Abstract
Considerable evidence has been collected indicating that histamine can modulate proliferation of different normal and malignant cells. High histamine biosynthesis and content together with histamine receptors have been reported in different human neoplasias including melanoma, colon and breast cancer, as well as in experimental tumours in which histamine has been postulated to behave as an important paracrine and autocrine regulator of proliferation. The discovery of the human histamine H4 receptor in different tissues has contributed to our understanding of histamine role in numerous physiological and pathological conditions revealing novel functions for histamine and opening new perspectives in histamine pharmacology research. In the present review we aimed to briefly summarize current knowledge on histamine and histamine receptor involvement in cancer before focusing on some recent evidence supporting the novel role of histamine H4 receptor in cancer progression representing a promising molecular target and avenue for cancer drug development.
LINKED ARTICLES
BJP has previously published a Histamine themed issue (2009). To view this issue visit http://dx.doi.org/10.1111/bph.2009.157.issue-1
Keywords: histamine, histamine H4 receptor, cancer pharmacology, breast cancer, melanoma, colon cancer, histamine H4 receptor ligands, anticancer drugs, cell proliferation, malignancy
Introduction
Cancer continues to be a major health problem for those in developed countries being a leading cause of death worldwide and accounting for 7.9 million deaths in 2007. That number is slated to increase to 11.5 million by the year 2030. Lung, stomach, liver, colon and breast cancer cause the most cancer deaths each year (Parkin et al., 2005; Mathers and Loncar, 2006).
A large body of literature indicates that tumorigenesis in humans is a multistep process and that these steps reflect genetic and epigenetic alterations that drive the progressive transformation of normal cells into highly malignant derivatives. More than 100 distinct types of cancer have been described and subtypes of tumours can be found within specific organs representing added challenges to cancer treatments (Hanahan and Weinberg, 2000; Frank and Knowles, 2005; Hall and Giaccia, 2006).
Surgery and radiation therapy are by far the most widely used local treatments for cancer and remain mainstays of the effective treatment of cancer to remove the primary tumour or by combining these treatments with chemotherapy, a systemic drug therapy, which are aimed to eradicate micrometastatic disease. The term chemotherapy, in its broadest definition, covers any therapeutic intervention utilizing chemicals and includes the use of any pharmaceutical compounds. In this fashion, chemotherapeutic agents comprise the traditional cytotoxic drugs, immunotherapy, hormonal therapy, monoclonal antibodies, signal transduction inhibitors, antiangiogenic drugs and differentiating drugs. The ideal chemotherapeutic drug would target and destroy only cancer cells however; most drugs exhibit several toxic effects resulting in serious adverse effects to patients (Camidge and Jodrell, 2005; Fentiman, 2005). Anticancer drug discovery, development and administration are changing in the post-genomics era. Advances in the understanding of cancer biology and the molecular basis of response and resistance to treatments are helping to create novel drugs that can contribute to improve efficacy. The war against cancer might be far from being won, but the era of molecular-targeted treatments could prove to be one of the most important turning points in determining the outcome. Pharmaceuticals are now screened against these targets rather than looking for crude cell turnover or tumour growth effects straightaway (Camidge and Jodrell, 2005; Fentiman, 2005).
A huge number of molecules involved in cell proliferation, a key event in tumour development and progression, have been extensively investigated including histamine (Rivera et al., 2000; Darvas et al., 2003; Pós et al., 2004). Histamine [2-(4-imidazolyl)-ethylamine] is an endogenous biogenic amine widely distributed throughout the organism and is known since long to be a pleiotropic mediator in different (patho) physiological conditions (Kahlson and Rosengren, 1968; Hill et al., 1997; Dy and Schneider, 2004; Pós et al., 2004; De Esch et al., 2005).
Notably, most malignant cell lines and experimental tumours express the histamine-synthesizing enzyme, L-histidine decarboxylase (HDC, EC 4.1.1.22) and contain high concentration of endogenous histamine that can be released to the extracellular media and via a paracrine or autocrine regulation, histamine may regulate diverse biological responses related to tumour growth (Bartholeyns and Fozard, 1985; Garcia-Caballero et al., 1994; Engel et al., 1996; Rivera et al., 2000; Falus et al., 2001; Pós et al., 2004). These events include angiogenesis, cell invasion, migration, differentiation, apoptosis and modulation of the immune response, indicating that histamine may be a crucial mediator in cancer development and progression. Since the first report in 1984 (Bartholeyns and Bouclier, 1984) showing that the inhibition of HDC with monofluormethylhistidine resulted in antitumoural effects on experimental tumours in rodents, a large body of experimental evidence has supported the critical role of HDC and histamine in cellular proliferation. The employment of specific HDC antisense oligonucleotides suppressed melanoma cell proliferation (Hegyesi et al., 2001), and the overexpression of the enzyme with an up-regulated histamine production in murine melanoma cells enhanced metastatic capacity and induced the expression of a more aggressive phenotype (Pós et al., 2005). In addition, in diverse human tumours histamine concentration showed to be higher compared with surrounding normal tissue including melanomas, colon and breast cancer (Garcia-Caballero et al., 1994; Reynolds et al., 1997; Hegyesi et al., 2001; Sieja et al., 2005; von Mach-Szczypiński et al., 2009). In a high number of human cell lines derived from different neoplasias, as well as in tumoural tissues, the expression of histamine receptors with the ability to regulate cell proliferation has been demonstrated to support the role of histamine as a growth factor (Tilly et al., 1990; Davio et al., 1993; 1996; Rivera et al., 1993; Cricco et al., 1994; Lemos et al., 1995; Wang et al., 1997; Falus et al., 2001; Molnár et al., 2001; 2002; Cianchi et al., 2005; Hegyesi et al., 2005; Medina et al., 2006; Cricco et al., 2008; Davenas et al., 2008; Medina et al., 2008). Histamine receptors in numerous malignant cell types can be associated with multiple signalling pathways. The regulation of receptor density at cell surface can strongly affect the receptor ability to functionally couple and regulate different signal transduction pathways (Mitsuhashi et al., 1989; Davio et al., 1995; Fitzsimons et al., 2002).
Furthermore, the discovery of the histamine H4 receptor (H4R) with functional presence in a wide range of tissues including tumours revealed novel functions for histamine leading to reconsideration of new perspectives in histamine pharmacology research (Huang and Thurmond, 2008; Leurs et al., 2009; Tiligada et al., 2009; Zampeli and Tiligada, 2009).
In the present review we aimed to briefly summarize current knowledge on histamine and histamine receptor involvement in cancer before focusing on some recent evidence supporting the novel role of H4R in cancer progression representing a promising therapeutic target for cancer drug development.
Characteristics of H4R and its ligands
The identification by genomics-based approach of the human H4R by several groups has helped refine our understanding of histamine roles. It appeared to have a selective expression pattern restricted to medullary and peripheral haematopoietic cells including eosinophils, mast cells, dendritic cells, T cells and monocytes. Therefore, growing attention is directed towards the therapeutic development of H4R ligands for inflammation and immune disorders. In addition, H4R was reported to be present on other cell types including intestinal epithelium, spleen, lung, stomach, central nervous system, nerves of nasal mucosa, enteric neurons and interestingly in cancer cells (Nakamura et al., 2000; Oda et al., 2000; Coge et al., 2001; Liu et al., 2001a; Morse et al., 2001; Nguyen et al., 2001; Zhu et al., 2001; Cianchi et al., 2005; Medina et al., 2006; Connelly et al., 2009; Leurs et al., 2009). The significance of the H4R presence in various human tissues remains to be elucidated and therefore, new roles of H4R are still unrevealed (Leurs et al., 2009; Zampeli and Tiligada, 2009). The H4R cDNA was finally identified in the human genome database on the basis of its overall homology (37%, 58% in transmembrane regions) to the H3R sequence and it has a similar genomic structure. On the other hand, the homology with H1R and H2R is of approximately 19%. The human H4R gene that mapped to chromosome 18 is interrupted by two large introns and encodes a protein of 390 amino acids (Coge et al., 2001; Dy and Schneider, 2004; De Esch et al., 2005,Akdis and Simons, 2006; Leurs et al., 2009). H4R is coupled to Gαi/o proteins, therefore inhibiting forskolin-induced cAMP formation (Nakamura et al., 2000; Oda et al., 2000; Dy and Schneider, 2004; De Esch et al., 2005). Additionally, stimulation of H4R leads to activation of mitogen-activated protein kinase and also increased calcium mobilization via Pertussis toxin-sensitive pathway (Morse et al., 2001; Hofstra et al., 2003; Akdis and Simons, 2006; Leurs et al., 2009).
