Abstract
Cell migration affects all morphogenetic processes and contributes to numerous diseases, including cancer and cardiovascular disease. For most cells in most environments, movement begins with protrusion of the cell membrane followed by the formation of new adhesions at the cell front that link the actin cytoskeleton to the substratum, generation of traction forces that move the cell forwards and disassembly of adhesions at the cell rear. Adhesion formation and disassembly drive the migration cycle by activating Rho GTPases, which in turn regulate actin polymerization and myosin II activity, and therefore adhesion dynamics.
The morphological features of migrating cells can vary considerably. Round, highly protrusive or blebbing cells (for example, lymphocytes and cancer cells in some environments) are at one extreme and seem to migrate using weak adhesions. Highly spread cells (for example, fibroblasts and endothelial cells) are at the other extreme and have many large adhesions; their migration is often referred to as being mesenchymal. In reality, there is a continuum of migration modes that seem to be determined by several factors, among the most important being substrate compliance (and perhaps dimensionality) and the intrinsic contractility of the cells.
Directional migration is initiated by extracellular cues such as a gradient of growth factors or chemokines. However, directional cues can also include mechanical forces (for example, cell stretching or fluid flow), extracellular matrix (ECM) proteins (for example, collagen and fibronectin), the topography and mechanics of the ECM3–6 and electrochemical gradients7. Cells initiate the migration cycle by polarizing and extending protrusions of the cell membrane towards the cue11. These protrusions comprise large, broad lamellipodia, spike-like filopodia or both and are driven by the polymerization of actin filaments12. Protrusions are then stabilized by adhesions that link the actin cytoskeleton to the underlying ECM proteins, and actomyosin contraction generates traction forces on the substratum. Contractility also promotes the disassembly of adhesions at the cell rear to allow the cell to move forwards18. Signals from both newly formed and more stable adhesions influence cytoskeletal organization and actin polymerization, and cytoskeletal structures in turn influence the formation and disassembly of the adhesions18. These bidirectional interactions coordinate adhesion, signalling, mechanical stresses and the spatial dynamics of cytoskeletal organization, leading to directional cell movement. The spatially segregated migration machinery and the signalling processes that regulate them are integrated by the cytoskeleton and vesicle trafficking, which span the entire cell.
Although cells express various cell surface adhesion receptors (including integrins, syndecans and other proteoglycans, cadherins and cell adhesion molecules), the integrin family of transmembrane heterodimeric receptors is the best studied and plays a prominent part in cell migration20. Integrin extracellular domains bind to specific sequence motifs present in proteins such as fibronectin, collagen and other ECM proteins. The binding of integrins to their extracellular ligands induces a conformational change that unmasks their short cytoplasmic tails, which promotes their linkage to the actin cytoskeleton through multiprotein complexes4,5,20. The integrin–actin linkage is mediated by several proteins, some of which bind directly to actin (BOX 1). The best studied are talin, which transitions integrins to an active state by binding to their cytoplasmic domain through its `head domain' and to filamentous actin (F-actin) and vinculin through sites in the `tail domain'1, vinculin, which also binds F-actin directly, and the actin cross-linking protein α-actinin10,14. Although the linkage of integrins to actin has been recognized for many years, the hierarchal structure of the linkage is probably complex. The network of protein interactions that potentially link integrins to the actin cytoskeleton has been intensely studied and globally organized into a structure termed the adhesome21. The most recent version of the adhesome includes 180 protein–protein interaction nodes, defining a network that is rich in complexity and connectivity22.
Box 1 | Key proteins linking integrins to actin.
Talin
Talin is an actin-binding protein that forms antiparallel homodimers. The amino-terminal FERM (protein 4.1, ezrin, radixin and moesin) domain binds β-integrin tails and is sufficient to activate integrins. The carboxy-terminal rod domain interacts with vinculin and filamentous actin1.
Vinculin
Vinculin is an actin-binding protein that is associated with cell–cell and cell–extracellular matrix junctions. It is comprised of a globular head domain linked to a tail domain by a short Pro-rich sequence. The intramolecular interaction between the head and tail masks binding sites for talin, actin and other effectors10.
α-actinin
α-actinin is an actin cross-linking protein that belongs to the spectrin superfamily. It forms antiparallel homodimers in a rod-like structure, with one actin-binding domain on each side of the rod. It can therefore cross link two filaments of actin14.
Kindlins
The kindlins are members of a family of conserved FERM domain–containing proteins named after the gene mutated in Kindler syndrome, a rare skin blistering disease. Although it is not clear exactly how kindlins activate integrins, they seem to act synergistically with talins to do so16,17.
The integrins also recruit, indirectly, scaffold and signalling proteins such as paxillin23 and the protein Tyr kinase focal adhesion kinase (FAK)24, respectively, which in turn associate with additional molecules that regulate signalling to Rho GTPases. The Rho GTPases act as a regulatory convergence node that dictates cytoskeletal and adhesion assembly and organization. Importantly, integrin signalling networks regulate the activation state of the Rho-family small GTPases — Rac, Rho and CDC42 — by recruiting guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) to adhesion complexes. In turn, Rho GTPases regulate adhesion assembly and disassembly by activating pathways that lead to contraction and actin polymerization.
The main objective of this Review is to describe the importance of the interplay between actin, contraction and adhesion dynamics (the formation and disassembly of adhesions), and how the dynamics of this process orchestrate the reiterative cycle of membrane protrusion, cell adhesion, forward movement and rear retraction — the canonical steps in cell migration. we review the evidence linking adhesion assembly and disassembly to the process of integrin binding to extracellular ligands and how adhesion dynamics are coupled to actin polymerization and myosin II-generated tension — processes that are in turn regulated by the activation of Rho GTPases and protein Tyr phosphorylation.
Adhesion: a dynamic structural continuum
Historically, integrin-dependent adhesions have been classified based on size, stability and location in the cell. However, the relative cellular distribution of the different types of adhesions is in fact dependent on the cell type and the composition and mechanical properties of the ECM substrate5,18 (FIG. 1; see Supplementary information S1 (movie)). Indeed, as we describe below, adhesion formation, maturation and disassembly is a continuous process driven by the balance of actin polymerization and actomyosin contraction.
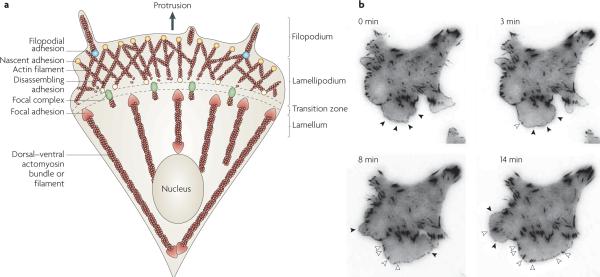
Figure 1. Structural elements of a migrating cell.
a | Adhesion is closely coupled with the protrusions of the leading edge of the cell (filopodia and lamellipodia). Adhesions (nascent adhesions) initially form in the lamellipodium (although adhesions may also be associated with filopodia) and the rate of nascent adhesion assembly correlates with the rate of protrusion. Nascent adhesions either disassemble or elongate at the convergence of the lamellipodium and lamellum (the transition zone). Adhesion maturation to focal complexes and focal adhesions is accompanied by the bundling and cross-bridging of actin filaments, and actomyosin-induced contractility stabilizes adhesion formation and increases adhesion size. b | TIRF micrographs of a Chinese hamster ovary (CHO) cell expressing paxillin–mEGFP (monomeric enhanced green fluorescent protein) on glass coated with fibronectin (5 μg ml−1). Images were acquired every 5 seconds, and representative images from 0, 3, 8 and 14 minutes are shown (see REF. 49). Closed arrow heads denote nascent adhesions assembling and turning over in protrusions. Open arrow heads indicate maturing adhesions that begin to elongate centripetally (that is, towards the cell centre) when protrusion pauses or halts. For a movie of this experiment see supplementary information S1 (movie).
