Abstract
BACKGROUND AND PURPOSE
Maternal infections are one of the main causes of adverse developmental outcomes including embryonic resorption and preterm labour. In this study a mouse model of inflammation-associated preterm delivery was developed, and used to study the relationship between nitric oxide (NO) and prostaglandins (PGs).
EXPERIMENTAL APPROACH
The murine model of preterm labour was achieved by assaying different doses of bacterial lipopolysaccharides (LPS). Once established, it was used to analyse uterine levels of prostaglandins E2 and F2α (by radioimmunoassay), cyclooxygenases (COX) and NOS proteins (by Western blot) and NO synthase (NOS) activity. Effects of inhibitors of COX and NOS on LPS-induced preterm labour were also studied. In vitro assays with a nitric oxide donor (SNAP) were performed to analyse the modulation of prostaglandin production by NO.
KEY RESULTS
Lipopolysaccharide increased uterine NO and PG synthesis and induced preterm delivery. Co-administration of meloxicam, a cyclooxygenase-2 inhibitor, or aminoguanidine, an inducible NOS inhibitor, prevented LPS-induced preterm delivery and blocked the increase in PGs and NO. Notably, the levels of NO were found to determine its effect on PG synthesis; low concentrations of NO reduced PG synthesis whereas high concentrations augmented them.
CONCLUSIONS AND IMPLICATIONS
An infection-associated model of preterm labour showed that preterm delivery can be prevented by decreasing PG or NO production. NO was found to have a dual effect on PG synthesis depending on its concentration. These data contribute to the understanding of the interaction between NO and PGs in pregnancy and parturition, and could help to improve neonatal outcomes.
Keywords: nitric oxide, prostaglandins, preterm delivery, uterus
Introduction
Preterm delivery is the leading cause of neonatal mortality and contributes to delayed physical and cognitive development in children. Although a significant number of preterm cases would benefit from delaying labour, there is considerable debate on the safety and efficacy of currently available medications for the maintenance of tocolysis.
A large body of evidence suggests that intra-amniotic infection may be a significant and potentially preventable cause of preterm birth (Romero et al., 1988). Bacterial lipopolysaccharides (LPS) have been associated with adverse developmental outcome, including embryonic resorption, intra-uterine foetal death, intra-uterine growth retardation and preterm delivery in rodents (Silver et al., 1995). Bacteria could reach the uterus with ejaculate or by intestinal absorption (Hamrick et al., 2003) and treatments with antibiotics that reduce intestinal flora prevent preterm labour (PTL) (Lamont et al., 2003).
Premature activation of uterine pro-inflammatory pathways can lead to preterm birth. Primary pro-inflammatory cytokines induce the production of prostaglandins (PGs) (Pollard and Mitchell, 1996). These, in turn, act synergistically to promote cervical ripening (Kelly, 2002), and prostaglandins E2 and F2α stimulate uterine contractility (Baggia et al., 1996).
Prostaglandins are generated from arachidonic acid (AA) by cyclooxygenases (COX). COX-1 and COX-2 catalyse the conversion of AA into PGH2, which is then converted to various PGs by specific synthases (Smith and Marnett, 1991).
Nitric oxide (NO) is one of the main inflammatory mediators. It is important for host defense and is a regulatory molecule for various physiological functions such as neurotransmission and vasodilatation (Mac Micking et al., 1997). NO is generated during the nitric oxide synthase (NOS)-catalysed conversion of arginine to citrulline. NOS exists in three isoforms, namely endothelial NOS (eNOS), neuronal NOS (nNOS), both constitutive and inducible NOS (iNOS), and all of them have been shown to be present in pregnant rat and mouse uterus (Farina et al., 2001; Ogando et al., 2003a). Although NO plays a pivotal role in many body functions, its excessive production can lead to cytotoxicity, inflammation, carcinogenicity, autoimmune disorders and embryonic resorption (Liu and Hotchkiss, 1995; Ogando et al., 2003b).
Nitric oxide is an important regulator of the biology and physiology of the organs of the reproductive system, including the uterus. Myometrial smooth muscle cells express eNOS, and the uterus is included in the list of ‘tissue exceptions’ and thus expresses iNOS even under unstimulated conditions (Nakaya et al., 1996).
It is well known that NO fulfills important functions during pregnancy; however, in high concentrations it could exert toxic effects as it works as a free radical. In a murine model of embryonic resorption decidual and uterine production of NO was increased by the treatment with LPS, and this increase is due to the induction of iNOS expression in decidua and uterus, and nNOS expression only in decidua. LPS also caused fibrinolysis and infiltration of mesometrial decidua by positive iNOS macrophages (Ogando et al., 2003b).
A relationship between NO biosynthesis and PGs generation has been shown to exist (Kenneth, 1994; Cella et al., 2006); particularly in the uterus, where it was found that NO donors augment PGs synthesis (Franchi et al., 1994) and epidermal growth factor and interleukin (IL)-1α enhance PGs production by stimulation of iNOS activity (Ribeiro et al., 1999; Farina et al., 2000).
It has also been reported that almost 33% of all cases of PTL are related to infections (Romero et al., 1989). In the present study, we developed a mouse model of infection-associated PTL, and used it to study the participation of NO and PGs and to determine if the administration of inhibitors of COX and NOS prolongs the duration of pregnancy in this model. We also determined the effect of NO on the synthesis of PGs in this model.
Methods
Receptor nomenclature throughout the manuscript conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2008)
Test systems used
Female mice of the BALB/c strain (20–24 g body weight) from our own colony were used (n≥ 4 for each day or treatment assayed). They were housed in groups in cages under controlled conditions of light (14 h light, 10 h dark) and temperature (23–25°C). Animals received rat chow and water ad libitum.
Animals were observed closely (every 30 min) for any signs of morbidity (piloerection, decreased movement), vaginal bleeding, and/or preterm delivery (pups present in the cage) after LPS or saline administration. The beginning of preterm delivery was defined by the delivery of the first pup.
In all cases, mice were killed by cervical dislocation. The uterus was removed immediately, cleaned of fat, placenta, fetuses, foetal membranes and blood vessels, and frozen until used.
Time-mated pregnant animals (n = 6 for each day) were killed at 10 h on different days of gestation (13, 15, 16, 18 and 19). Spontaneous term labour occurred on the morning of day 19 (day 0: day sperm plug observed).
Lipopolysaccharide group: mice received two doses of LPS on day 15 of pregnancy, the first one at 10 h (10 µg in 0.1 mL of sterile saline solution, this is equivalent to 0.4 mg·kg−1 of body weight) and the second at 13 h (20 µg in 0.1 mL of sterile saline solution, this is equivalent to 0.8 mg·kg−1 of body weight).