Isoforms have been described for the H4R, which have different ligand binding and signalling characteristics. H4R splice variants [H4R (67) and H4R (302)] have a dominant negative effect on H4R (390) functionality, being able to retain it intracellularly and to inactivate a population of H4R (390) presumably via hetero-oligomerization (van Rijn et al., 2008; Leurs et al., 2009). In addition, H4R dimeric structures that include homo- and hetero-oligomer formation and post-translational changes of the receptor might contribute to added pharmacological complexity for H4R ligands (van Rijn et al., 2006; 2008; Leurs et al., 2009).
In accordance with the homology between the two receptors, various H3R ligands are recognized by the H4R, albeit with different affinities. Initially compounds contain an imidazole heterocycle and have H3R and H4R dual activity. They include the H3R and H4R agonist, imetit; and the H3R antagonist and H4R agonist, clobenpropit (Lim et al., 2005; Leurs et al., 2009) (Table 1). The H4R selective agonists include compounds such as OUP-16 and 4-methylhistamine (Hashimoto et al., 2003; Lim et al., 2005; Leurs et al., 2009) (Table 1). The antipsychotic agent clozapine was the first non-imidazole H4R agonist; however, it has affinity for numerous G-protein coupled receptors (Oda et al., 2000; Leurs et al., 2009). VUF8430 is a non-imidazole full agonist at human H4R and is considered a new useful tool to evaluate H4R pharmacology (Leurs et al., 2009; Lim et al., 2009) (Table 1).
Table 1.
Compounds most widely used in H4R investigation
| Compound | Chemical name | Human H4R Ki (nmol·L−1) |
|---|---|---|
| Histamine | 2-(1H-imidazol-5-yl)ethanamine | 16 |
| Imetit | 2-(1H-imidazol-5-yl)ethyl carbamimidothioate | 1.6 |
| Clobenpropit | 3-(1H-imidazol-5-yl)propyl N′-[(4-chlorophenyl)methyl]carbamimidothioate | 13 |
| OUP-16 | (-)-2-cyano-1-methyl-3-{(2R,5R)-5-[1H-imidazol-4(5)-yl]tetrahydrofuran-2-yl}methyl-guanidine | 125 |
| 4-Methylhistamine | 2-(5-methyl-1H-imidazol-4-yl)ethanamine | 50 |
| Clozapine | 3-chloro-6-(4-methylpiperazin-1-yl)-5H-benzo[c][1,5]benzodiazepine | 625 |
| VUF8430 | 2-(2-guandinoethyl)isothiourea | 32 |
| Thioperamide | N-cyclohexyl-4-(1H-imidazol-5-yl)piperidine-1-carbothioamide | 125 |
| JNJ7777120 | 1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine | 4 |
| VUF6002 | (5-chloro-1H-benzo[d]imidazol-2-yl)(4-methylpiperazin-1-yl)methanone | 26 |
| A-987306 | cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine | 6 |
| A-940894 | 4-piperazin-1-yl-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-d]pyrimidin-2-ylamine | 71 |
Values (Ki) are taken from Leurs et al. (2009) except for imetit (Wulff et al., 2002) and A-940894 (Strakhova et al., 2009). More detailed information on these and other H4R compounds is reviewed at Lim et al. (2005), Leurs et al. (2009), Lim et al. (2009), Smits et al. (2009), Koenig et al. (2010) and Smits et al. (2010).
H4R, histamine H4 receptor.
The first H4R antagonist was also an imidazole compound with H3R and H4R dual activity such as thioperamide that behaves as an inverse agonist at the H4R (Hofstra et al., 2003; Damaj et al., 2007; Leurs et al., 2009). After subsequent medicinal chemistry efforts by Johnson and Johnson Pharmaceuticals the selective non-imidazole neutral antagonist JNJ7777120 was discovered and it became an H4R reference antagonist with more than a thousand fold selective over other histamine receptor subtypes (Jablonowski et al., 2003; Leurs et al., 2009). Recently, other compounds such as A-987306 and A-940894 that have shown to exhibit good pharmacokinetic properties including oral bioavailability and half-life have been reported by Abbot Laboratories (Liu et al., 2008; Strakhova et al., 2009) (Table 1).
Moreover, differences in pharmacological activities of H4R ligands have been described between different species restricting the preclinical development of future H4R drugs (Liu et al., 2001b; Lim et al., 2008; 2010; Leurs et al., 2009).
Histamine receptors and breast cancer
Breast cancer is the most common neoplastic disease in women, accounting for over one-fifth of the estimated annual 4.7 million cancer diagnoses in women and continues to rise in incidence (Bray et al., 2004; Parkin et al., 2005). Despite advances in early detection and continuous contributions to the understanding of the molecular bases of breast cancer biology, about 30% of patients with early-stage breast cancer have recurrent disease, which is metastatic in most cases and whose cure is very limited showing a 5 year survival rate of 20% (Gonzalez-Angulo et al., 2007).
The identification of genes and biochemical pathways involved in breast carcinogenesis are of utmost importance for the development of rational molecularly based preventive and therapeutic approaches that offer increased efficacy and low toxicity (Camidge and Jodrell, 2005; Fentiman, 2005).
Considerable evidence has been accumulated indicating that histamine can modulate proliferation of different normal and malignant cells (Tilly et al., 1990; Wang et al., 1997; Rivera et al., 2000; Falus et al., 2001; Pós et al., 2004). Histamine plays a critical role in the pathological and physiological aspects of the mammary gland. Histamine is involved in growth regulation, differentiation and functioning during development, pregnancy and lactation (Malinski et al., 1993; Davio et al., 1994; Kierska et al., 1997; Wagner et al., 2003). In addition, histamine is increased in plasma and cancerous tissue derived from breast cancer patients compared with healthy group, which is associated with an imbalance between synthesis and degradation of this monoamine (Garcia-Caballero et al., 1994; Reynolds et al., 1998; Sieja et al., 2005; von Mach-Szczypiński et al., 2009). A pilot study revealed that in samples of the same invasive ductal carcinoma patient, histamine peripheral blood levels tended to be reduced post-operatively (Kyriakidis et al., 2009). Further studies support the role of histamine in breast cancer development. It was reported that in experimental mammary carcinomas, histamine becomes an autocrine growth factor capable of regulating cell proliferation via H1R and H2R, as one of the first steps responsible for the onset of malignant transformation. In this light, the in vivo treatment with H2R antagonists produced the complete remission of 70% of experimental tumours (Rivera et al., 1993; 2000; Cricco et al., 1994; Davio et al., 1995). Although many reports indicate the presence of H1R and H2R in normal and malignant tissues as well as in different cell lines derived from human mammary gland (Davio et al., 1993; 1996; Lemos et al., 1995), the clinical trials that have been carried out with H2R antagonists in cancer patients demonstrated controversial results for breast cancer (Bolton et al., 2000; Parshad et al., 2005) (Table 2).
Table 2.