Focal complexes, focal adhesions and fibrillar adhesions
Fibroblasts migrating on fibronectin- or collagen-coated surfaces exhibit small, short-lived adhesions (hereafter referred to as nascent adhesions) in the lamellipodium, which form immediately behind the leading edge. Nascent adhesions (which are optimally visualized using TIRF microscopy) can either turn over rapidly, in ~60 seconds, or mature to larger, dot-like adhesions referred to as focal complexes. Focal complexes reside slightly further back from the leading edge, at the lamellipodium–lamellum interface, are slightly larger in size (approximately 1 μm in diameter) and persist for several minutes (FIG. 1). As the migration cycle continues, focal complexes can continue to mature into larger, elongated focal adhesions, which are typically 2 μm wide and 3–10 μm long and reside at the ends of large actin bundles or stress fibres25 that extend from near the front of the cell along the sides to the cell centre or the rear. As traction forces move the cell forwards, focal adhesions at the rear of the cell disassemble. Fibroblasts grown in fibronectin-rich environments for extended times form fibrillar adhesions, which are characterized by long lifetimes and a highly elongated structure. These specialized adhesions are involved in fibronectin matrix assembly and reorganization of the ECM and are not prominent in rapidly migrating cells.
Although focal complexes, focal adhesions and fibrillar adhesions show quantitative differences in the levels of protein components such as phosphotyrosine, zyxin and tensin26, they seem to be in a continuum of structures rather than distinct classes. Furthermore, not all cells exhibit the full range of adhesion structures. For example, cells of the myeloid lineage, such as neutrophils and macrophages, have small, highly dynamic adhesions (nascent adhesions and focal complexes) that facilitate their rapid movement on ECM substrates, whereas more contractile cells, such as migrating fibroblasts, endothelial and smooth muscle cells, have more prominent, stable adhesions (focal complexes and focal adhesions).
The contractile nature of many cells combined with the mechanical properties of the matrix (such as compliance, dimensionality and fibre orientation) plays an important part in determining the nature of the adhesions3,27. For example, fibroblasts or epithelial cells can be grown in or on materials of variable mechanical stiffness28,29. Cells propagated on softer substrates contain smaller and more dynamic adhesions, whereas cells on stiffer substrates exhibit larger and more stable adhesions that are typical of cells on matrix-coated glass or plastic. Thus, adhesion size and distribution reflect the contractile state of the cell, which emphasizes the importance of the interaction between pliability and contraction in shaping adhesion dynamics. The size of adhesions in cells in three-dimensional matrices resembles those formed by cells on more pliable substrates; however, it is likely that the dimensionality of the matrix also contributes to adhesion organization. The adhesions of cells attached to large collagen fibres are large and oriented along the fibres in a two-dimensional (2D)-like organization30. These observations underscore how the composition, organization and mechanical properties of the matrix combine to regulate adhesion.
Podosomes and invadopodia
Podosomes and invadapodia are yet another class of adhesions and arecharacteristically found in leukocytes of the monocytic lineage, endothelial and smooth muscle cells, and in tumour cells, respectively31,32. Podosomes are small, circular, highly dynamic adhesions comprised of a central actin core, with integrins and other adhesion-associated proteins arranged in a ring around the centre. In osteoclasts, and sometimes in other cells, podosomes reside in clusters that form circular rings at the cell periphery. Although each podosome is highly transient, with a typical lifetime of 2–10 minutes, the rings can be quite stable33. Invadapodia resemble podosomes, but they do not arrange into rings, are much more stable and can protrude further into the ECM34. Both podosomes and invadapodia contact the substratum and function as sites of localized protease secretion and ECM degradation35. Localized ECM degradation is thought to contribute to the invasiveness of normal leukocytes and cancer cells, as well as to bone resorption31. Although it is likely that the formation and disassembly of podosomes and invadapodia share many features with the other classes of integrin-dependent adhesions described above, we will not consider them further in this Review (for recent reviews see REFS 32,35,36).
Processes coupled to adhesion dynamics
The formation and disassembly of the different types of adhesions have been studied mainly in 2D culture systems using spreading or migrating fibroblasts or fibroblast-like cells plated on fibronectin, collagen or other purified ECM proteins37,38. The mechanistic insights derived from these studies, however, seem to apply to a wide range of other cell types including mesenchymal, epithelial and endothelial cells, as well as leukocytes and neuronal cells. There is abundant evidence that adhesion assembly and disassembly is closely coupled with two fundamental cellular processes: actin polymerization and myosin II-generated tension.
Actin polymerization in adhesion dynamics
The initial step in the migration cycle, protrusion of a leading edge, is driven by actin polymerization in filaments organized into two distinct zones, the lamellipodium and the lamellum39. In the lamellipodium, actin is arranged in a dendritic, or branched, structure that is localized beneath the membrane12. Polymerization of lamellipodial actin is catalysed by the ARP2/3 complex, the activity of which is regulated by the Rho GTPases Rac and CDC42 through downstream effectors belonging to the Wiskott–Aldrich syndrome protein (WASP) and WASP-family verprolin homologue (WAVE; also known as SCAR) families of proteins12,40. Lamellipodial actin undergoes rapid retrograde flow driven by the resistance of the membrane to actin polymerization at the leading edge41. In the lamellum, actin filaments reside in parallel bundles that undergo a slower retrograde movement largely owing to myosin II contraction (see below). In the region of convergence between the lamellipodium and the lamellum, known as the transition zone, dendritic actin depolymerizes and reorganizes into bundles42–44.
Actin filaments in the central and rear regions of migrating cells are often organized into thick bundles called stress fibres45 (FIG. 1). Dorsal stress fibres connect to the substrate through focal adhesions at one end. Transverse arcs, which are not directly anchored to the substrate, are generated by the annealing of myosin-II–actin bundles and ARP2/3-nucleated actin bundles at the lamella. Finally, ventral stress fibres arise from dorsal stress fibres and transverse arcs and are anchored to focal adhesions at both ends44. Each of these structures depend on the activity of Rho and its effectors Rho-associated protein kinase (ROCK) and the formin mouse diaphanous 1 (mDia1), contain myosin II and α-actinin and are contractile18,44,46.