Meloxicam groups: BALB/c females on day 15 of pregnancy were injected with LPS (following our LPS-induced PTL model), meloxicam (4 i.p. doses of 40 µg – equivalent to 1.6 mg·kg−1 of body weight – at 10 h, 13 h, 16 h and 19 h of day 15 of pregnancy) or LPS and meloxicam and killed at 22 h.
Aminoguanidine groups: females BALB/c on day 15 of pregnancy were injected with LPS (following our LPS-induced PTL model), aminoguanidine (3 i.p. doses of 6 mg – equivalent to 240 mg·kg−1 of body weight – at 7 h, 1 h and 19 h of day 15 of pregnancy) or LPS and aminoguanidine and killed at 22 h.
For i.p. administration of LPS, meloxicam, aminoguanidine or SNAP the animals where manually restrained and no anaesthesia was used.
S-nitroso-N-acetyl-penicillamine studies: In vivo, two doses (i.p., 60 µg) of SNAP were administered at 0 and 6 h to female BALB/c mice on day 19 of pregnancy and they were killed at 9 h.
In vitro, female BALB/c mice on day 19 of pregnancy were killed at 9 h. Uterine strips were prepared and incubated with SNAP 10 µM, 1 mM or 3 mM for 1 h at 37°C before PG levels measured.
The experimental procedures reported here were approved by the Animal Care Committee of the Center for Pharmacological and Botanical Studies of the National Research Council and by the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL, Comité Institucional Para el Cuidado y Uso de Animales de Laboratorio) from the School of Medicine of the University of Buenos Aires, and were carried out in accordance with the Guide for Care and Use of Laboratory Animals (NIH).
Measurements
Determination of total NOS activity
NOS enzyme activity was quantified in uterine strips by the modified method of Bredt & Snyder, which measures the conversion of [14C]-L-arginine to [14C]-L-citrulline (Bredt and Snyder, 1989).
Briefly, samples were weighed and homogenized in a buffer containing 20 mM HEPES, 4.5 µM CaCl2 and 100 mM DTT and 25 mM valine. After homogenization, 10 µM [14C]-arginine (0.3 µCi) and 0.12 mM NADPH were added. Samples were incubated for 15 min in a 5%-CO2 atmosphere at 37°C and immediately centrifuged at 28 980 × g for 10 min (4°C). Then the supernatants were applied to 1 mL DOWEX AG50W-X8 columns (Na+ form) equilibrated with HEPES medium and citrulline. Finally [14C]-citrulline was eluted in 3 mL of water.
The radioactivity was measured by liquid scintillation counting. Enzyme activity is reported in fmol of [14C]-citrulline produced mg−1 of tissue in 15 min.
Prostaglandin radioimmunoassay
PGE2 and PGF2α were measured in uterine samples as described by Ribeiro et al., (2005). First, uterine strips were incubated for 1 h in Krebs-Ringer bicarbonate solution in a 5%-CO2 atmosphere at 37°C. After incubation, the medium was acidified and PGs were extracted twice with ethyl acetate. PG concentrations were determined by radioimmunoassay. Sensitivity was 5–10 pg per tube, and values are expressed as pg PGs mg−1 of tissue.
Western blot
The uteri were homogenized in an Ultra-Turrax homogenizer in 20 mM Tris-Buffer (pH = 7.4), containing 1 mM EDTA, 2 µg·mL−1 aprotinin, 10 µg·mL−1 leupeptin, 10 µg·mL−1DTT, 100 µg·mL−1 STY, 1 mg·mL−1 caproic acid and 1 mg·mL−1 benzamidine. The homogenates were sonicated and centrifuged at 3000 × g for 10 min to remove cellular debris. The supernatants were collected and kept at −70°C until the Western blot was performed. The concentration of each sample was measured by the Bradford method (1976) and 100 µg of protein were loaded in each lane The samples were separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane (Sigma Chemical Co, St. Louis, Mi, USA). The membrane was reacted with a rabbit antiserum against nNOS, iNOS, eNOS, COX-1 or COX-2 followed by a horse radish peroxidase-conjugated anti-rabbit IgG as the secondary antibody and developed by ECL. The protein bands were identified by molecular weight markers (BIO-RAD). Each blot was repeated three times with different samples. Blots were scanned using a scanning densitometer and the intensity of bands determined using the Image J (NIH) program.
Drugs, chemical reagents and other materials
Aminoguanidine (AG), S-nitroso-N-acetyl-penicillamine (SNAP), L-valine, LPS of Escherichia coli 05:B55, PGE2 and PGF2α standards and antiserums were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Meloxicam was purchased from Boehringer Ingelheim. [14C]-arginine was purchased from Amersham Corp. (Arlington Heights, II, USA). Dowex AG50W-X8 cationic exchange resin was obtained from Bio-Rad Laboratories (Alfatron, SRL, Buenos Aires, Argentina). The Western blot reagents were obtained from Sigma and Bio-Rad Chemicals. The antibodies for Western blot were obtained from Transduction Laboratories (NJ, USA). All other chemicals were analytical grade.
Data analysis and statistical procedures
Statistical analysis was performed using the GraphPad Prism Software (San Diego, CA, USA). Comparisons between values of different groups were performed using one way anova. Significance was determined using Tukey's multiple comparison tests for unequal replicates. All values presented in this study represent means ± SEM. Differences between means were considered significant when P was 0.05 or less.
Results
Murine model of preterm delivery
We developed a murine model in which mice were induced to deliver prematurely after exposure to LPS on day 15 of pregnancy.
Initially we assayed a single intraperitoneal (i.p.) dose of LPS. A singular 50 µg dose was initially chosen based on previous work where PTL was induced with this dose in C3H/HeN pregnant mice (Fidel et al., 1994) and in our model of LPS-induced embryonic resorption with a similar dose of endotoxin (Aisemberg et al., 2007). The dose of 50 µg per mouse caused PTL in 70% of the mice but also killed 50% of the mice (maternal death). We found that doses over 50 µg per mouse administered at 10 h on day 15 of pregnancy resulted in a high number of maternal deaths. We also observed that LPS doses under 50 µg per mouse did not induce PTL (Table 1A). So we decided to examine the effect of two doses of LPS administered with a 3 h interval, according to a publication by Kaga et al. (1997). Based on the results obtained, we finally established our PTL model; it consisted of administration of 10 µg LPS (i.p.) at 10 h on day 15 of pregnancy plus another of 20 µg (i.p.) at 13 h on this day, as this treatment resulted in PTL and delivery of live pups around 10 h after the last LPS-injection in 100% of the animals, with 95% maternal survival (Table 1B). These pups survived for less than 1 h. This treatment did not change the number of pups delivered (Table 1C) or damage the ability of the mother to become pregnant in the future. To study this, ten mothers of the LPS group, after 1 month rest, were mated and it was found that a similar number of mice became pregnant (around 50%) as the control animals and the litter sizes (9 ± 3 pups) were similar. Although treatment with two doses of 20 µg of LPS also resulted in 100% PTL 10 h after the last injection, has the same pups survival and morbidity of the pups was the same as with the previous dosing regime, it caused a maternal death rate of 33%.