Expression and functional characteristics of histamine receptors in different cancer types
| Cancer type | Histamine receptor expression and function | References |
|---|---|---|
| Breast cancer | H1R and H2R expression in experimental tumours. H2R antagonist inhibited proliferation. | Cricco et al. (1994); Rivera et al. (2000) |
| H1R and H2R expression in human benign and malignant lesions. No correlation between H2R and hormone or EGF receptors. | Davio et al. (1993); Lemos et al. (1995) | |
| H3R and H4R expression in human benign and malignant lesions. Correlation between H3R and proliferation marker and histamine production. | Medina et al. (2008) | |
| H1R and H2R expression in human non-tumorigenic (MCF-10A, HBL-100) and tumorigenic (MCF-10T, MCF-7, MDA-MB-231) cell lines. Histamine did not modulate proliferation of HBL-100. H1R and H2R agonist decreased proliferation of MCF-7 and MDA-MB-231 cells. | Davio et al. (1996; 2002); Medina et al. (2006; 2008); | |
| H3R and H4R expression in HBL-100, MCF-7 and MDA-MB-231 cell lines. H3R agonists increased MDA-MB-231 cell growth and migration. H4R agonists inhibited proliferation, increasing cell apoptosis and senescence of MCF-7 and MDA-MB-231 cells. | Medina et al. (2006; 2008; unpubl. data) | |
| Melanoma | H2R expression in syngeneic or xenogenic melanoma grafts in mice. H2R antagonists inhibited tumour growth. | Szincsák et al. (2002a,b); Tomita et al. (2005) |
| H1R, H2R and H3R expression in human melanoma cell lines (WM35, WM983/B, HT-168, HT-168/M1). H1R agonist reduced proliferation in all cell lines. H2R agonist increased proliferation and Ets-1 expression in WM35 cells. | Hegyesi et al. (2005); Molnár et al. (2001; 2002); | |
| H2R expression in B16-C3 mouse and A375P and C32 human melanoma cells. H2R antagonists inhibited proliferation while stimulated melanogenesis only in B16-C3 cells. | Uçar (1991) | |
| Colon cancer | H2R expression in syngeneic or xenogenic colon cancer grafts in mice. H2R antagonists inhibited tumour growth by inhibiting angiogenesis and by attenuating antitumour cytokine expression in the tumour microenvironment. | Adams et al. (1994); Takahashi et al. (2001); Tomita et al. (2003); Tomita and Okabe (2005) |
| H1R, H2R and H4R expression in human normal colon mucosa and tumour tissue. Decreased H1R and H4R expression in tumours compared with normal colonic mucosa. | Boer et al. (2008); Cianchi et al. (2005) | |
| H1R, H2R and H4R expression in human colon cancer cells (HT29, Caco-2 and HCT116). H2R and H4R antagonists suppressed histamine-induced proliferation of the three cell lines while reduced histamine-induced COX-2 and VEGF expression in HT29 and Caco-2 cells. H1R antagonist inhibited growth and radiosensitized HT29 cells. | Cianchi et al. (2005); Soule et al. (2010) | |
| Pancreatic cancer | H1R and H2R expression in Panc-1 human cancer cell line. Histamine inhibited proliferation through the H1R and H2R, which was associated with a partial differentiation. Through the H2R histamine induced G0/G1 phase arrest, modulation of MAPK and Bcl-2 family proteins. | Cricco et al. (2000; 2004; 2006); Martín et al. (2002) |
| H3R and H4R expression in Panc-1 cells. H3R agonist increased while H4R agonist decreased proliferation. | Cricco et al. (2008) |
EGF, epidermal growth factor; Ets-1, v-ets erythroblastosis virus E26 oncogene homolog 1; COX-2, cyclooxygenase-2; VEGF, vascular endothelial growth factor; MAPK, mitogen-activated protein kinase; Bcl-2, B-cell lymphoma 2.
Recently, it was demonstrated that H3R and H4R are expressed in cell lines derived from human mammary gland (Medina et al., 2006). Histamine is capable of modulating cell proliferation exclusively in malignant cells while no effect on proliferation or expression of oncogenes related to cell growth is observed in non-tumorigenic HBL-100 cells (Davio et al., 2002; Medina et al., 2006). Furthermore, histamine modulated the proliferation of MDA-MB-231 breast cancer cells in a dose-dependent manner producing a significant decrease at 10 µmol·L−1 concentration whereas at lower concentrations increased proliferation moderately. The negative effect on proliferation was associated with the induction of cell cycle arrest in G2/M phase, differentiation and a significant increase in the number of apoptotic cells (Medina et al., 2006). Accordingly, by using pharmacological tools, results demonstrated that histamine increased MDA-MB-231 cell proliferation and also migration via H3R. In contrast, clobenpropit and VUF8430 treatments significantly decreased proliferation to 45.5% ± 14.8% and to 76.7% ± 5.3% respectively. This outcome was associated with an induction of apoptosis assessed after 48 h of H4R agonist treatment. Clobenpropit and VUF8430 produced a threefold increase in the number of apoptotic cells determined by Annexin-V staining and this effect was blocked by the specific H4R antagonist JNJ7777120. This result was additionally confirmed by TdT-mediated UTP-biotin Nick End labelling (TUNEL) assay. In accordance to this, clobenpropit produced the disruption of the mitochondrial transmembrane potential (Δψm, 81.5%) that is associated with apoptosis, and also exerted a 2.5-fold increase in the cell senescence while reduced migration (Medina et al., 2008). The biological responses triggered by histamine in a more differentiated breast cancer cell line (MCF-7) were further investigated. Results showed that histamine at all doses tested, decreased the proliferation of MCF-7 breast cancer cells through the stimulation of the four histamine receptor subtypes exhibiting a higher effect through the H4R. Treatment of MCF-7 cells with the H4R agonists, inhibited proliferation by 50% increasing the exponential doubling time from 32.6 to 47.2 h and 44.1 h in clobenpropit and VUF8430 treated cells respectively. This negative effect on proliferation was related to an increase in Annexin-V and TUNEL positive cells (P < 0.01), a decrease in the Δψm (59.5%) and a twofold increase in cell senescence (Medina et al., unpubl. data). These results represent the first report about the expression of H3R and H4R in human breast cells and interestingly show that the H4R is involved in the regulation of breast cancer cell proliferation, apoptosis and senescence.
In agreement with this, recent data indicate that H3R and H4R are expressed in human biopsies of benign lesions and breast carcinomas being the level of their expression significantly higher in carcinomas, confirming that H3R and H4R are present not only in cell lines but also in the human mammary tissue. Furthermore, the expression of H3R is highly correlated with proliferation and histamine production in malignant lesions while the 50% of malignant lesions expressed H4R, all of them corresponding to metastases or high invasive tumours (Medina et al., 2008) (Figure 1).
Figure 1.
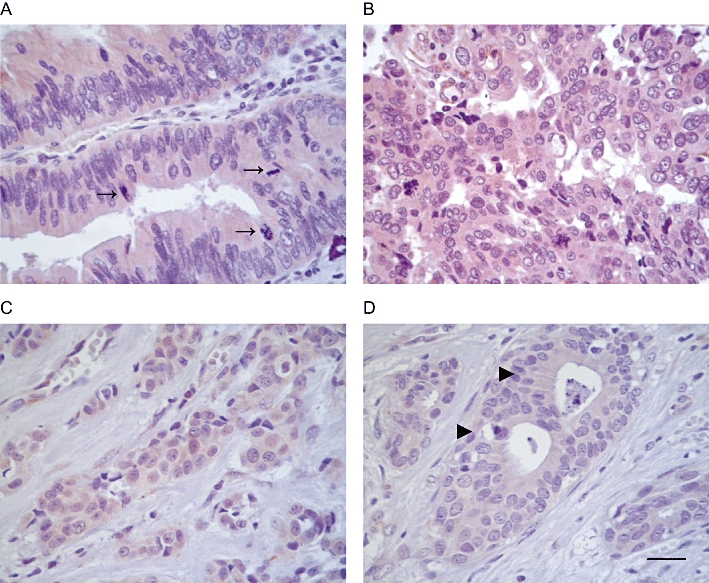
Immunological evidence for the presence of histamine H4 receptor (H4R) in human cancers. Fixed specimens were permeabilized and subjected to immunohistochemical analysis as described in Medina et al. (2008), probed with rabbit anti-H4R antibodies (Alpha Diagnostic, 10 µg·mL−1). (A) Atypical glands from a gallbladder adenocarcinoma showing multistratified nuclei with frequent mitosis (→) and marked cytoplasmic positive staining for H4R. (B) Malignant glands of a lymph node metastasis from ovary adenocarcinoma exhibiting atypical nuclei, and very strong cytoplasmic positive staining for H4R. (C) Infiltrating lobular carcinoma cells displaying positive immunoreactivity for H4R. (D) Atypical ducts (▸) derived from a ductal breast adenocarcinoma with positive staining for H4R. Magnification ×630; scale bar = 20 µm.