Myosin II-generated tension in adhesion dynamics
Contraction of actin stress fibres is mediated by myosin II, which moves antiparallel actin filaments past each other and thereby provides the force that rearranges the actin cytoskeleton (FIG. 2). Myosin II also bundles actin filaments owing to its oligomeric nature and actin-binding properties8,47. Myosin II is comprised of two heavy chains, two regulatory light chains (RLCs) and two essential light chains and has three isoforms (myosin IIA, myosin IIB and myosin IIC), which are specified by the different heavy chains that they contain. Myosin IIA and myosin IIB are present in most cells, whereas myosin IIC is not widely expressed and may have a role in cancer8. Myosin II activity (ATP hydrolysis and actin filament formation) is regulated by the reversible phosphorylation of Thr18 and Ser19 of the RLC of the myosin II molecule. This phosphorylation is controlled by several protein kinases and phosphatases, many of which are regulated by Rho GTPases. Although myosin II is not present in the lamelli podium, its activity influences membrane protrusion at the leading edge. For example, knockdown of myosin II with small interfering RNAs or treatment of cells with blebbistatin (a small molecule inhibitor of myosin II) reduces actin bundling in the protrusion, increases the protrusion rate and decreases the size of adhesions43,47, underscoring the requirement for myosin II activity in maintaining these structures.
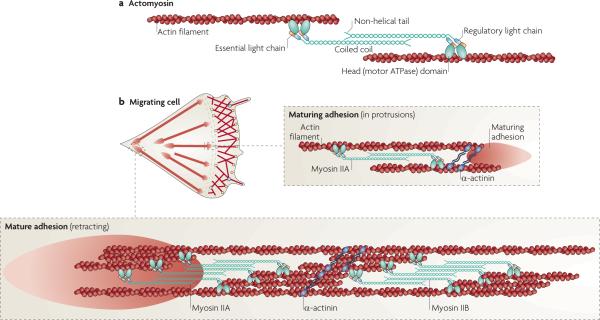
Figure 2. Myosin ii and adhesion maturation and turnover.
a | Adhesions elongate along actin filaments that contain myosin IIA, which cross links the actin filaments and exerts tension on them. This leads to tension on the conformational sensitivity, and clustering of, adhesion molecules that are directly or indirectly associated with actin. Myosin II activity is regulated by phosphorylation on the regulatory light chain at Thr18 and Ser19, although other regulatory sites in the heavy chain are also implicated in its activities. For a more complete discussion of myosin II structure and function see REF. 8. b | In a migrating cell, myosin IIA acts at a distance to regulate adhesion maturation and turnover as it is juxtaposed to, but not directly associated with, the maturing adhesion at the cell front. α-actinin cross links actin filaments. Adhesions at the rear are associated with large actin filament bundles that contain both myosin IIA and myosin IIB. Their activity mediates rear retraction and adhesion disassembly.
Adhesion dynamics
In motile cells, the earliest detectable adhesions (nascent adhesions) form in the lamellipodium just behind the leading edge (FIG. 1). Their assembly is independent of myosin II activity but is proportional to the protrusion rate of the leading edge and requires ARP2/3 complex-mediated actin polymerization48,49. These adhesions contain integrins, talin, vinculin, α-actinin, paxillin and FAK, among other proteins, and are enriched in phosphotyrosine49, an indication that these adhesions are active signalling complexes (see below). As the leading edge of a migrating cell moves forwards, nascent adhesions either elongate and grow or disassemble, depending on the cell type. Disassembly occurs when the nascent adhesions encounter the zone of depolymerizing actin at the juncture of the lamellipodium and lamellum. Adhesion turnover in this region is therefore coincident with actin severing and the disassembly of branched actin structures. Nascent adhesions can also mature into focal complexes coincident with periodic or occasional pauses of the forward movement of the leading edge. These pauses correlate with, and depend on, myosin II-dependent contractile events47–49. The actin cross-linking protein α-actinin has also been implicated as a crucial component of adhesion maturation as it is the earliest component detected in maturing adhesions and it accumulates with actin filaments before other adhesion components49. Either new actin polymerization or the reorganization of existing actin filaments at the junction of the lamellipodium and lamellum creates templates for maturation49. The time of appearance and spatial organization of α-actinin suggests that it is crucial for orienting these actin templates and linking the actin filaments to the adhesions.
Although tension is clearly important for adhesion maturation, different adhesion components show differential sensitivity to tension50. For example, the incorporation of paxillin, talin and integrin are independent of myosin II activity, whereas FAK, zyxin and α-actinin are dependent on it. Paxillin phosphorylation seems to be tension-sensitive and a key regulator of maturation, in part through its effect on vinculin binding50.
Models of nascent adhesion nucleation
The mechanisms by which nascent adhesions are nucleated, elongate and disassemble are not yet clear. Two possible models for nascent adhesion nucleation have been proposed (FIG. 3). In the first model, nucleation of adhesions is initiated by the binding of integrins to ECM proteins, their ligand-mediated clustering and the subsequent assembly of new adhesion complexes on their clustered cytoplasmic domains (FIG. 3a). In the second model, the assembly is initiated by actin polymerization and uses dendritic actin as a template for the nucleation of adhesion complexes (FIG. 3b). Evidence for the first model comes from the presence of activated integrins near the leading edge of protrusions in migrating cells and the juxtaposition of nascent adhesions forming beneath the lamellipodium in contact with the ECM. Ligand-bound, clustered integrins would then form a `multivalent' scaffold that binds other adhesion components (such as vinculin and talin) and recruits additional integrins, all of which ultimately link to actin filaments. This general model also gains strong support from studies using ligand or anti-integrin antibodies coupled to beads, which induce the clustering of adhesion components around the bead51. The second model is suggested by evidence that adhesion formation is coupled to actin polymerization, and that vinculin and FAK bind directly to ARP2/3 complexes and colocalize with ARP2/3 before adhesion formation47–49. These complexes could therefore nucleate integrin-containing complexes before integrin binds to the ECM. In reality, these models are not mutually exclusive; there is likely to be some degree of integrin clustering before ligation, with ligation increasing the clustering and signalling. Detailed molecular studies are needed to fully understand the possible mechanisms.
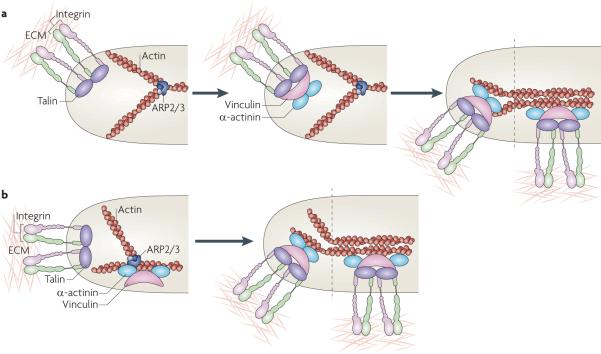
Figure 3. Models for the assembly of nascent adhesions.
a | In one model, adhesion nucleation is initiated by the binding of integrins to extracellular matrix (ECM) proteins, their ligand-mediated clustering and the coordinate assembly of new adhesion complexes on the clustered integrin cytoplasmic domains, which are depicted here as a complex with talin, vinculin, α-actinin and dendritic actin (middle panel). Maturation of the adhesions is mediated by increased tension on them and the bundling and cross-bridging of the actin filaments (right panel). b | A second model posits that adhesion formation is coupled to actin polymerization and that vinculin (and perhaps focal adhesion kinase (FAK)) bind directly to actin-related protein 2/3 (ARP2/3) complexes and colocalize before adhesion formation (left panel). These complexes then bind integrins (depicted here in association with talin), stabilizing the nascent adhesion (right panel). As in part a, maturation of the adhesions is mediated by increased tension on the adhesions and the bundling and cross-bridging of the actin filaments.