Table 1.
Setup for preterm labour (PTL) model in mice
| Dose | n | PTL | TL | DM | |
|---|---|---|---|---|---|
| A | |||||
| One dose | 80 µg | 4 | – | 1 | 3 |
| 60 µg | 4 | 1 | 1 | 2 | |
| 50 µg | 10 | 7 | 3 | 5a | |
| 40 µg | 4 | – | 2 | 2 | |
| sham (1 dose) | 4 | – | 4 | – | |
| B | |||||
|---|---|---|---|---|---|
| Two doses | 2 × 40 µg | 4 | – | – | 4 |
| 2 × 30 µg | 4 | – | – | 4 | |
| 2 × 20 µg | 6 | 6 | – | 2a | |
| 1 × 10 µg + 1 × 20 µg | 20 | 20 (100%) | – | 1a | |
| 2 × 10 µg | 4 | 1 | 3 | – | |
| sham (2 doses) | 4 | – | 4 | – | |
| C | |||||
|---|---|---|---|---|---|
| Control | Sham | PTL | |||
| Beginning of partum | Day 19 morning | Day 19 morning | Day 15 nightb | ||
| Number of pups | 10 ± 3 | 10 ± 3 | 9 ± 2 | ||
BALB/c female mice on day 15 of pregnancy were injected (A) once (at 10 h) or (B) twice (at 10 h and 13 h) with different doses of LPS (i.p.). Sham animals were treated with vehicle (physiological solution). n ≥ 4 for each treatment.
(C) Females on day 15 of pregnancy received no treatment (control) or were administered vehicle (physiological solution, sham group) or LPS twice (i.p., at 10 h and 13 h) and were observed up to the partum. Beginning of partum and number of pups were recorded. n≥ 4 for each treatment.
During preterm labour.
10 ± 2 h post LPS.
DM, dead mothers; PTL, preterm labour; TL, term labour.
Animals that were injected with saline (sham group) delivered at term (Table 1C).
Uterine synthesis of PGs and NOS activity near labour
We evaluated PGE2 and PGF2α synthesis during the last days of pregnancy. The production of both prostaglandins was maximal at day 19 of gestation (Figure 1A). In contrast, a considerable amount of NOS activity was present on every day of the pregnancy assessed, but became minimal during the last days of gestation (days 18 and 19) (Figure 1B).
Figure 1.
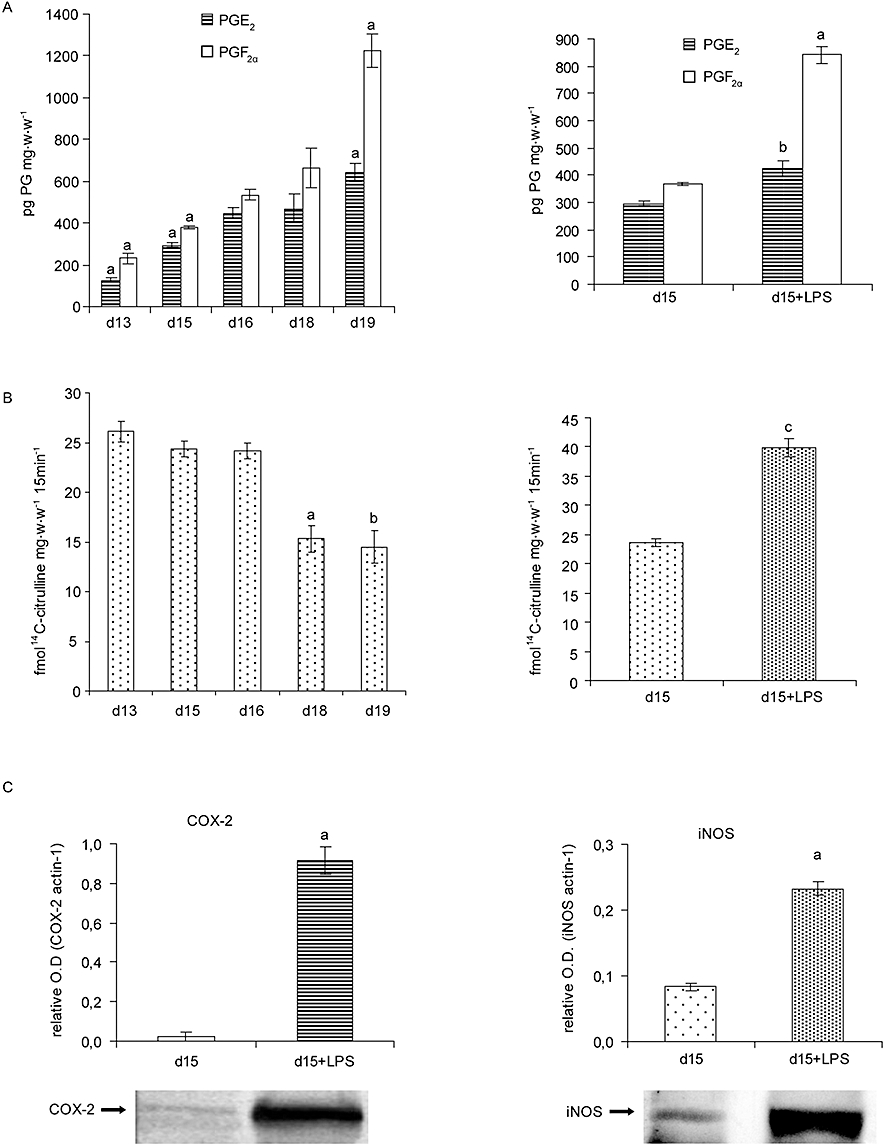
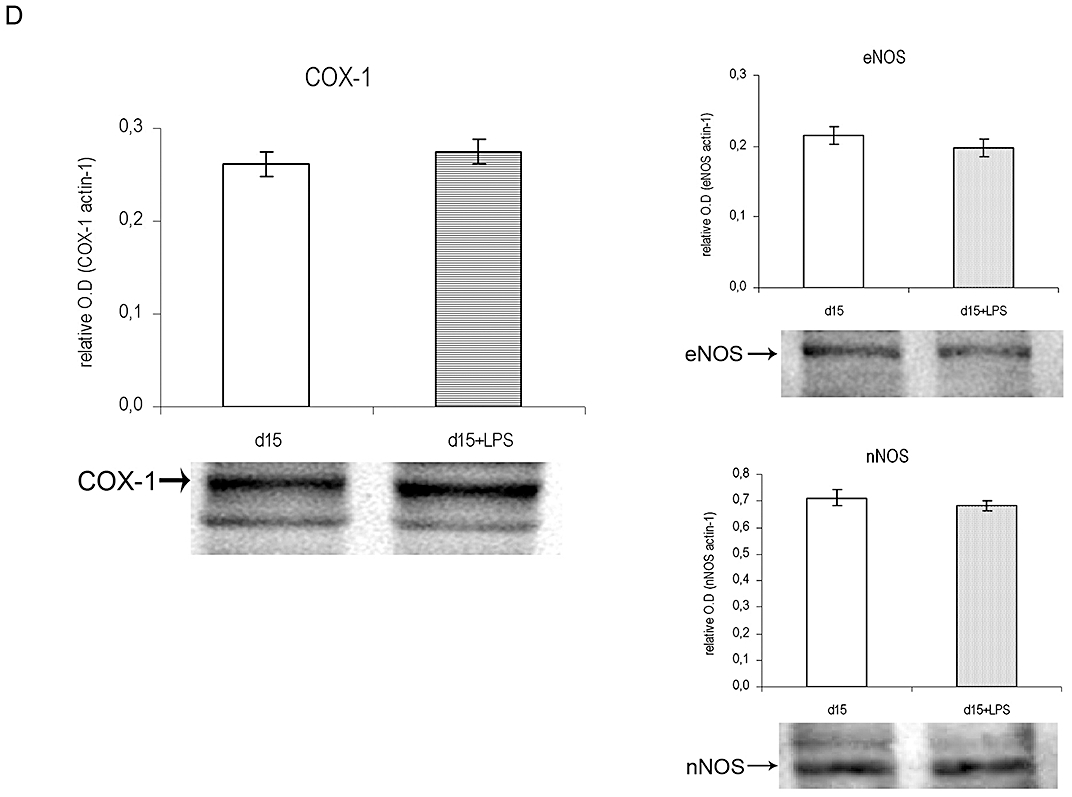
The NO and PG systems during the last part of pregnancy and during LPS-induced preterm labour (PTL). Female mice were killed at 10 h on days 13, 15, 16, 18 and 19 of pregnancy. Mice on day 15 of pregnancy treated with LPS (day 15 + LPS) (i.p., 10 µg at 10 h and 20 µg at 13 h) were killed at 22 h. Uterine strips were collected to measure: (A) Uterine