, et al. Characterization of long-term endogenous cardiac repair in children after heart transplantation. Eur Heart J. 2008;29:1867–1872. doi: 10.1093/eurheartj/ehn223. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Aviles F, San Roman J, Garcia-Frade J, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 18.Kinnaird T, Stabile E, Burnett M, Epstein S. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95:354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 19.Kinnaird T, Stabile E, Burnett M, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 20.Kocher A, Schuster M, Szabolcs M, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 21.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 22.Shake J, Gruber P, Baumgartner W, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 23.Tomita S, Li R, Weisel R, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 24.Yoon Y, Wecker A, Heyd L, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel-Latif A, Bolli R, Tleyjeh I, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Latif A, Bolli R, Zuba-Surma EK, Tleyjeh IM, Hornung CA, Dawn B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2008;156:216–226. doi: 10.1016/j.ahj.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865–873. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuba-Surma EK, Kucia M, Abdel-Latif A, et al. Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12:292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuhara S, Tomita S, Nakatani T, Yutani C, Kitamura S. Endogenous bone-marrow-derived stem cells contribute only a small proportion of regenerated myocardium in the acute infarction model. J Heart Lung Transplant. 2005;24:67–72. doi: 10.1016/j.healun.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Tendera M, Wojakowski W, Ruzyllo W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 31.Wojakowski W, Tendera M, Kucia M, et al. Cardiomyocyte differentiation of bone marrow-derived Oct-4+CXCR4+SSEA-1+ very small embryonic-like stem cells. Int J Oncol. 2010;37:237–247. doi: 10.3892/ijo_00000671. [DOI] [PubMed] [Google Scholar]
- 32.Dawn B, Tiwari S, Kucia MJ, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratajczak M, Zuba-Surma E, Wysoczynski M, Wan W, Ratajczak J, Kucia M. Hunt for pluripotent stem cell e Regenerative medicine search for almighty cell. J Autoimmun. 2008;30:151–162. doi: 10.1016/j.jaut.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seta N, Kuwana M. Human circulating monocytes as multipotential progenitors. Keio J Med. 2007;56:41–47. doi: 10.2302/kjm.56.41. [DOI] [PubMed] [Google Scholar]
- 35.Deans R, Moseley A. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 36.Zangrossi S, Marabese M, Broggini M, et al. Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells. 2007;25:1675–1680. doi: 10.1634/stemcells.2006-0611. [DOI] [PubMed] [Google Scholar]
- 37.Zuba-Surma E, Kucia M, Izabela Klich I, et al. Optimization of isolation and further molecular and functional characterization of SSEA-4+/Oct-4+/CD133+/CXCR4+/LINneg/CD45neg Very Small Embryonic-Like (VSEL) stem cells isolated from umbilical cord blood. Blood. 2008;112:807. [Google Scholar]
- 38.Kucia M, Halasa M, Wysoczynski M, et al. Morphological and molecular characterization of novel population of CXCR4(+) SSEA-4(+) Oct-4(+) very small embryonic-like cells purified from human cord blood - preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 39.Kucia M, Dawn B, Hunt G, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wojakowski W, Tendera M, Michałowska A, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 41.Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4+ tissue-committed stem cells. Biol Cell. 2005;97:133–146. doi: 10.1042/BC20040069. [DOI] [PubMed] [Google Scholar]
- 42.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 43.Pasquet S, Sovalat H, Henon P, et al. Long-term benefit of intracardiac delivery of autologous granulocyte-colony-stimulating factor-mobilized blood CD34+ cells containing cardiac progenitors on regional heart structure and function after myocardial infarct. Cytotherapy. 2009;11:1002–1015. doi: 10.3109/14653240903164963. [DOI] [PubMed] [Google Scholar]
- 44.Xu RX, Chen X, Chen JH, Han Y, Han BM. Mesenchymal stem cells promote cardiomyocyte hypertrophy in vitro through hypoxia-induced paracrine mechanisms. Clin Exp Pharmacol Physiol. 2009;36:176–180. doi: 10.1111/j.1440-1681.2008.05041.x. [DOI] [PubMed] [Google Scholar]
- 45.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 46.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.