Myosin II promotes adhesion maturation and stability
The activity of myosin II and the resulting tension exerted on adhesions seem to be important factors in determining the balance between adhesion disassembly and maturation3,4,47,52. In Chinese hamster ovary (CHO) cells, in which myosin II activity is low, nascent adhesions are readily seen in the lamellipodium, whereas in more contractile cells, nascent adhesions are scarce and most rapidly mature to focal complexes47,49. Inhibiting myosin II with blebbistatin prevents adhesion maturation and greatly increases nascent adhesions. Conversely, myosin IIA overexpression in CHO cells inhibits leading edge protrusion and increases nascent adhesion maturation to focal complexes47. Thus, initial adhesion assembly is mechanistically and kinetically linked to actin polymerization in the lamellipodium, whereas myosin II activity and tension exerted on actin in the lamellum contribute to the maturation of newly formed adhesions to focal complexes and focal adhesions.
How does myosin II promote adhesion maturation and stability? One way is through the generation of tension, which directly perturbs the conformation of proteins in the adhesion complex. For example, the application of forces in vitro to single talin rods exposes cryptic binding sites for vinculin. Because the talin head domain interacts with integrins while its tail binds actin filaments, talin bears the force transmitted from the actin cytoskeleton to the matrix. Thus, actomyosin contraction would trigger force-dependent talin unfolding and increase talin–vinculin binding to reinforce the adhesion53. The vinculin tail domain also provides a linkage to actin54. In cells, recruitment of vinculin to adhesions is driven by changes in tension55. This recruitment is probably controlled at the molecular level, at least in part, by tension-induced conformation changes that result in the perturbation of the interaction between its amino- and carboxy-terminal domains10. Other adhesion-associated molecules, such as paxillin and CRK-associated SRC substrate (CAS; also known as p130cas), may also change conformation under tension (or tension-induced signals) to reveal new protein-binding and/or phosphorylation sites21,56,57. The ensuing protein–protein interactions and/or phosphorylation would activate these scaffold proteins to recruit additional signalling proteins (see below). Recently, α5β1 integrin was reported to undergo a conformational change in response to myosin II-generated cytoskeletal force, suggesting that this force, combined with ECM stiffness, triggers an integrin switch that is required to generate signals through the adhesion complex58.
A second action of myosin II on adhesion maturation occurs through its cross-linking properties. Phosphorylation of myosin II RLCs increases myosin II's assembly into bipolar myosin filaments, which bundle actin. Indeed, myosin II mutants that assemble into filaments and bind actin but lack the motor activity required to produce tension, still induce focal adhesions47, indicating that both contractility and actin bundling probably contribute to the maturation and stabilization of adhesions.
Adhesion linkages: the clutch
The tension exerted on adhesions depends on the efficiency of the linkage between actin and the ECM, namely the efficiency of the adhesion `clutch'5,59–61. Actin in the lamellipodium undergoes retrograde flow from two sources. One is the force from membrane resistance at the leading edge, which is created by actin polymerization itself and causes rearward actin flow. The other is from myosin II-mediated contraction of actin filaments in the lamellum. Thus, the net rate of forward protrusion of the leading edge is determined by the rate of actin polymerization minus these rearward forces. Adhesions function as traction points that resist the force arising from the rearward flow of actin filaments and shunt the force to the substratum, resulting in increased protrusion. However, the efficiency of this shunting, or resistance, seems to be variable, as some adhesion components move in a retrograde direction with the actin but not at the same rate, pointing to a `slippage' in the actin–adhesion linkage. This has led to the idea that the link between adhesions and actin is regulated by a clutch-like mechanism. When the clutch between adhesions and rearward flowing actin is engaged, rates of forward protrusion of the leading edge increase while the adhesions undergo force-dependent maturation61,62. The efficiency of this clutch seems to differ among cells, suggesting that it is regulated; this idea has important implications for the efficiency of tension-induced adhesive signalling59,60. Interestingly, myosin II localizes on actin filaments several micrometres away from the adhesions, suggesting that the contractile forces generated by myosin II are transmitted down the filament to the adhesions; that is, myosin II acts at a distance47.
Adhesion disassembly at the cell front and rear
Finally, tension and other factors contribute to adhesion disassembly at both the front and the rear of the cell63. At the front, disassembly occurs most prominently at the lamellum–lamellipodium interface, presumably owing to actin depolymerization and reorganization. Disassembly also occurs in regions undergoing retraction at both the cell front (as a part of the extension and retraction cycle of a protrusion) and the rear. Disassembly associated with retraction is usually accompanied by an apparent `sliding' of adhesions, which accompanies the inward movement of the cell edge, and then the `dispersal' of adhesion structures. Although not fully understood, adhesion sliding seems to be a Rho GTPase- and myosin II-dependent form of treadmilling, in which the peripheral edge of the adhesion disassembles while the central edge assembles64,65. Thus, although the whole adhesion moves, individual components exchange in and out of it but otherwise remain stationary. Interestingly, integrins, but not the cytoplasmic components of adhesions, are sometimes seen on the substratum behind migrating cells, indicating a severing between integrin and the cytoplasmic components of the adhesion during release66. This effect is blocked by a myosin II inhibitor67, suggesting that it is also tension-dependent.
Box 2 | Key regulators of adhesion dynamics: Rho GEFs and Rho GAPs.
PIX proteins
PAK-interacting exchange factor (PIX) proteins were originally identified as binding partners for the CDC42 and Rac target and effector, p21-activated kinase (PAK). PIX proteins (PIXα and PIXβ) contain a DBL homology (DH) domain, but only PIXα has significant guanine nucleotide exchange factor (GEF) activity for Rac, which is under tight control through intramolecular interactions involving several binding partners2.
DOCK180
180 kDa protein downstream of CRK (DOCK180; also known as DOCK1) is a GEF that, following integrin receptor activation, forms a complex with CRK-associated SRC substrate (CAS; also known as p130cas) and CRK which is targeted to focal adhesions. DOCK180 interacts with the small GTPase RAC1, but not with Rho or CDC42, and functions as a GEF to activate Rac. The CRKII–DOCK180–Rac cascade promotes the reorganization of the actin network, membrane ruffling, lamellipodial protrusion and phagocytosis of apoptotic cells9.
GIT
G protein-coupled receptor (GPCR) kinase-interacting protein (GIT) is a member of a family of ADP-ribosylation factor GTPase activating proteins (ARFGAPs). Members of this family share common binding partners, including paxillin, PIX, GPCR kinase (GRK) and focal adhesion kinase (FAK)2. The role of their association with focal adhesion proteins is still poorly understood but it may be a point of convergence for ARF and integrin signalling.