production of prostaglandins E2 and F2α by RIA. aP < 0.001 versus all the rest, bP < 0.05 versus day 15. (B) Uterine NOS activity. aP < 0.001 versus days 13, 15 and 16; bP < 0.01 versus days 13, 15 and 16; cP < 0.001 versus day 15. (C) COX-2 and iNOS protein levels on LPS-induced PTL. Each blot was repeated three times with different samples. One representative blot is presented. aP < 0.001 versus day 15. (D) COX-1, eNOS and nNOS protein levels on LPS-induced PTL. Each blot was repeated three times with different samples. One representative blot of protein is presented. aP < 0.001 versus day 15. Values are expressed as means ± SEM (n ≥ 4). COX, cyclooxygenases; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; NOS, nitric oxide synthase; PG, prostaglandin.
LPS treatment increased uterine PGs and NO synthesis
The effects of the administration of LPS on day 15 of gestation on PGE and PGF synthesis are presented in Figure 1A. The results show that administration of LPS on day 15 significantly augmented PGE2 and PGF2α synthesis.
When NOS activity was assayed, we found that LPS also increased NOS activity by 80% compared with day 15 of control animals (Figure 1B).
LPS treatment increased uterine COX-2 and iNOS protein levels
To investigate the possible source of the elevated levels of PGE2, PGF2α and NO synthesis we used Western blot to determine the protein levels of the different COX and NOS isoforms. In our model of LPS-induced PTL, COX-2 and iNOS were significantly increased (Figure 1C), whereas nNOS, eNOS and COX-1 remained unchanged (Figure 1D).
Meloxicam prevented LPS-induced preterm delivery
We hypothesized that an increased synthesis of PGs induced by LPS could be involved in the mechanisms that trigger PTL. As COX-2 activity was increased by LPS, we determined whether meloxicam, a COX-2 selective inhibitor, could prevent PTL.
In this series of experiments, animals were injected on day 15 with LPS (10 µg at 10 h and 20 µg at 13 h), meloxicam (4 i.p. doses of 40 µg at 10 h, 13 h, 16 h and 19 h) or LPS and meloxicam. Pregnancy was monitored until delivery. Co-injection of meloxicam prevented LPS-induced preterm delivery in 90% of cases, whereas the injection of meloxicam alone did not alter pregnancy length (Figure 2A).
Figure 2.