Recent results obtained with the orthotopic xenograft tumours of the highly invasive human breast cancer line MDA-MB-231 in immune deficient nude mice xenotransplanted tumours indicate that the H4R was the major histamine receptor expressed in the tumour. Remarkably, in vivo JNJ7777120 treatment (10 mg·kg−1, p.o., daily administration) significantly decreased lung metastases indicating that H4R may be involved in the metastatic process (Medina et al., unpubl. data).
The identification of H4R and the elucidation of its role in the development and growth of human mammary carcinomas may represent an essential clue for advances in breast cancer treatment. The presented evidences contribute to the identification of molecules involved in breast carcinogenesis and confirm the role of H4R in the regulation of breast cancer growth and progression representing a novel molecular target for new therapeutic approach.
Histamine receptors and melanoma
Malignant melanoma is an aggressive, therapy-resistant malignancy of melanocytes. The incidence of melanoma has been steadily growing worldwide, resulting in an increasing public health problem (Markovic et al., 2007). In its early stages malignant melanoma can be cured by surgical resection, but once it has progressed to the metastatic stage it is extremely difficult to treat and does not respond to current therapies. Thus, the development of effective treatments is imperative in order to improve survival and quality of life of melanoma patients (Gray-Schopfer et al., 2007; Cashin et al., 2008). The immunoactivating cytokine interleukin 2 is employed in the treatment of stage IV melanoma in many European countries and in the USA and induces complete regression of melanoma metastases in 3%–5% of treated patients. However, toxicity limits its use. Clinical trials are being performed with the cytokine alone or combined with histamine dihydrochloride suggesting that the combined treatment may specifically prolong the survival of melanoma patients with liver metastases (Mitchell, 2003; Agarwala et al., 2004; Galmarini, 2004).
Melanoma cells and tissues but not normal melanocytes express HDC and contain large amounts of histamine, present histamine receptors, and also both endogenous and exogenous histamine have the ability to regulate melanoma cell growth suggesting the existence of autocrine and paracrine regulation mediated by histamine (Tilly et al., 1990; Reynolds et al., 1996; Haak-Frendscho et al., 2000; Hegyesi et al., 2001; 2005; Molnár et al., 2001; Darvas et al., 2003).
Numerous in vivo studies employing animal models bearing syngeneic or xenogenic melanoma grafts demonstrated that both endogenous and exogenous histamine have the ability to stimulate tumour growth while H2R antagonists (e.g. cimetidine, famotidine, roxatidine) inhibited this effect (Uçar, 1991; Szincsák et al., 2002a,b; Pós et al., 2005; Tomita et al., 2005). Overexpression of HDC markedly accelerated tumour growth and increased metastatic colony-forming potential along with rising levels of local histamine production that was correlated with tumour H2R and rho-C expression in mouse melanoma (Pós et al., 2005). Additionally, H2R antagonists stimulated melanogenesis and inhibited proliferation in B16-C3 mouse melanoma cells (Uçar, 1991) (Table 2).
Accordingly, it was previously described that histamine exerts a dual effect on proliferation of the human primary melanoma cell line, WM35, increasing proliferation at low concentrations through the H2R receptor while decreasing it at higher concentrations (10 µmol·L−1) via the H1R, and no evidence of mitogenic signalling through the H3R has been demonstrated (Falus et al., 2001; Molnár et al., 2001; 2002; Hegyesi et al., 2005). The inhibitory effect of histamine on proliferation was associated with the stimulation of the production of hydrogen peroxide and the induction of senescence of WM35 cells (Medina et al., 2009).
In this line, a number of small clinical trials have investigated the effects of cimetidine alone or in combination with other agents, such as leukocyte interferon, on malignant melanoma with debatable results (Siegers et al., 1999).
Recently, the expression of H4R and its associated biological responses in the human malignant melanoma cell lines, WM35 (primary melanoma) and M1/15 (derived from liver metastasis), were investigated. Results demonstrated that melanoma cells express H4R at the mRNA and protein level. By using histamine agonists and antagonists it was shown that the inhibitory effect of histamine on proliferation was in part mediated through the stimulation of the H4R (clobenpropit IC50 = 1.7 µmol·L−1; VUF8430 IC50 = 1.66 µmol·L−1 in WM35 cells, and clobenpropit IC50 = 4.7 µmol·L−1; VUF8430 IC50 = 4.8 µmol·L−1 in M1/15 cells). Treatment with a specific H4R antagonist, JNJ7777120, blocked the decrease in proliferation triggered by the H4R agonists. Furthermore, the decrease in proliferation exerted by H4R agonists was associated with a twofold induction of cell senescence and an increase in melanogenesis that is a differentiation marker on these cells (Massari et al., unpubl. data).
Current studies indicate that the H4R is expressed in human melanoma biopsies, confirming that the H4R is present not only in these cell lines but also in human melanoma tissue (Massari et al., unpubl. data). The identification of H4R and the clarification of its role in human malignant melanoma progression may contribute for advances in the treatment of this disease.
Histamine receptors and colon cancer
Colorectal cancer is one of the leading causes of cancer death among both men and women worldwide. Mortality has remained constant during the past decades even though the incidence in fact has increased (Parkin et al., 2005). Surgery remains the mainstay of treatment for colon cancer and resulting in 5 year survival in more than 60% of patients (Jemal et al., 2003).
It has been shown that HDC enzymatic activity is significantly increased in colorectal carcinoma compared with normal mucosa (Garcia-Caballero et al., 1988; Reynolds et al., 1997; Cianchi et al., 2005). Furthermore, it was reported that the activity of the histamine catabolizing enzymes, diamine oxidase or histamine N-methyltransferase, was significantly lower in adenoma tissue than in healthy mucosa in the same patients (Kuefner et al., 2008). These studies suggest that histamine may be involved in tumour development and progression. In this line, loratadine, an H1R antagonist, inhibited growth and enhanced the effect of radiation of human colon carcinoma cell lines (Soule et al., 2010). Furthermore, earlier studies demonstrated that histamine induced in vitro and in vivo cell proliferation and this outcome could be blocked by H2R antagonists (Adams et al., 1994; Cianchi et al., 2005). This effect could be associated with the attenuation of antitumour cytokine expression in the tumour microenvironment exerted by histamine, thus resulting in stimulated colorectal cancer growth (Takahashi et al., 2001; Tomita and Okabe, 2005). In addition, H2R antagonist significantly suppressed the growth of tumour implants in mice by inhibiting angiogenesis via reducing vascular endothelial growth factor (VEGF) expression (Tomita et al., 2003). Accordingly, some beneficial effects of cimetidine and other H2R antagonists on survival in colorectal cancer patients have been clinically demonstrated (Adams and Morris, 1994; Kelly et al., 1999; Kapoor et al., 2005) (Table 2).
More recently, the expression of H4R not only in cell lines but also in tissue derived from colon carcinoma was described (Cianchi et al., 2005; Boer et al., 2008). Interestingly, the H4R antagonist, JNJ7777120, prevented the cell growth-promoting activity of histamine in three colon cancer cell lines without affecting the basal growth of the cells and also inhibited the histamine-mediated increase in VEGF in two cell lines. Combination treatment with zolantidine (an H2R antagonist) and JNJ7777120 determined an additive effect on reducing the histamine-induced VEGF production and histamine-stimulated proliferation (Cianchi et al., 2005). Furthermore, a significant decrease in H4R levels in neoplastic samples compared with normal colonic tissue was demonstrated, suggesting the involvement of H4R in colon carcinogenesis (Boer et al., 2008).