ARHGAP22
ARHGAP22 (also known as RHOGAP2) is a Rho GAP that converts RAC1 to an inactive GDP-bound state. Expression of ARHGAP22 inhibits RAC1-dependent lamellipodium formation13.
p190RhoGEF
p190RhoGEF (also known as RGNEF) is a brain-enriched, RHOA-specific GEF, the highly interactive carboxy-terminal domain of which provides potential linkage to multiple pathways in a cell15.
p190RhoGAPs
p190RhoGAPs exist in two isoforms, A and B. p190RhoGAPs regulate actin cytoskeleton dynamics, membrane ruffling, neurite retraction, smooth muscle contraction, cytokinesis, cellular morphology, cellular motility and invasion, embryonic neuronal development and vascular permeability19.
The Ca2+-activated protease calpain has also emerged as an important mediator of adhesion disassembly in retracting regions68. Calpain inhibition by chemical inhibitors, biological agents (such as calpastatin) and genetic deletion block disassembly. Both talin 1 and the integrin β3 cytoplasmic domain have been identified as key calpain substrates in adhesion disassembly, although there are many others with a functional significance that is less well investigated69–71.
Regulation of adhesion dynamics
The Rho GTPases Rac, Rho and CDC42 together regulate adhesion by directly controlling the balance between actin-mediated protrusion and myosin II-mediated contraction72–75.
Rac, Rho and CDC42 activity in adhesion dynamics
As expected, Rac and CDC42 are activated at the front of migrating cells, but with distinct spatial and temporal characteristics76. RHOA is prominently activated at the cell rear and also, unexpectedly, at the front77–80. Current evidence indicates that Rac and CDC42 probably have partially overlapping functions in mediating the formation of actin-rich protrusions at the leading edge81. Whereas the expression of activated CDC42 alone produces filopodia, and expression of activated Rac stimulates broad lamellipodia, leading-edge protrusion in most cells probably involves both. The activation of Rho at the leading edge was surprising, as Rho was thought mainly to activate myosin II in the rear of the cell80. However, colocalization data suggest that Rho in this region couples selectively to the formin mDia1 (REFS 77–79), which binds actin barbed ends, and promotes polymerization82 through mDia1 rather than through myosin II activation.
Rac and CDC42 induce protrusions in most cells by activating the WASP homologue (WH) domain-containing proteins neural WASP (NWASP) and WAVE, which in turn induce actin polymerization by directly activating the ARP2/3 complex. Rac and CDC42 also bind and activate the PAK Ser/Thr kinases (PAK1, PAK2 and PAK3). PAKs have multiple cytoskeletal targets, including LIM kinase, which is activated by PAK and enhances actin polymerization by inactivating cofilin — a protein that disassembles actin filaments83,84. PAK also activates myosin II by phosphorylating its RLCs. RHOA activation leads to the maturation of focal adhesions through its ability to activate myosin II, which promotes adhesion maturation and stability, as discussed above. Rho activates myosin through ROCK1 and ROCK2, which act mainly by inactivating a subunit of myosin phosphatases (myosin phosphatase-targeting subunit 1 (MYPT1; also known as PPP1R12A)), thus sustaining myosin II RLC phosphorylation. As mentioned above, Rho also activates the formin mDia1 to promote actin polymerization. Both of these effector pathways contribute to actin polymerization, bundling and adhesion formation46.
Adhesion dynamics are regulated by complex feedback loops with the Rho proteins and a poorly understood reciprocity between Rac and Rho activation that is presumably mediated through the action of GEFs and GAPs (BOX 2). Recently, it was shown that the activation of a novel photoactivatable RAC1 (PA-RAC1) was sufficient to produce cell motility and control the direction of cell movement. Importantly, local activation of PA-RAC1 inhibited RHOA activation in protrusions of migrating fibroblasts85. The molecular basis for the reciprocal regulation of Rac and Rho is not understood. On the one hand, Rac can inhibit Rho through the activation of p190RhoGAP (also known as GRLF1) to adhesions86, which can reduce tension at the leading edge to allow more continuous forward protrusion87. On the other hand, maturation of focal complexes into focal adhesions involves activation of Rho downstream of Rac, perhaps through the recruitment of a Rho GEF to adhesions88. Conversely, Rho can inhibit Rac through a pathway that involves ROCK, possibly through mechanical tension stimulating a Rac GAP such as ARHGAP22 (REFS 13,89). Understanding this reciprocity and these feedback loops is an important issue that needs to be addressed.
The spatial and temporal activation of Rac, Rho and CDC42 at the leading edge of migrating cells has been examined recently using the simultaneous visualization of two GTPase biosensors paired with computational multiplexing approaches80. Surprisingly, RHOA is activated near the cell edge concomitant with leading-edge advancement. In contrast, CDC42 and RAC1 are activated distal to the leading edge with a delay of ~ 40 seconds. Thus, both the timing and spatial characteristics of RAC1, CDC42 and RHOA activation are distinct. The spatial localization of RAC1 and CDC42 activation are consistent with the fact that these GTPases stimulate dendritic actin polymerization, which is essential for the leading edge. The role of RHOA is less clear but, as mentioned above, mDia1 activation is an attractive pathway as it promotes polymerization of the initial actin filaments needed for ARP2/3-mediated dendritic polymerization. mDia1 also attaches an actin barbed end to the membrane and allows the insertion of actin monomers at the end of the filament. However, active Rac or CDC42 can induce protrusions when RHOA is inhibited; thus, cooperation between these GTPases is not essential. Overall, much remains to be learned about how these proteins regulate dynamics at the leading edge.
Regulation of Rho GTPases in adhesion dynamics
Various scaffold proteins organize signalling complexes that regulate Rho GTPases. Protein Tyr kinases (PTKs), such as SRC, FAK, Abelson kinase 1 (ABL1) and ABL2, and their adhesion-associated substrates, function as scaffolds to differentially organize the regulatory proteins that control the activity of the Rho GTPases and, therefore, actin and adhesion dynamics and organization24,88,90,91. For example, PTKs phosphorylate adhesion proteins such as paxillin and CAS, which then bind and localize activated forms of GEFs and GAPs for Rho GTPases, as well as SH2-containing adaptor proteins such as CRK and NCK92. These SH2-containing adaptor proteins recruit additional regulators of downstream kinases, including extracellular signal-regulated kinases (ERKs) and PAKs92–94. Thus, paxillin, FAK and CAS are examples of adhesion-associated proteins that function as `switchable' scaffolds, in which phosphorylation of their Tyr residues leads to the recruitment of functional regulators of Rho GTPases and other signalling proteins. Other adhesion proteins such as zyxin and tensin may also be switchable scaffolds, although their role in regulating adhesion dynamics is less clear95–97
Adhesion assembly seems to be a key regulator of scaffold phosphorylation. For example, the catalytic activity of both FAK and SRC is stimulated by recruitment to newly formed adhesions98. Adhesion-dependent autophosphorylation of FAK leads to the recruitment and activation of SRC, which mediates Tyr phosphorylation of FAK itself, paxillin and other adhesion molecules99,100. Notably, paxillin and CAS undergo conformational changes concomitant with phosphorylation on their Tyr residues and recruitment into adhesion structures23,56. The importance of Tyr phosphorylation in the activation of the paxillin scaffold was revealed by the observations that phosphorylation of paxillin on Tyr31 and Tyr118 regulates the coordinated formation of lamellipodia or the induction of myosin II-dependent contraction21. Overexpression of phosphomimetic paxillin (Tyr31Glu and Tyr118Glu) enhances lamellipodial protrusion and the formation of nascent adhesions, whereas overexpression of non-phosphorylatable paxillin (Tyr31Phe and Tyr118Phe) induces large focal adhesions, prominent fibrillar adhesions and fibronectin fibrillogenesis, which are characteristic of highly contractile cells. These observations are consistent with Tyr-phosphorylated paxillin being a scaffold for the recruitment of positive regulators of Rac and CDC42. Similarly, Tyr phosphorylation of CAS recruits the SH2-containing adaptor protein CRK, which in turn recruits or activates the Rac GEF DOCK180 (180 kDa protein downstream of CRK; also known as DOCK1) and the RAP1 GEF C3G (also known as RAPGEF1)92. Ser phosphorylation of paxillin has also been reported to regulate adhesion turnover and protrusion dynamics in migrating cells101.