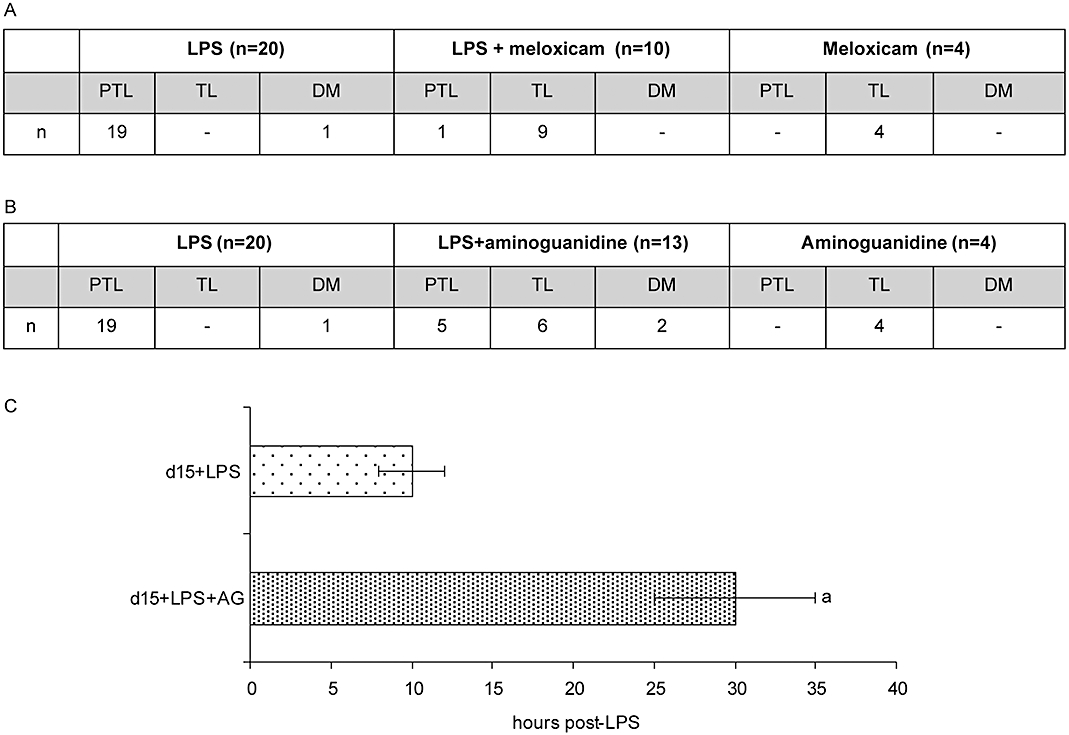
Effects of meloxicam and aminoguanidine on LPS-induced preterm labour (PTL). (A) Pregnant females BALB/c on day 15 of pregnancy were injected with LPS (following our LPS-induced PTL model), meloxicam (4 i.p. doses of 40 µg at 10 h, 13 h, 16 h and 19 h of day 15 of pregnancy) or LPS and meloxicam and the moment of delivery was registered. (B) Pregnant females BALB/c on day 15 of pregnancy were injected with LPS (following our LPS-induced PTL model), aminoguanidine (3 i.p. doses of 6 mg, at 7 h, 13 h and 19 h on day 15 of pregnancy) or LPS and aminoguanidine and the moment of delivery was recorded. (C) Time of beginning of delivery in animals treated with LPS and animals treated with LPS and aminoguanidine that had PTL. aP < 0.01 versus day 15 + LPS. DM, dead mothers; LPS, lipopolysaccharide; TL, term labour.
Aminoguanidine delayed LPS-induced preterm delivery
To evaluate whether the increase in NOS activity, specifically iNOS levels, induced by LPS is involved in PTL, we studied the ability of aminoguanidine (AG), a selective iNOS inhibitor, to prevent LPS-induced PTL.
Animals were injected on day 15 with LPS (10 µg at 10 h and 20 µg at 13 h), aminoguanidine (3 i.p. doses of 6 mg at 7 h, 13 h and 19 h) or LPS and aminoguanidine. Pregnancy was monitored until delivery. Administration of AG delayed preterm delivery around 20 h in 40% of the mice (Figure 2C) and prevented it in 45% of the cases (Figure 2B). AG alone did not have any effect on the beginning of delivery.
Aminoguanidine diminished LPS-induced augmentation of PGs
Prostaglandins stimulate myometrial contractions and are capable of inducing abortion. Here we showed that uterine explants from LPS-treated animals produced more PGs than those from controls and that meloxicam prevented LPS-induced PTL. A significant body of experimental evidence suggests that there is a relationship between NO biosynthesis and the generation of PGs. Thus, we hypothesized that NO could affect the synthesis of PGs in this model, so the effects of LPS and AG administration on the synthesis of PGs were examined. AG totally inhibited LPS-induced augmentation of PGE2 and PGF2α synthesis (Figure 3).
Figure 3.
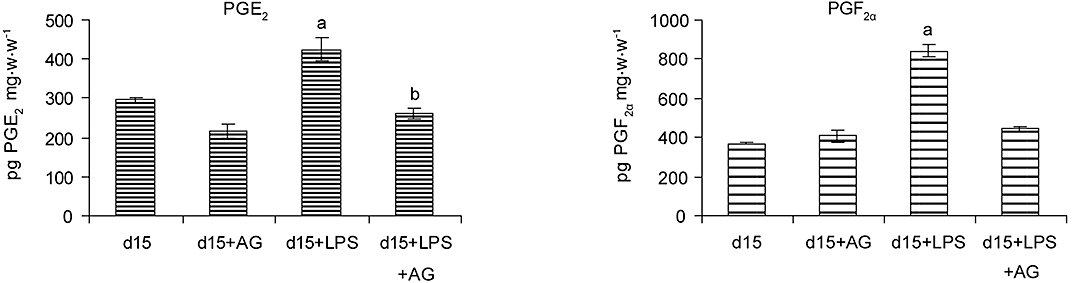
Effect of aminoguanidine on uterine prostaglandin production in LPS-induced preterm labour. Three doses of aminoguanidine were administered (i.p., 6 mg) at 7, 13, and 19 h to control or LPS-treated female BALB/c on day 15 of pregnancy. Uterine strips were collected and the production of PGE2 and PGF2α was measured by RIA. aP < 0.001 versus day 15, bP < 0.01 versus day 15 + LPS. Values are expressed as means ± SEM (n ≥ 4). LPS, lipopolysaccharide.
In vivo administration of a NO donor decreased the synthesis of PGs
As the treatment with AG inhibited the LPS-induced increase in uterine PG synthesis on day 15, we investigated whether S-nitroso-N-acetylpenicillamine (SNAP) (i.p., 60 µg per mouse), a NO donor, could also regulate PG production on day 19 of pregnancy, when NO synthesis was minimum. Surprisingly, the administration of SNAP decreased the production of PGs suggesting that increasing the concentration of NO had a negative effect on the synthesis of PGs (Figure 4A).
Figure 4.