Histamine receptors and pancreatic and other cancers
As in other human neoplasias, HDC expression and histamine content have been reported in pancreatic cancer (Rivera et al., 2000; Tanimoto et al., 2004). Furthermore, it was previously reported that H1R and H2R are expressed and associated with cell proliferation in Panc-1, a cell line derived from a human ductal pancreatic carcinoma. Histamine concentrations higher than 1 µmol·L−1 inhibited clonogenic growth through the H1R and H2R. On the other hand, nanomolar histamine doses stimulated cell proliferation (Cricco et al., 2000). The antiproliferative effect exerted by histamine through the H2R is associated with a G0/G1 phase arrest, a decrease in phosphoactivated ERK1/ERK2 and an increase in phosphoactivated P38 expression, and also a modulation of Bcl-2 family proteins. However, apoptosis is not significantly induced while a partial cell differentiation was associated with this inhibitory action (Cricco et al., 2000; 2004; 2006; Martín et al., 2002).
More recently, it was described the expression of H3R and H4R in Panc-1 cells. Proliferation studies indicated that the H3R and H4R are involved in pancreatic carcinoma cell growth, being proliferation augmented through H3R and diminished by H4R (Cricco et al., 2008) (Table 2).
Moreover, histamine content increased unequivocally in other human cancer types such as ovarian, cervical and endometrial carcinoma in comparison with their adjoining normal tissues suggesting the participation of histamine in carcinogenesis. Besides, exogenous histamine, at micromolar concentration, stimulated proliferation of human ovarian cancer cell line SKOV-3 (Batra and Fadeel, 1994; Chanda and Ganguly, 1995). Preliminary results show that H4R is expressed in primary and metastatic ovarian carcinoma and also in gallbladder cancer (Figure 1).
Conclusions and perspectives
A significant body of research has contributed to the elucidation of the functional capacities of histamine in tumour cell growth and development. The discovery of the human H4R has helped to refine our understanding of histamine role in (patho) physiological conditions.
Recent findings indicate that H4R is expressed in human breast tissues and cell lines exhibiting a key role in histamine-mediated biological processes such as cell proliferation, senescence, apoptosis and metastatic potential in malignant cells. Similar responses were observed in the human cell lines of pancreatic carcinoma and melanoma where histamine, via H4R, inhibits proliferation and modulates cell differentiation and migration. In addition, H4R was detected in both colorectal cancer and adjacent normal colonic specimens, and in human colon cancer cell lines in which histamine exerts both a proproliferative and a proangiogenic effect via H2R/H4R activation. Although these reports make unquestionably the presence of functional H4R in human cancer tissues, the precise role of H4R in cell proliferation seems to be cancer type dependent and must be further investigated. The presented data suggest a novel and complex role of H4R in carcinogenesis that might represent a new molecular target and avenue potentially useful for the design of more specific and effective therapies for cancer; however, further investigation is need to fully understand its function in the diverse types of tumours. Therefore, the identification of H4R with functional presence in different cancers opens new perspectives in histamine pharmacology research aimed to develop a new generation of antihistamines targeting H4R that may contribute for advances in the treatment of cancer.
Acknowledgments
This work has been supported by grants from the University of Buenos Aires B061, from the National Agency of Scientific and Technological Promotion PICT-2007-01022 and from the EU-FP7 COST Action BM0806 (recent advances in histamine receptor H4R research). We thank Miss Natalia Rivera for proof-reading the manuscript.
Glossary
Abbreviations
- Δψm
mitochondrial transmembrane potential
- H1R
histamine H1 receptor
- H2R
histamine H2 receptor
- H3R
histamine H3 receptor
- H4R
histamine H4 receptor
- HDC
L-histidine decarboxylase
- MAPK
mitogen-activated protein kinase
- TUNEL
TdT-mediated UTP-biotin Nick End labelling
Conflict of interest
The authors state no conflict of interest.
Supplemental material
References
- Adams WJ, Morris DL. Short-course cimetidine and survival with colorectal cancer. Lancet. 1994;344:1768–1769. [PubMed] [Google Scholar]
- Adams WJ, Lawson JA, Morris DL. Cimetidine inhibits in vivo growth of human colon cancer and reverses histamine stimulated in vitro and in vivo growth. Gut. 1994;35:1632–1636. doi: 10.1136/gut.35.11.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwala SS, Hellstrand K, Gehlsen K, Naredi P. Immunotherapy with histamine and interleukin 2 in malignant melanoma with liver metastasis. Cancer Immunol Immunother. 2004;53:840–841. doi: 10.1007/s00262-004-0537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis CA, Simons FER. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Bartholeyns J, Bouclier M. Involvement of histamine in growth of mouse and rat tumors: antitumoral properties of monofluormethylhistidine, an enzyme-activated irreversible inhibitor of histidine decarbolxylase. Cancer Res. 1984;44:639–645. [PubMed] [Google Scholar]
- Bartholeyns J, Fozard J. Role of histamine in tumor development. Trends Pharmacol Sci. 1985;6:123–125. [Google Scholar]
- Batra S, Fadeel I. Release of intracellular calcium and stimulation of cell growth by ATP and histamine in human ovarian cancer cells (SKOV-3) Cancer Lett. 1994;77:57–63. doi: 10.1016/0304-3835(94)90348-4. [DOI] [PubMed] [Google Scholar]
- Boer K, Helinger E, Helinger A, Pocza P, Pos Z, Demeter P, et al. Decreased expression of histamine H1 and H4 receptors suggests disturbance of local regulation in human colorectal tumours by histamine. Eur J Cell Biol. 2008;87:227–236. doi: 10.1016/j.ejcb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bolton E, King J, Morris DL. H2-antagonists in the treatment of colon and breast cancer. Seminar Cancer Biol. 2000;10:3–10. doi: 10.1006/scbi.2000.0301. [DOI] [PubMed] [Google Scholar]
- Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge DR, Jodrell DI. Chemotherapy. In: Knowles M, Selby P, editors. Introduction to the Cellular and Molecular Biology of Cancer. New York: Oxford University Press; 2005. pp. 399–413. [Google Scholar]
- Cashin RP, Lui P, Machado M, Hemels ME, Corey-Lisle PK, Einarson TR. Advanced cutaneous malignant melanoma: a systematic review of economic and quality-of-life studies. Value Health. 2008;11:259–271. doi: 10.1111/j.1524-4733.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- Chanda R, Ganguly AK. Diamine-oxidase activity and tissue di- and poly-amine contents of human ovarian, cervical and endometrial carcinoma. Cancer Lett. 1995;89:23–28. doi: 10.1016/0304-3835(95)90153-1. [DOI] [PubMed] [Google Scholar]
- Cianchi F, Cortesini C, Schiavone N, Perna F, Magnelli L, Fanti E, et al. The role of cyclooxygenase-2 in mediating the effects of histamine on cell proliferation and vascular endothelial growth factor production in colorectal cancer. Clin Cancer Res. 2005;11:6807–6815. doi: 10.1158/1078-0432.CCR-05-0675. [DOI] [PubMed] [Google Scholar]