The scaffold functions of FAK seem to be important in the recruitment of Rho GAPs and Rho GEFs15,88. The association of FAK with p190RhoGAP seems to be important for RHOA inhibition during fibronectin-stimulated cell spreading, which facilitates lamellipodial protrusion19,102. Less clearly understood is the interaction of FAK with p190RhoGEF (also known as RGNEF). In cells plated on fibronectin for long periods of time, FAK seems to selectively associate with p190RhoGEF, suggesting that as cells become more contractile, positive regulation of RHOA is the dominant activity in more mature adhesions88. Additional scaffold proteins, other kinases and protein phosphatases (for example, tensin, zxyin, integrin-linked kinase (ILK), particularly interesting new Cys-His protein 1 (PINCH; also known as LIMS1), parvin, Abl, FYN and SH2 domain-containing Tyr phosphatase 2) are reported to associate with focal adhesions91,95,96,103–106. Numerous studies have shown that knock down, knock out or overexpression of these proteins modulate adhesion structures and dynamics in complex ways; however, the mechanisms and regulation are poorly understood.
Unifying the principles of adhesion
As discussed above, the adhesive steps in the migration cycle — assembly, maturation and disassembly — are tightly coupled to actin polymerization and organization and to actin–myosin contraction, which are in turn regulated by Rho GTPases and PTKs5 (FIG. 4). The first step in the cycle is formation of nascent adhesions beneath the lamellipodium near the leading edge. These adhesions not only stabilize the leading edge through contact of the cell with the ECM, but their formation leads to the generation of signals that activate Rac and CDC42, reinforcing the actin polymerization at the leading edge and subsequent membrane protrusion.
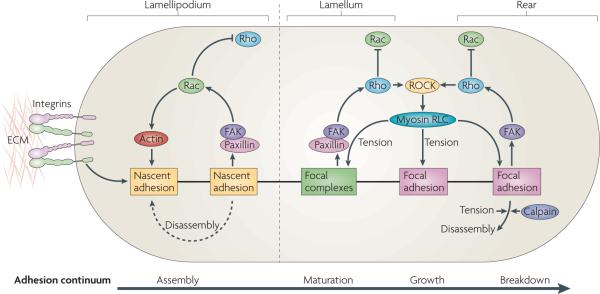
Figure 4. adhesion maturation and rho gTPase activation.
Nascent adhesion formation and disassembly are coupled with the forward movement of the lamellipodium. Maturation of adhesions is dependent on actomyosin in the lamellum, where adhesions become larger. Adhesion formation and disassembly in the lamellipodium is driven by the activation of Rac (and perhaps the localized suppression of Rho activity), which involves activation of the Tyr-phosphorylated scaffolds, paxillin and focal adhesion kinase (FAK). In the lamellum, adhesion maturation is accompanied by localized activation of Rho, perhaps through FAK-dependent recruitment of Rho guanine nucleotide exchange factors (GEFs) and Rho GTPase-activating proteins (GAPs). Rho activation sustains the activation of myosin II through the action of Rho-associated protein kinase (ROCK), which controls the kinases and phosphatases that regulate its regulatory light chain (RLC) phosphorylation. Myosin II-generated tension sustains adhesion maturation by cross linking- and tension-induced conformational changes in various adhesion proteins (see main text). Disassembly of adhesions at the cell rear is Rho GTPase- and myosin II-dependent, and may also involve the action of proteases, such as calpains, on adhesion-linked proteins. ECM, extracellular matrix.
Nascent adhesions disassemble as the lamellipodium moves forwards, unless they connect with actomyosin in the lamellum, in which case they mature and become larger. Adhesion maturation is probably accompanied by localized activation of Rho, perhaps through FAK-dependent recruitment of Rho GEFs. Rho activation sustains the activation of myosin II through the action of ROCK on the kinases and phosphatases that regulate myosin II RLC phosphorylation. Myosin II-generated tension sustains adhesion maturation through cross-linking and tension-induced conformational changes in various adhesion proteins. Although myosin II controls adhesion maturation and disassembly, the extent to which these processes occur probably reflects the efficiency of the linkage between actin and the ECM by the adhesion clutch and/or pliability of the matrix, two factors that contribute to myosin II activity and intracellular tension. Cells on rigid surfaces coated with high densities of ECM proteins exhibit large, myosin II-dependent focal adhesions, whereas cells on pliable substrates coated with low densities of adhesion molecules tend to have smaller adhesions3. Indeed, artificially increasing integrin clustering can make cells behave on soft substrates as if adhered to rigid ones107. These data suggest that cells sense the mechanical properties of the substratum and subsequently modulate myosin II activity, integrin clustering, adhesion size and composition, and downstream signalling. However, the model also implies that myosin II is regulated through changes in integrin signalling. Indeed, the feedback loop that connects adhesion, contractility and signalling almost certainly involves Rho GTPases and/or the regulation of myosin II activity and actomyosin tension.
Cell adhesion, contractility and signalling play central parts in the front–back polarization of migrating cells and hence in regulating directional motility. In fibro blasts, adhesions at the leading edge generate signals that activate Rac, which in turn leads to dendritic actin poly merization and establishment of the cell front. Conversely, actomyosin bundles and stable adhesions are crucial for generating the cell rear108,109. An attractive hypothesis is that the cross-linking and bundling by myosin IIb generates large, stable actin filaments and adhesions, which inhibits adhesion signalling to Rac (FIG. 3). Recent studies have shown that actin filament bundles in the cell rear contain activated myosin IIb, which is crucial for the formation and stabilization of the rear47,110. The partitioning defective 3 (PAR3)– or PAR6–protein kinase Cα (PKCα) complex is also implicated in cell polarity111; however, the role of adhesion in this process remains to be clarified.