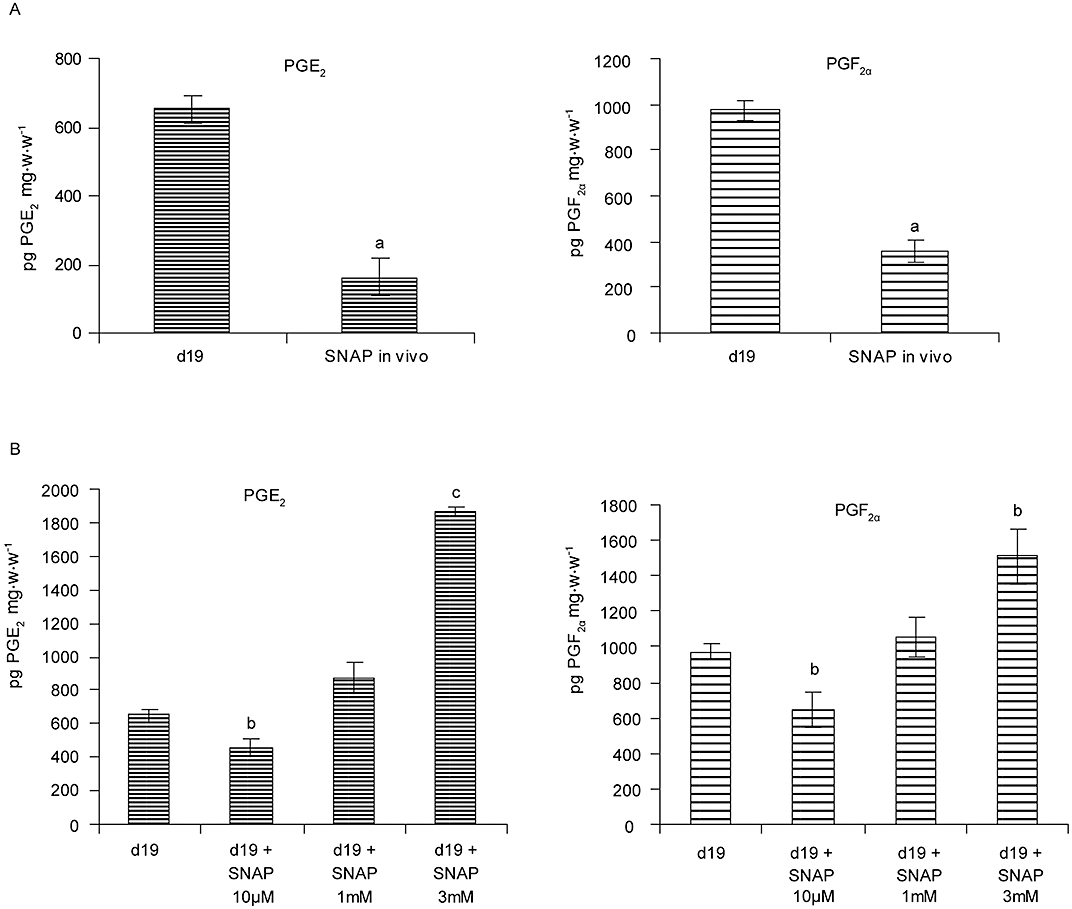
Effect of SNAP on uterine prostaglandin production in term labour. (A) In vivo: two doses (i.p., 60 µg) of SNAP were administered at 0 and 6 h to female BALB/c mice on day 19 of pregnancy and they were killed at 9 h. Uterine strips were collected and the production of PGE2 and PGF2α was measured by RIA. aP < 0.001 versus day 19. (B) In vitro: female BALB/c mice on day 19 of pregnancy were killed at 9 h. The uterine strips collected were incubated with SNAP 10 µM, 1 mM or 3 mM for 1 h at 37°C and production of PGE2 and PGF2α was measured by RIA. bP < 0.05 versus day 19, cP < 0.01 versus day 19. Values are expressed as means ± SEM (n ≥ 6). SNAP, S-nitroso-N-acetyl-penicillamine.
Dual effect of NO on PGs synthesis
Previous studies have shown differential effects of NO on the synthesis of PGs (Franchi et al., 1994; Tunctan et al., 2006). To investigate whether the amount of NO determines its effect on PG synthesis, explants from mouse uteri on day 19 of pregnancy were incubated with different concentrations of SNAP and the production of PGs was quantified.
A low concentration of SNAP (10 µM) decreased the uterine synthesis of PGE2 and PGF2α. However, a higher concentration of SNAP (3 mM) significantly augmented the synthesis of these PGs (Figure 4B).
Discussion
The initial aim of this study was to develop a mouse model of inflammation-associated preterm delivery that resembles the clinical presentation in humans. Numerous studies have shown that infection is causally linked to preterm birth (Romero et al., 2002). It has been reported that murine and human decidua respond similarly to cytokines and this finding validates the use of murine models of PTL to study the mechanism by which microbial pathogens induce labour (Swaisgood et al., 1997).
Even though animal models that utilize bacterial products are not useful to study spontaneous PTL syndrome, they may be relevant for infection-associated preterm birth (Mitchell and Taggart, 2009). Furthermore, other authors have also described murine models of PTL (Gross et al., 2000; Pirianov et al., 2009).
Human preterm delivery is associated with a range of severity of inflammation, but only a small proportion of women with infection-associated PTL have clinical signs of infection. Thus, this model of LPS-induced preterm delivery was designed to mimic, as closely as possible, the clinical consequences of preterm infection.
Compared with other mice models (Gross et al., 2000; Pirianov et al., 2009) our model has the advantages of effectiveness and a simple study design. We obtained 100% incidence of preterm birth with minimal fatal effects on mothers (5%).
In the PTL models described previously, induced by cytokines or LPS (Fidel et al., 1994; Gross et al., 2000), the kinetics observed are similar to those presented in our model, where pregnant animals delivered the first pup as early as 10 h after the last injection of LPS.
The mechanisms by which microbial pathogens induce labour have not been fully elucidated; however, there is evidence supporting key roles for several factors, including NO and PGs (Swaisgood et al., 1997; Keelan et al., 2003). Changes in the production of uterine PGs and NO synthesis appear to be involved in the regulation of myometrial activity during pregnancy and labour. We observed that the synthesis of PGs was augmented and NO production diminished at term. In accord with this, we and others have demonstrated that uterine PGF2α synthesis increases abruptly just before the onset of labour (Unezaki et al., 1996; Farina et al., 2004). As presented here, other studies have shown that the generation of NO is up-regulated during pregnancy and down-regulated during delivery and after labour (Dong et al., 1996; Farina et al., 2001).
As observed in the present study, several authors have indicated that LPS stimulates NOS activity and the synthesis of PGs during pregnancy (Aisemberg et al., 2007; Anbe et al., 2007). We and others have successfully prevented abortion and PTL by administering PG synthesis inhibitors (Aisemberg et al., 2007; Rac et al., 2007; Wang et al., 2008). The finding that meloxicam, a selective COX-2 inhibitor, almost completely prevented PTL induced by LPS is strong evidence that PGs, especially those synthesized by COX-2, are critically involved in the onset of labour. In accordance with our results, COX-2 has been found to be up-regulated in the uterus of LPS-treated mice (Swaisgood et al., 1997; Gross et al., 2000).
As Olson and Ammann (2007) reported, although COX inhibitors are usually successful in suppressing PTL in animal and human studies, these drugs have adverse effects on foetal physiology and development, so others ways to prevent PTL need to be investigated. In this sense, the contribution of the predominant myometrial PG produced just prior to human labour, prostacyclin, to the enhancement of the contractile response of human pregnant myometrium to oxytocin and the up-regulation of the contractile apparatus protein expression and the gap junction protein connexin 43, should be take in account (Fetalvero et al., 2008). We think that in future studies the changes in NO and PG synthesis observed in the present work, and the effects of different drugs on these changes, should be related to altered cytokine and steroid hormone/receptor levels in uterine tissues.