- Coge F, Guenin SP, Rique H, Boutin JA, Galizzi JP. Structure and expression of the human histamine H4-receptor gene. Biochem Biophy Res Commun. 2001;284:301–309. doi: 10.1006/bbrc.2001.4976. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Shenton FC, Lethbridge N, Leurs R, Waldvogel HJ, Faull RL, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol. 2009;157:55–63. doi: 10.1111/j.1476-5381.2009.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cricco GP, Davio CA, Fitzsimons CP, Engel N, Bergoc RM, Rivera ES. Histamine as an autocrine growth factor in experimental carcinomas. Agents Actions. 1994;43:17–20. doi: 10.1007/BF02005757. [DOI] [PubMed] [Google Scholar]
- Cricco G, Martin G, Labombarda F, Cocca C, Bergoc R, Rivera E. Human pancreatic carcinoma cell line Panc-I and the role of histamine on cell growth. Inflamm Res. 2000;49(Suppl 1):S68–S69. doi: 10.1007/PL00000188. [DOI] [PubMed] [Google Scholar]
- Cricco GP, Martin GA, Medina VA, Núñez M, Gutiérrez AS, Cocca C, et al. Histamine regulates the MAPK pathway via the H2 receptor in PANC-1 human cells. Inflamm Res. 2004;53(Suppl 1):S65–S66. doi: 10.1007/s00011-003-0331-4. [DOI] [PubMed] [Google Scholar]
- Cricco G, Martín G, Medina V, Núñez M, Mohamad N, Croci M, et al. Histamine inhibits cell proliferation and modulates the expression of Bcl-2 family proteins via the H2 receptor in human pancreatic cancer cells. Anticancer Res. 2006;26:4443–4450. [PubMed] [Google Scholar]
- Cricco GP, Mohamad NA, Sambuco LA, Genre F, Croci M, Gutiérrez AS, et al. Histamine regulates pancreatic carcinoma cell growth through H3 and H4 receptors. Inflamm Res. 2008;57(Suppl 1):S23–S24. doi: 10.1007/s00011-007-0611-5. [DOI] [PubMed] [Google Scholar]
- Damaj BB, Barrena Becerra C, Esber HJ, Wen Y, Maghazachi AA. Functional expression of H4 histamine receptor in human natural killer cells, monocytes, and dendritic cells. J Immunol. 2007;179:7907–7915. doi: 10.4049/jimmunol.179.11.7907. [DOI] [PubMed] [Google Scholar]
- Darvas Z, Sakurai E, Schwelberger HG, Hegyesi H, Rivera E, Othsu H, et al. Autonomous histamine metabolism in human melanoma cells. Melanoma Res. 2003;13:239–246. doi: 10.1097/00008390-200306000-00003. [DOI] [PubMed] [Google Scholar]
- Davenas E, Rouleau A, Morisset S, Arrang JM. Autoregulation of McA-RH7777 hepatoma cell proliferation by histamine H3 receptor. J Pharmacol Exp Ther. 2008;326:406–413. doi: 10.1124/jpet.107.135368. [DOI] [PubMed] [Google Scholar]
- Davio CA, Cricco GP, Andrade N, Bergoc RM, Rivera ES. H1 and H2 histamine receptors in human mammary carcinomas. Agents Actions. 1993;38:C172–C174. [Google Scholar]
- Davio CA, Cricco GP, Martin G, Fitzsimons CP, Bergoc RM, Rivera ES. Effect of histamine on growth and differentiation of the rat mammary gland. Agents Actions. 1994;41:C115–C117. doi: 10.1007/BF02007792. [DOI] [PubMed] [Google Scholar]
- Davio CA, Cricco GP, Bergoc RM, Rivera ES. H1 and H2 histamine receptors in experimental carcinomas with an atypical coupling to signal transducers. Biochem Pharmacol. 1995;50:91–96. doi: 10.1016/0006-2952(95)00108-c. [DOI] [PubMed] [Google Scholar]
- Davio C, Madlovan A, Shayo C, Lemos B, Baldi A, Rivera E. Histamine receptors in neoplastic transformation. Studies in human cell lines. Inflamm Res. 1996;45(Suppl 1):S62–S63. doi: 10.1007/BF03354090. [DOI] [PubMed] [Google Scholar]
- Davio C, Mladovan A, Lemos B, Monczor F, Shayo C, Rivera E, et al. H1 and H2 histamine receptors mediate the production of inositol phosphates but not cAMP in human breast epithelial cells. Inflamm Res. 2002;51:1–7. doi: 10.1007/pl00000276. [DOI] [PubMed] [Google Scholar]
- De Esch JP, Thurmond RL, Jongejan A, Leurs R. The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol Sci. 2005;26:462–469. doi: 10.1016/j.tips.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Dy M, Schneider E. Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rev. 2004;15:393–410. doi: 10.1016/j.cytogfr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Engel N, Cricco G, Davio C, Martin G, Croci M, Fitzsimons C, et al. Histamine regulates the expression of histidine decarboxylase in NMU-induced tumors in rats. Inflamm Res. 1996;45(Suppl 1):S64–S65. doi: 10.1007/BF03354091. [DOI] [PubMed] [Google Scholar]
- Falus A, Hegyesi H, Lazar-Molnar E, Pos Z, Laszlo V, Darvas Z. Paracrine and autocrine interactions in melanoma: histamine is a relevant player in local regulation. Trends Immunol. 2001;22:648–652. doi: 10.1016/s1471-4906(01)02050-6. [DOI] [PubMed] [Google Scholar]
- Fentiman IS. Local treatment of cancer. In: Knowles M, Selby P, editors. Introduction to the Cellular and Molecular Biology of Cancer. New York: Oxford University Press; 2005. pp. 390–398. [Google Scholar]
- Fitzsimons C, Engel N, Policastro L, Durán H, Molinari B, Rivera E. Regulation of phospholipase C activation by the number of H(2) receptors during Ca(2+)-induced differentiation of mouse keratinocytes. Biochem Pharmacol. 2002;63:1785–1796. doi: 10.1016/s0006-2952(02)00975-9. [DOI] [PubMed] [Google Scholar]
- Frank LM, Knowles MA. What is cancer? In: Knowles M, Selby P, editors. Introduction to the Cellular and Molecular Biology of Cancer. New York: Oxford University Press; 2005. pp. 1–24. [Google Scholar]
- Galmarini CM. Histamine dihydrochloride (subcutaneous) Maxim. Curr Opin Investig Drugs. 2004;5:1298–1310. [PubMed] [Google Scholar]
- Garcia-Caballero M, Neugebauer E, Campos R, Nunez de Castro I, Vara-Thorbeck C. Increased histidine decarboxylase (HDC) activity in human colorectal cancer: results of a study on ten patients. Agents Actions. 1988;23:357–360. doi: 10.1007/BF02142587. [DOI] [PubMed] [Google Scholar]
- Garcia-Caballero M, Neugebauer E, Campos R, Nuñez de Castro I, Vara-Thorbeck C. Histamine synthesis and content in benign and malignant breast tumors. Surg Oncol. 1994;3:167–173. doi: 10.1016/0960-7404(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- Haak-Frendscho M, Darvas A, Hegyesi H, Karpati S, Hoffman R, Laszló V, et al. Histidine decarboxylase expression in human melanoma. J Invest Dermatol. 2000;115:345–352. doi: 10.1046/j.1523-1747.2000.00054.x. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. Cancer biology. In: Hall EJ, Giaccia AJ, editors. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 274–302. [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Harusawa S, Araki L, Zuiderveld OP, Smit MJ, Imazu T, et al. A selective human H(4)-receptor agonist: (-)-2-cyano-1-methyl-3-[(2R,5R)-5- [1H-imidazol-4(5)-yl]tetrahydrofuran-2-y] methylguanidine. J Med Chem. 2003;46:3162–3165. doi: 10.1021/jm0300025. [DOI] [PubMed] [Google Scholar]
- Hegyesi H, Somlai B, Varga VL, Toth G, Kovacs P, Mólnar EL, et al. Suppression of melanoma cell proliferation by histidine decarboxylase specific antisense oligonucleotides. J Invest Dermatol. 2001;117:151–153. doi: 10.1046/j.0022-202x.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- Hegyesi H, Horváth B, Pállinger E, Pós Z, Molnár V, Falus A. Histamine elevates the expression of Ets-1, a protooncogen in human melanoma cell lines through H2 receptor. FEBS Lett. 2005;579:2475–2479. doi: 10.1016/j.febslet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–284. [PubMed] [Google Scholar]
- Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- Huang JF, Thurmond RL. The new biology of histamine receptors. Curr Allergy Asthma Rep. 2008;8:21–27. doi: 10.1007/s11882-008-0005-y. [DOI] [PubMed] [Google Scholar]