Migration in disease
Migration is a prominent feature of many diseases, including cancer and chronic inflammation. It is also important in stem cell transplantation strategies, where injected cells may need to migrate into target tissues, and in wound repair, where enhanced cell migration contributes to wound closure. Although adhesion receptors and ECM ligands have been studied as potential targets for therapeutic strategies, differences in adhesion dynamics and maturation may also play a part in disease and therefore offer targets for intervention and diagnosis. For example, myosin II activity regulates migration through its effects on adhesion maturation and signalling. Thus, strategies directed at specific regulators of myosin II, such as Rho kinase and myosin light chain kinase (MlCK), provide another route to the regulation of migration. In addition, the activation (phosphorylation) status of key effectors of the Rho kinases might be a parameter for predicting invasive potential; that is, whether the cells are primed for migration. The feasibility of this strategy derives from the relatively small number of molecules downstream of Rac and Rho that regulate adhesion dynamics and signalling for migration. Thus, there are provocative new opportunities for both therapeutic and diagnostic techniques that may play an important part in clinical medicine.
Some remaining questions
The model for adhesion dynamics described here supports contemporary views of directed cell migration18, in which the compartmentalization of Rac and/or CDC42 and Rho activity maintains the direction of cell movement109. The balance of actin polymerization and myosin II-generated contractility provide both feedforward and feedback loops that regulate adhesion formation and disassembly. In the framework of this model, several important questions remain. what regulates the efficiency of coupling between adhesions and rearward flowing actin? what determines whether an adhesion strengthens under force, as occurs in the front of migrating cells, versus disassembles, as occurs in the rear? How do cells sense the rigidity of the ECM to control myosin II activation and how does this feed back to regulate signalling by adhesions? Do different adhesions generate distinct signals and, if so, how, when and where? what is the role of Rho at the leading edge? How do changes in adhesion and migration pathways underlie immune disorders, developmental defects and cancer cell invasion and metastasis? Clearly, understanding the fundamental mechanisms that govern adhesion signalling offers unique opportunities to design and implement therapeutic interventions that may have a considerable impact on the treatment of human disease.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the help of M. Vicente-Manzanares and C. Choi in the preparation of the figures and C. Choi for making the supplementary movie. The authors acknowledge support from the Cell Migration Consortium (U54 GM64346), NCI CA40042 (to J.T.P), NIGMS-GM23244 (to A.R.H) and GM47214 (to M.A.S).
Glossary
- Blebbing cell
A cell that extends a round, dynamic process from the its membrane.
- Substrate compliance
A measure of the elasticity of the material to which cells adhere and is related to the distance a material deforms under force. It is the inverse of stiffness and is given in units of 1 per Pascal.
- Extracellular matrix
The fibrillar material made of collagens, laminin, fibronectin or other glycoproteins, and proteoglycans, which forms a solid substratum under or around cells in vivo and in culture.
- Lamellipodium
A broad, flat protrusion at the leading edge of a cell that moves owing to actin polymerization that is generally induced by Rac activation.
- Filopodium
A long, thin protrusion at the periphery of cells and growth cones. Filopodia are composed of F-actin bundles and are often induced by the activation of CDC42.
- Actomyosin
A complex of myosin and actin filaments. Activation of the myosin motor leads to shortening of the filaments and subsequent cellular movements.
- Guanine nucleotide exchange factor
A protein that activates specific small GTPases by catalysing the exchange of bound GDP for GTP.
- GTPase-activating protein
A protein that inactivates small GTP-binding proteins, including Ras and Rho family members, by increasing their rate of GTP hydrolysis.
- TIRF
(Total internal reflection fluorescence). A microscope exploiting evanescent wave excitation of the thin region (~100 nm) at the contact area between a specimen and the glass coverslip (of a distinct refractive index). It provides improved signal to noise ratios for the observation of events near the coverslip–water interface.
- Lamellum
A distinct region of dense actin behind the lamellipodium.
- Three-dimensional matrix
(3D matrix). Cells that migrate on top of a thin layer of ECM are considered to be in 2D, whereas cells that are inside and surrounded by ECM on all sides are considered to be in 3D.
- Osteoclast
A mesenchymal cell with the capacity to differentiate into bone tissue.
- ARP2/3
A complex consisting of seven subunits, including the actin-related proteins ARP2 and ARP3, that, on activation by WASP-family proteins, binds to the sides of existing actin filaments and nucleates the growth of new filaments to form a dendritic network.
- Retrograde flow
The movement of actin filaments or other cell components from the cell edge towards the centre, generally driven by actin polymerization at the leading edge.
- Transverse arc
A bundle of actin filaments that forms parallel to the leading edge and undergoes retrograde movement towards the cell centre.
- Barbed end
The fast-polymerizing end of an actin filament, which is defined by the arrowhead-shaped decoration of actin filaments with myosin fragments.
- Computational multiplexing
A mathematical method to correlate multiple time-dependent variables obtained during time-lapse imaging of cells.
Footnotes
Competing interests statement The authors declare no competing financial interests.
SUPPLEMENTARY INFORMATION See online article: S1 (movie)
References
- 1.Campbell ID, Ginsberg MH. The talin–tail interaction places integrin activation on FERM ground. Trends Biochem. Sci. 2004;29:429–435. doi: 10.1016/j.tibs.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Premont RT. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell. Signal. 2004;16:1001–1011. doi: 10.1016/j.cellsig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nature Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 4.Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J. Cell Sci. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration — the actin connection. J. Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nature Rev. Mol. Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 8.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nature Rev. Mol. Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Cell. Signal. 2007;17:383–393. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 12.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 13.Sanz-Moreno V, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Otey CA, Carpen O. α-actinin revisited: a fresh look at an old player. Cell. Motil. Cytoskeleton. 2004;58:104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- 15.Lim Y, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J. Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montanez E, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goult BT, et al. The structure of the N-terminus of kindlin-1: a domain important for αIIbβ3 integrin activation. J. Mol. Biol. 2009;394:944–956. doi: 10.1016/j.jmb.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 19.Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK–p120RasGAP–p190RhoGAP complex regulates polarity in migrating cells. J. Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 21.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J. Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 22.Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J. Cell Sci. 2010;123:1385–1388. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the interactions among adhesion proteins comprising the cell adhesome.
- 23.Brown MC, Turner CE. Paxillin: adapting to change. Physiol. Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 24.Parsons JT. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 25.Zimerman B, Volberg T, Geiger B. Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading. Cell. Motil. Cytoskeleton. 2004;58:143–159. doi: 10.1002/cm.20005. [DOI] [PubMed] [Google Scholar]
- 26.Zamir E, et al. Molecular diversity of cell–matrix adhesions. J. Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- 27.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell–matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 29.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 30.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Linder S. Invadosomes at a glance. J. Cell Sci. 2009;122:3009–3013. doi: 10.1242/jcs.032631. [DOI] [PubMed] [Google Scholar]
- 33.Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J. Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- 34.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 35.Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J. Cell Sci. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 37.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 38.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells — over and over and over again. Nature Cell Biol. 2002;4:E97–E100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 39.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 40.Nicholson-Dykstra S, Higgs HN, Harris ES. Actin dynamics: growth from dendritic branches. Curr. Biol. 2005;15:R346–R357. doi: 10.1016/j.cub.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 41.Vallotton P, Danuser G, Bohnet S, Meister JJ, Verkhovsky AB. Tracking retrograde flow in keratocytes: news from the front. Mol. Biol. Cell. 2005;16:1223–1231. doi: 10.1091/mbc.E04-07-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shemesh T, Verkhovsky AB, Svitkina TM, Bershadsky AD, Kozlov MM. Role of focal adhesions and mechanical stresses in the formation and progression of the lamellum interface. Biophys. J. 2009;97:1254–1264. doi: 10.1016/j.bpj.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 44.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amano M, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 46.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 47.Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J. Cell Biol. 2008;183:543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexandrova AY, et al. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE. 2008;3:e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi CK, et al. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nature Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the transition from small nascent adhesions to larger myosin II-dependent focal complexes and focal adhesions through actin filament clustering rather than myosin II motor activity.