As iNOS protein levels and NOS activity are increased in the uterus during PTL, we studied the effect of AG on the length of pregnancy. The administration of AG delayed PTL, and prevented it in 50% of the animals, suggesting that NO is part of the mechanism of LPS-induced PTL.
These results appear to contradict the idea that NO is a uterine relaxant. So we studied the effect of AG on the synthesis of PGs, so far described as the principal molecules involved in labour. AG attenuated the increase in the synthesis of PGs induced by LPS suggesting a positive modulation of the production of PGs by NO. To further confirm this we studied the effect of in vivo administration of a NO donor on the synthesis of PGs, and surprisingly it decreased the production of PGs suggesting a negative modulation of the synthesis of PGs by NO. As it has been reported in several studies that NO, depending on its concentration, can exert dual effects in different systems (Liew, 1995; Kaneko et al., 2003; Lee et al., 2006), we decided to determine whether this could be occurring in our model. To address this question we investigated whether the amount of NO determines its effect on the synthesis of PGs using the NO donor SNAP. We found that a low concentration of SNAP (10 µM) was able to decrease PGs synthesis whereas higher concentrations of SNAP (3 mM) augmented them.
As mentioned above, NO could be a uterine relaxant, and have a negative effect on the synthesis of PGs. Also, diminished serum NO levels are associated with abortion in women (Paradisi et al., 2007). However, it is important to remember that large quantities of NO, particularly those generated by iNOS, in an inflammatory setting such as sepsis, could increase the synthesis of PGs and contribute to uterine contractility and PTL. In this context, it has been reported that high concentrations of NO metabolites are present in amniotic fluid of women with intra-amniotic infection (Hsu et al., 1998) and that NO may contribute to PTL in LPS-challenged pregnant mice (Swaisgood et al., 1997).
In conclusion, we present an animal model in which it is possible to study preterm delivery and the regulation of important mediators of pregnancy and parturition. Additionally this work also suggests that the role of NO in pregnancy and labour is far more complex than was originally appreciated.
Acknowledgments
We thank Mrs Ramona Morales and Mrs Ana Inés Casella for their excellent technical support. This work was supported by FONCYT (PICT 2004/20207) and by Proyecto UBACYT 2008–10. (M411). Universidad de Buenos Aires.
Glossary
Abbreviations
- AG
aminoguanidine
- COX
cyclooxygenases
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PGs
prostaglandins
- PTL
preterm labour
- SNAP
S-nitroso-N-acetyl-penicillamine
Conflicts of interest
None.
Supplemental material
References
- Aisemberg J, Vercelli C, Billi S, Ribeiro ML, Ogando D, Meiss R, et al. Nitric oxide mediates prostaglandins' deleterious effect on lipopolysaccharide-triggered murine fetal resorption. Proc Natl Acad Sci USA. 2007;104:7534–7539. doi: 10.1073/pnas.0702279104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbe H, Okawa T, Sugawara N, Takahashi H, Sato A, Vedernikov YP, et al. Influence of progesterone on myometrial contractility in pregnant mice treated with lipopolysaccharide. J Obstet Gynaecol Res. 2007;33:765–771. doi: 10.1111/j.1447-0756.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3:121–126. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Aisemberg J, Sordelli MS, Billi S, Farina M, Franchi AM, et al. Prostaglandins modulate nitric oxide synthase activity early in time in the uterus of estrogenized rat challenged with lipopolysaccharide. Eur J Pharmacol. 2006;534:218–226. doi: 10.1016/j.ejphar.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Dong YL, Gangula PR, Yallampalli C. Nitric oxide synthase isoforms in the rat uterus: differential regulation during pregnancy and labor. J Reprod Fertil. 1996;107:249–254. doi: 10.1530/jrf.0.1070249. [DOI] [PubMed] [Google Scholar]
- Farina M, Ribeiro ML, Ogando D, Gimeno M, Franchi AM. IL1alpha augments prostaglandin synthesis in pregnant rat uteri by a nitric oxide mediated mechanism. Prostaglandins Leukot Essent Fatty Acids. 2000;62:243–247. doi: 10.1054/plef.2000.0150. [DOI] [PubMed] [Google Scholar]
- Farina M, Ribeiro ML, Franchi A. Nitric oxide synthases in pregnant rat uterus. Reproduction. 2001;121:403–407. doi: 10.1530/rep.0.1210403. [DOI] [PubMed] [Google Scholar]
- Farina M, Ribeiro ML, Weissmann C, Estevez A, Billi S, Vercelli C, et al. Biosynthesis and catabolism of prostaglandin F2alpha (PGF2alpha) are controlled by progesterone in the rat uterus during pregnancy. J Steroid Biochem Mol Biol. 2004;91:211–218. doi: 10.1016/j.jsbmb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Fetalvero KM, Zhang P, Shyu M, Young BT, Hwa J, Young RC, et al. Prostacyclin primes pregnant human myometrium for an enhanced contractile response in parturition. J Clin Invest. 2008;118:3966–3979. doi: 10.1172/JCI33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel PL, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- Franchi AM, Chaud M, Rettori V, Suburo A, McCann SM, Gimeno M. Role of nitric oxide in eicosanoid synthesis and uterine motility in estrogen-treated rat uteri. Proc Natl Acad Sci USA. 1994;91:539–543. doi: 10.1073/pnas.91.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Imamura T, Vogt SK, Wozniak DF, Nelson DM, Sadovsky Y, et al. Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1415–R1423. doi: 10.1152/ajpregu.2000.278.6.R1415. [DOI] [PubMed] [Google Scholar]
- Hamrick TS, Horton JR, Spears PA, Havell EA, Smoak IW, Orndorff PE. Influence of pregnancy on the pathogenesis of listeriosis in mice inoculated intragastrically. Infect Immun. 2003;71:5202–5209. doi: 10.1128/IAI.71.9.5202-5209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CD, Meaddough E, Aversa K, Hong SF, Lee IS, Bahodo-Singh RO, et al. Dual roles of amniotic fluid nitric oxide and PGE2 in preterm labor with intra-amniotic infection. Am J Perinatol. 1998;15:683–687. doi: 10.1055/s-2007-999302. [DOI] [PubMed] [Google Scholar]