- Jablonowski JA, Grice CA, Chai W, Dvorak CA, Venable JD, Kwok AK, et al. The first potent and selective non-imidazole human histamine H4 receptor antagonists. J Med Chem. 2003;46:3957–3960. doi: 10.1021/jm0341047. [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- Kahlson G, Rosengren E. New approaches to the physiology of histamine. Physiol Rev. 1968;48:155–196. doi: 10.1152/physrev.1968.48.1.155. [DOI] [PubMed] [Google Scholar]
- Kapoor S, Pal S, Sahni P, Dattagupta S, Kanti Chattopadhyay T. Effect of pre-operative short course famotidine on tumor infiltrating lymphocytes in colorectal cancer: a double blind, placebo controlled, prospective randomized study. J Surg Res. 2005;129:172–175. doi: 10.1016/j.jss.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Kelly MD, King J, Cherian M, Dwerryhouse SJ, Finlay IG, Adams WJ, et al. Randomized trial of preoperative cimetidine in patients with colorectal carcinoma with quantitative assessment of tumor-associated lymphocytes. Cancer. 1999;85:1658–1663. doi: 10.1002/(sici)1097-0142(19990415)85:8<1658::aid-cncr3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kierska D, Fogel W, Malinski C. Histamine concentration and metabolism in mouse mammary gland during estrous cycle. Inflamm Res. 1997;46(Suppl 1):S63–S64. [PubMed] [Google Scholar]
- Koenig JR, Liu H, Drizin I, Witte DG, Carr TL, Manelli AM, et al. Rigidified 2-aminopyrimidines as histamine H4 receptor antagonists: effects of substitution about the rigidifying ring. Bioorg Med Chem Lett. 2010;20:1900–1904. doi: 10.1016/j.bmcl.2010.01.131. [DOI] [PubMed] [Google Scholar]
- Kuefner MA, Schwelberger HG, Hahn EG, Raithel M. Decreased histamine catabolism in the colonic mucosa of patients with colonic adenoma. Dig Dis Sci. 2008;53:436–442. doi: 10.1007/s10620-007-9861-x. [DOI] [PubMed] [Google Scholar]
- Kyriakidis K, Zampeli E, Tiligada E. Histamine levels in whole peripheral blood from women with ductal breast cancer: a pilot study. Inflamm Res. 2009;58(Suppl 1):S73–S74. doi: 10.1007/s00011-009-2014-2. [DOI] [PubMed] [Google Scholar]
- Lemos B, Davio C, Gass H, Gonzales P, Cricco G, Martín G, et al. Histamine receptors in human mammary gland, different benign lesions and mammary carcinomas. Inflamm Res. 1995;44(Suppl 1):S68–S69. doi: 10.1007/BF01674400. [DOI] [PubMed] [Google Scholar]
- Leurs R, Chazot PL, Shenton FC, Lim HD, de Esch IJ. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol. 2009;157:14–23. doi: 10.1111/j.1476-5381.2009.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- Lim HD, Jongejan A, Bakker RA, Haaksma E, de Esch IJ, Leurs R. Phenylalanine 169 in the second extracellular loop of the human histamine H4 receptor is responsible for the difference in agonist binding between human and mouse H4 receptors. J Pharmacol Exp Ther. 2008;327:88–96. doi: 10.1124/jpet.108.140343. [DOI] [PubMed] [Google Scholar]
- Lim HD, Adami M, Guaita E, Werfel T, Smits RA, de Esch IJ, et al. Pharmacological characterization of the new histamine H4 receptor agonist VUF 8430. Br J Pharmacol. 2009;157:34–43. doi: 10.1111/j.1476-5381.2009.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HD, de Graaf C, Jiang W, Sadek P, McGovern PM, Istyastono EP, et al. Molecular determinants of ligand binding to H4R species variants. Mol Pharmacol. 2010;77:734–743. doi: 10.1124/mol.109.063040. [DOI] [PubMed] [Google Scholar]
- Liu C, Ma XJ, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, et al. Cloning and pharmacological characterization of a fourth histamine receptor (H4) expressed in bone marrow. Mol Pharmacol. 2001a;59:420–426. doi: 10.1124/mol.59.3.420. [DOI] [PubMed] [Google Scholar]
- Liu C, Wilson SJ, Kuei C, Lovenberg TW. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther. 2001b;299:121–130. [PubMed] [Google Scholar]
- Liu H, Altenbach RJ, Carr TL, Chandran P, Hsieh GC, Lewis LG, et al. cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (A-987306), a new histamine H4R antagonist that blocks pain responses against carrageenan-induced hyperalgesia. J Med Chem. 2008;51:7094–7098. doi: 10.1021/jm8007618. [DOI] [PubMed] [Google Scholar]
- von Mach-Szczypiński J, Stanosz S, Sieja K, Stanosz M. Metabolism of histamine in tissues of primary ductal breast cancer. Metabolism. 2009;58:867–870. doi: 10.1016/j.metabol.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Malinski C, Kierska D, Fogel W, Kinnunum A, Panula P. Histamine: its metabolism and localization in mammary gland. Comp Biochem Physiol C. 1993;105:269–273. doi: 10.1016/0742-8413(93)90206-z. [DOI] [PubMed] [Google Scholar]
- Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364–380. doi: 10.4065/82.3.364. [DOI] [PubMed] [Google Scholar]
- Martín G, Cricco G, Darvas Z, Croci M, Núñez M, Bergoc R, et al. Histamine inhibits proliferation of a pancreatic carcinoma cell line without inducing apoptosis significantly. Inflamm Res. 2002;51(Suppl 1):S67–S68. doi: 10.1007/pl00022452. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina V, Cricco G, Nuñez M, Martín G, Mohamad N, Correa-Fiz F, et al. Histamine-mediated signaling processes in human malignant mammary cells. Cancer Biol Ther. 2006;5:1462–1471. doi: 10.4161/cbt.5.11.3273. [DOI] [PubMed] [Google Scholar]
- Medina V, Croci M, Crescenti E, Mohamad N, Sanchez-Jiménez F, Massari N, et al. The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment. Cancer Biol Ther. 2008;7:28–35. doi: 10.4161/cbt.7.1.5123. [DOI] [PubMed] [Google Scholar]
- Medina VA, Massari NA, Cricco GP, Martín GA, Bergoc RM, Rivera ES. Involvement of hydrogen peroxide in histamine-induced modulation of WM35 human malignant melanoma cell proliferation. Free Radic Biol Med. 2009;46:1510–1515. doi: 10.1016/j.freeradbiomed.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Mitchell MS. Immunotherapy as part of combinations for the treatment of cancer. Int Immunopharmacol. 2003;3:1051–1059. doi: 10.1016/S1567-5769(03)00019-5. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi M, Mitsuhashi T, Payan D. Multiple signaling pathways of histamine H2 receptors. Identification of an H2 receptor-dependent Ca2+ mobilization pathway in human HL-60 promyelocytic leukemia cells. J Biol Chem. 1989;264:18356–18362. [PubMed] [Google Scholar]
- Molnár EL, Cricco G, Martin G, Darvas Z, Hegyesi H, Fitzsimons C, et al. Histamine as a potential autocrine regulator of melanoma. Inflamm Res. 2001;50(Suppl 1):S102–S103. doi: 10.1007/PL00020786. [DOI] [PubMed] [Google Scholar]
- Molnár EL, Hegyesi H, Pállinger E, Kovács P, Tóth S, Fitzsimons C, et al. Inhibition of human primary melanoma cell proliferation by histamine is enhanced by interleukin-6. Eur J Clin Invest. 2002;32:743–749. doi: 10.1046/j.1365-2362.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- Morse KL, Behan J, Laz TM, West RE, Jr, Greenfeder SA, Anthes JC, et al. Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther. 2001;296:1058–1066. [PubMed] [Google Scholar]
- Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun. 2000;279:615–620. doi: 10.1006/bbrc.2000.4008. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Shapiro DA, George SR, Setola V, Lee DK, Cheng R, et al. Discovery of a novel member of the histamine receptor family. Mol Pharmacol. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Parshad R, Hazrah P, Kumar S, Gupta SD, Ray R, Bal S. Effect of preoperative short course famotidine on TILs and survival in breast cancer. Indian J Cancer. 2005;42:185–190. [PubMed] [Google Scholar]