- 50.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 2010;188:877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyamoto S, et al. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz MA. Cell biology. The force is with us. Science. 2009;323:588–589. doi: 10.1126/science.1169414. [DOI] [PubMed] [Google Scholar]
- 53.del Rio A, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper confirms the prediction from structural studies that applying force to talin exposes vinculin binding sites.
- 54.Humphries JD, et al. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen DM, Kutscher B, Chen H, Murphy DB, Craig SW. A conformational switch in vinculin drives formation and dynamics of a talin-vinculin complex at focal adhesions. J. Biol. Chem. 2006;281:16006–16015. doi: 10.1074/jbc.M600738200. [DOI] [PubMed] [Google Scholar]
- 56.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai X, et al. Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol. Cell Biol. 2008;28:201–214. doi: 10.1128/MCB.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper developed a fluorescence-based biosensor for FAK activation and used it to describe a stimulus-dependent local FAK activation in cells that depends on FAK binding to acidic phospholipids.
- 58.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]; This paper presents evidence that α5β1 integrin responds to mechanical tension by conversion to a high affinity state that is required for binding to the synergy site in fibronectin.
- 59.Brown CM, et al. Probing the integrin–actin linkage using high-resolution protein velocity mapping. J. Cell Sci. 2006;119:5204–5214. doi: 10.1242/jcs.03321. [DOI] [PubMed] [Google Scholar]
- 60.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 61.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 62.Jay DG. The clutch hypothesis revisited: ascribing the roles of actin-associated proteins in filopodial protrusion in the nerve growth cone. J. Neurobiol. 2000;44:114–125. doi: 10.1002/1097-4695(200008)44:2<114::aid-neu3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 63.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr. Opin. Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential αVβ3-integrin turnover regulates focal adhesion behavior. J. Cell Biol. 2001;155:1319–1332. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Digman MA, Brown CM, Horwitz AR, Mantulin WW, Gratton E. Paxillin dynamics measured during adhesion assembly and disassembly by correlation spectroscopy. Biophys. J. 2008;94:2819–2831. doi: 10.1529/biophysj.107.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palecek SP, Huttenlocher A, Horwitz AF, Lauffenburger DA. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J. Cell Sci. 1998;111:929–940. doi: 10.1242/jcs.111.7.929. [DOI] [PubMed] [Google Scholar]
- 67.Crowley E, Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J. Cell Biol. 1995;131:525–537. doi: 10.1083/jcb.131.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J. Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 69.Franco SJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nature Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]; This paper identified talin, the proteolysis of which is required for focal adhesion turnover, as an important substrate for the protease calpain.
- 70.Flevaris P, et al. A molecular switch that controls cell spreading and retraction. J. Cell Biol. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan KT, Bennin DA, Huttenlocher A. Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK) J. Biol. Chem. 2010;285:11418–11426. doi: 10.1074/jbc.M109.090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 73.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 74.Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 75.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 76.Kurokawa K, Nakamura T, Aoki K, Matsuda M. Mechanism and role of localized activation of Rho-family GTPases in growth factor-stimulated fibroblasts and neuronal cells. Biochem. Soc. Trans. 2005;33:631–634. doi: 10.1042/BST0330631. [DOI] [PubMed] [Google Scholar]
- 77.Goulimari P, et al. Gα12/13 is essential for directed cell migration and localized Rho-Dia1 function. J. Biol. Chem. 2005;280:42242–42251. doi: 10.1074/jbc.M508690200. [DOI] [PubMed] [Google Scholar]
- 78.Kurokawa K, Matsuda M. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol. Biol. Cell. 2005;16:4294–4303. doi: 10.1091/mbc.E04-12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 80.Machacek M, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper uses multi-wavelength imaging of FRET sensors for Rho GTPases and sophisticated quantitative analysis to define the relationships between the activation of Rho, Rac and CDC42, and leading-edge dynamics.
- 81.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 82.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nature Rev. Mol. Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- 83.Yang N, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 84.Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nature Rev. Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nature Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 87.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 2001;12:2711–20. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr. Opin. Cell Biol. 2009;5:676–683. doi: 10.1016/j.ceb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katsumi A, et al. Effects of cell tension on the small GTPase Rac. J. Cell Biol. 2002;158:153–164. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 91.Peacock JG, et al. The Abl-related gene tyrosine kinase acts through p190RhoGAP to inhibit actomyosin contractility and regulate focal adhesion dynamics upon adhesion to fibronectin. Mol. Biol. Cell. 2007;18:3860–3872. doi: 10.1091/mbc.E07-01-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–63. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Bladt F, et al. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol. Cell Biol. 2003;23:4586–4597. doi: 10.1128/MCB.23.13.4586-4597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deakin NO, Turner CE. Paxillin comes of age. J. Cell Sci. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clark K, et al. Tensin 2 modulates cell contractility in 3D collagen gels through the RhoGAP DLC1. J. Cell Biochem. 2010;109:808–817. doi: 10.1002/jcb.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall EH, Daugherty AE, Choi CK, Horwitz AF, Brautigan DL. Tensin1 requires protein phosphatase-1α in addition to RhoGAP DLC-1 to control cell polarization, migration, and invasion. J. Biol. Chem. 2009;284:34713–34722. doi: 10.1074/jbc.M109.059592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hervy M, Hoffman L, Beckerle MC. From the membrane to the nucleus and back again: bifunctional focal adhesion proteins. Curr. Opin. Cell Biol. 2006;18:524–532. doi: 10.1016/j.ceb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 98.Lietha D, et al. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 100.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nature Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 101.Nayal A, et al. Paxillin phosphorylation at Ser273 localizes a GIT1–PIX–PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 2006;173:587–589. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ren XD, et al. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 103.Lin S-Y, et al. The Protein-tyrosine phosphatase SHP-1 regulates the phosphorylation of α-actinin. J. Biol. Chem. 2004;279:25755–25764. doi: 10.1074/jbc.M314175200. [DOI] [PubMed] [Google Scholar]
- 104.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nature Rev. Mol. Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 106.Burridge K, Sastry SK, Sallee JL. Regulation of cell adhesion by protein-tyrosine phosphatases. J. Biol. Chem. 2006;281:15593–15596. doi: 10.1074/jbc.R500030200. [DOI] [PubMed] [Google Scholar]
- 107.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Keymeulen A, et al. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J. Cell Biol. 2006;174:437–445. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu J, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 110.Yam PT, et al. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J. Cell Biol. 2007;178:1207–1221. doi: 10.1083/jcb.200706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.