- Kaga N, Katsuki Y, Kajikawa S, Shibutani Y. Preventive effect of ritodrine hydrochloride and/or urinary trypsin inhibitor against lipopolysaccharide-induced preterm delivery in mice. Acta Obstet Gynecol Scand. 1997;76:811–816. doi: 10.3109/00016349709024357. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Ishikawa T, Amano S, Koichi N. Dual effect of nitric oxide on cytosolic Ca2+ concentration and insulin secretion in rat pancreatic beta –cells. Am J Physiol Cell Physiol. 2003;284:C1215–C1222. doi: 10.1152/ajpcell.00223.2002. [DOI] [PubMed] [Google Scholar]
- Keelan A, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition – a review. Placenta. 2003;24(Suppl. A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol. 2002;57:217–224. doi: 10.1016/s0165-0378(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Kenneth K. Inducible cyclooxigenase and nitric oxide synthase. Adv Pharmacol. 1994;33:179–198. doi: 10.1016/s1054-3589(08)60669-9. [DOI] [PubMed] [Google Scholar]
- Lamont RF, Duncan SL, Mandal D, Bassett P. Intravaginal clindamycin to reduce preterm birth in women with abnormal genital tract flora. Obstet Gynecol. 2003;101:516–522. doi: 10.1016/s0029-7844(02)03054-5. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim HS, Lee HJ, Lee J, Jeon BH, Jun CD, et al. Dual effect of nitric oxide in immortalized and malignant human oral keratinocytes: induction of apoptosis and differentiation. J Oral Pathol Med. 2006;35:352–360. doi: 10.1111/j.1600-0714.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- Liew FY. Regulation of lymphocyte functions by nitric oxide. Curr Opin Immunol. 1995;7:396–399. doi: 10.1016/0952-7915(95)80116-2. [DOI] [PubMed] [Google Scholar]
- Liu RH, Hotchkiss JH. Potential genotoxicity of chronically elevated nitric oxide; a review. Mutat Res. 1995;339:73–89. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Mac Micking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297:R525–R545. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Yamamoto S, Hamada Y, Kamada M, Aono T, Niwa M. Inducible nitric oxide synthase in uterine smooth muscle. Life Sci. 1996;58:PL249–PL255. doi: 10.1016/0024-3205(96)00064-1. [DOI] [PubMed] [Google Scholar]
- Ogando D, Farina M, Ribeiro ML, Perez Martinez S, Cella M, Rettori V, et al. Steroid hormones augment nitric oxide synthase activity and expression in rat uterus. Reprod Fertil Dev. 2003a;15:269–274. doi: 10.1071/rd03013. [DOI] [PubMed] [Google Scholar]
- Ogando DG, Paz D, Cella M, Franchi AM. The fundamental role of increased production of nitric oxide in lipopolysaccharide-induced embryonic resorption in mice. Reproduction. 2003b;125:95–110. doi: 10.1530/rep.0.1250095. [DOI] [PubMed] [Google Scholar]
- Olson DM, Ammann C. Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Front Biosci. 2007;12:1329–1343. doi: 10.2741/2151. [DOI] [PubMed] [Google Scholar]
- Paradisi R, Fabbri R, Battaglia C, Facchinetti F, Venturoli S. Nitric oxide levels in women with missed and threatened abortion: results of a pilot study. Fertil Steril. 2007;88:744–748. doi: 10.1016/j.fertnstert.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Pirianov G, Waddington SN, Lindström TM, Terzidou V, Mehmet H, Bennett PR. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J(2) delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2009;150:699–706. doi: 10.1210/en.2008-1178. [DOI] [PubMed] [Google Scholar]
- Pollard JK, Mitchell MD. Intrauterine infection and the effects of inflammatory mediators on prostaglandin production by myometrial cells from pregnant women. Am J Obstet Gynecol. 1996;174:682–686. doi: 10.1016/s0002-9378(96)70450-7. [DOI] [PubMed] [Google Scholar]
- Rac VE, Scott CA, Small C, Adamson SL, Rurak D, Challis JR, et al. Dose-dependent effects of meloxicam administration on cyclooxygenase-1 and cyclooxygenase-2 protein expression in intrauterine tissues and fetal tissues of a sheep model of preterm labor. Reprod Sci. 2007;14:750–764. doi: 10.1177/1933719107309042. [DOI] [PubMed] [Google Scholar]
- Ribeiro ML, Perez Martinez S, Farina M, Ogando D, Gimeno M, Franchi AM. The effect of epidermal growth factor on prostaglandin synthesis of oestrogenized rat uterus is mediated by nitric oxide. Prostaglandins Leukot Essent Fatty Acids. 1999;61:353–358. doi: 10.1054/plef.1999.0110. [DOI] [PubMed] [Google Scholar]
- Ribeiro ML, Aisemberg J, Billi S, Farina MG, Meiss R, McCann S, et al. Epidermal growth factor prevents prepartum luteolysis in the rat. Proc Natl Acad Sci USA. 2005;102:8048–8053. doi: 10.1073/pnas.0502899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- Silver RM, Edwin SS, Trautman MS, Simmons DL, Branch DW, Dudley DJ, et al. Bacterial lipopolysaccharide-mediated fetal death: production of a newly recognized form of inducible cyclooxygenase (COX-2) in murine decidua in response to lipopolysaccharide. J Clin Invest. 1995;95:725–731. doi: 10.1172/JCI117719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, Marnett LJ. Prostaglandin endoperoxide synthase: structure and catalysis. Biochem Biophys Acta. 1991;1083:1–17. doi: 10.1016/0005-2760(91)90119-3. [DOI] [PubMed] [Google Scholar]
- Swaisgood CM, Zu HX, Perkins DJ, Wu S, Garver CL, Zimmerman PD, et al. Coordinate expression of inducible nitric oxide synthase and cyclooxygenase-2 genes in uterine tissues of endotoxin-treated pregnant mice. Am J Obstet Gynecol. 1997;177:1253–1262. doi: 10.1016/s0002-9378(97)70047-4. [DOI] [PubMed] [Google Scholar]
- Tunctan B, Ozveren E, Korkmaz B, Buharalioglu CK, Tamer L, Degirmenci U, et al. Nitric oxide reverses endotoxin-induced inflammatory hyperalgesia via inhibition of prostacyclin production in mice. Pharmacol Res. 2006;53:177–192. doi: 10.1016/j.phrs.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Unezaki S, Sugatani J, Masu Y, Watanabe K, Ito S. Characterization of prostaglandin F2 alpha production in pregnant and cycling mice. Biol Reprod. 1996;55:889–894. doi: 10.1095/biolreprod55.4.889. [DOI] [PubMed] [Google Scholar]
- Wang PH, Cheng MH, Lee WL. The choice of tocolytic drugs for preterm labor – comparison of COX-2 inhibitor and magnesium sulfate. J Obstet Gynaecol Res. 2008;34:439–440. doi: 10.1111/j.1447-0756.2008.00785.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