- Pós Z, Hegyesi H, Rivera ES. Histamine and cell proliferation. In: Falus A, editor. Histamine: Biology and Medical Aspects. Budapest: SpringMed Publishing; 2004. pp. 199–217. [Google Scholar]
- Pós Z, Sáfrány G, Müller K, Tóth S, Falus A, Hegyesi H. Phenotypic profiling of engineered mouse melanomas with manipulated histamine production identifies histamine H2 receptor and Rho-c as histamine-regulated melanoma progression markers. Cancer Res. 2005;65:4458–4466. doi: 10.1158/0008-5472.CAN-05-0011. [DOI] [PubMed] [Google Scholar]
- Reynolds JL, Akhter J, Morris DL. In vitro effect of histamine and histamine H1 and H2 receptor antagonists on cellular proliferation of human malignant melanoma cell lines. Melanoma Res. 1996;6:95–99. doi: 10.1097/00008390-199604000-00003. [DOI] [PubMed] [Google Scholar]
- Reynolds JL, Akhter J, Adams WJ, Morris DL. Histamine content in colorectal cancer. Are there sufficient levels of histamine to affect lymphocyte function? Eur J Surg Oncol. 1997;23:224–227. doi: 10.1016/s0748-7983(97)92388-x. [DOI] [PubMed] [Google Scholar]
- Reynolds JL, Akhter JA, Magarey CJ, Schwartz P, Adams WJ, Morris DL. Histamine in human breast cancer. Br J Surg. 1998;85:538–541. doi: 10.1046/j.1365-2168.1998.00625.x. [DOI] [PubMed] [Google Scholar]
- van Rijn RM, Chazot PL, Shenton FC, Sansuk K, Bakker RA, Leurs R. Oligomerization of recombinant and endogenously expressed human histamine H(4) receptors. Mol Pharmacol. 2006;70:604–615. doi: 10.1124/mol.105.020818. [DOI] [PubMed] [Google Scholar]
- van Rijn RM, van Marle A, Chazot PL, Langemeijer E, Qin Y, Shenton FC, et al. Cloning and characterization of dominant negative splice variants of the human histamine H4 receptor. Biochem J. 2008;414:121–131. doi: 10.1042/BJ20071583. [DOI] [PubMed] [Google Scholar]
- Rivera E, Davio C, Cricco G, Bergoc R. Histamine regulation of tumour growth. Role of H1 and H2 receptors. In: Garcia-Caballero M, Brandes L, Hosoda S, editors. Histamine in Normal and Cancer Cell Proliferation. Oxford: Adv. in Bioscience, Pergamon Press; 1993. pp. 299–317. [Google Scholar]
- Rivera ES, Cricco GP, Engel NI, Fitzimons CP, Martin GA, Bergoc RM. Histamine as an autocrine growth factor: an unusual role for a widespread mediator. Semin Cancer Biol. 2000;10:15–23. doi: 10.1006/scbi.2000.0303. [DOI] [PubMed] [Google Scholar]
- Siegers CP, Andresen S, Keogh JP. Does cimetidine improve prospects for cancer patients? A reappraisal of the evidence to date. Digestion. 1999;60:415–421. doi: 10.1159/000007686. [DOI] [PubMed] [Google Scholar]
- Sieja K, Stanosz S, Von Mach-Szczypinski J, Olewniezak S, Stanosz M. Concentration of histamine in serum and tissues of the primary ductal breast cancer in women. Breast. 2005;14:236–241. doi: 10.1016/j.breast.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Smits RA, Leurs R, de Esch IJ. Major advances in the development of histamine H4 receptor ligands. Drug Discov Today. 2009;14:745–753. doi: 10.1016/j.drudis.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Smits RA, Adami M, Istyastono EP, Zuiderveld OP, van Dam CM, de Kanter FJ, et al. Synthesis and QSAR of quinazoline sulfonamides as highly potent human histamine H4 receptor inverse agonists. J Med Chem. 2010;53:2390–2400. doi: 10.1021/jm901379s. [DOI] [PubMed] [Google Scholar]
- Soule BP, Simone NL, DeGraff WG, Choudhuri R, Cook JA, Mitchell JB. Loratadine dysregulates cell cycle progression and enhances the effect of radiation in human tumor cell lines. Radiat Oncol. 2010;5:8. doi: 10.1186/1748-717X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakhova MI, Cuff CA, Manelli AM, Carr TL, Witte DG, Baranowski JL, et al. In vitro and in vivo characterization of A-940894: a potent histamine H4 receptor antagonist with anti-inflammatory properties. Br J Pharmacol. 2009;157:44–54. doi: 10.1111/j.1476-5381.2009.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szincsák N, Hegyesi H, Hunyadi J, Falus A, Juhász I. Different h2 receptor antihistamines dissimilarly retard the growth of xenografted human melanoma cells in immunodeficient mice. Cell Biol Int. 2002a;26:833–836. doi: 10.1016/s1065-6995(02)90934-0. [DOI] [PubMed] [Google Scholar]
- Szincsák N, Hegyesi H, Hunyadi J, Martin G, Lázár-Molnár E, Kovács P, et al. Cimetidine and a tamoxifen derivate reduce tumour formation in SCID mice xenotransplanted with a human melanoma cell line. Melanoma Res. 2002b;12:231–240. doi: 10.1097/00008390-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanaka S, Ichikawa A. Effect of cimetidine on intratumoral cytokine expression in an experimental tumor. Biochem Biophys Res Commun. 2001;281:1113–1119. doi: 10.1006/bbrc.2001.4487. [DOI] [PubMed] [Google Scholar]
- Tanimoto A, Matsuki Y, Tomita T, Sasaguri T, Shimajiri S, Sasaguri Y. Histidine decarboxylase expression in pancreatic endocrine cells and related tumors. Pathol Int. 2004;54:408–412. doi: 10.1111/j.1440-1827.2004.01641.x. [DOI] [PubMed] [Google Scholar]
- Tiligada E, Zampeli E, Sander K, Stark H. Histamine H3 and H4 receptors as novel drug targets. Expert Opin Investig Drugs. 2009;18:1519–1531. doi: 10.1517/14728220903188438. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Tertoolen LGJ, Remorie R, Ladoux A, Verlaan I, De Laat SW, et al. Histamine as a growth factor and chemoattractant for human carcinoma and melanoma cells: action through Ca2+-mobilizing H1 receptors. J Cell Biol. 1990;110:1211–1215. doi: 10.1083/jcb.110.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Okabe S. Exogenous histamine stimulates colorectal cancer implant growth via immunosuppression in mice. J Pharmacol Sci. 2005;97:116–123. doi: 10.1254/jphs.fp0040691. [DOI] [PubMed] [Google Scholar]
- Tomita K, Izumi K, Okabe S. Roxatidine- and cimetidine-induced angiogenesis inhibition suppresses growth of colon cancer implants in syngeneic mice. J Pharmacol Sci. 2003;93:321–330. doi: 10.1254/jphs.93.321. [DOI] [PubMed] [Google Scholar]
- Tomita K, Nakamura E, Okabe S. Histamine regulates growth of malignant melanoma implants via H2 receptors in mice. Inflammopharmacology. 2005;13:281–289. doi: 10.1163/156856005774423917. [DOI] [PubMed] [Google Scholar]
- Uçar K. The effects of histamine H2 receptor antagonists on melanogenesis and cellular proliferation in melanoma cells in culture. Biochem Biophys Res Commun. 1991;177:545–550. doi: 10.1016/0006-291x(91)92018-f. [DOI] [PubMed] [Google Scholar]
- Wagner W, Ichikawa A, Tanaka S, Panula P, Fogel WA. Mouse mammary epithelial histamine system. J Physiol Pharm. 2003;54:211–223. [PubMed] [Google Scholar]
- Wang LD, Hoeltzel M, Butler K, Hare B, Todisco A, Wang M, et al. Activation of the human histamine H2 receptor is linked to cell proliferation and c-fos gene transcription. Am J Physiol. 1997;273:C2037–C2045. doi: 10.1152/ajpcell.1997.273.6.C2037. [DOI] [PubMed] [Google Scholar]
- Wulff BS, Hastrup S, Rimvall K. Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur J Pharmacol. 2002;453:33–41. doi: 10.1016/s0014-2999(02)02382-8. [DOI] [PubMed] [Google Scholar]
- Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol. 2009;157:24–33. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Michalovich D, Wu H, Tan KB, Dytko GM, Mannan IJ, et al. Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Mol Pharmacol. 2001;59:434–441. doi: 10.1124/mol.59.3.434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


