Abstract
BACKGROUND AND PURPOSE
Certain 5-lipoxygenase (5-LO) inhibitors exhibit anti-carcinogenic activities against 5-LO overexpressing tumour types and cultured tumour cells. It has been proposed therefore that 5-LO products significantly contribute to tumour cell proliferation. To date, the relationship between the inhibitory mechanisms of 5-LO inhibitors, which vary widely, and tumour cell viability has not been evaluated. This study addresses the anti-proliferative and cytotoxic potency of a number of 5-LO inhibitors with different inhibitory mechanisms in 5-LO-positive and 5-LO-negative tumour cells.
EXPERIMENTAL APPROACH
Cell viability was measured by the WST-1 assay; cell proliferation was assessed using the bromodeoxyuridine (BrdU) incorporation assay. Cell death was analysed by annexin V staining, Western blot analysis of PARP (poly ADP-ribose polymerase) cleavage and a cytotoxicity assay. 5-LO product formation was quantified by a 5-LO activity assay.
KEY RESULTS
The common 5-LO inhibitors AA-861, Rev-5901 and MK-886 induced cytotoxic and anti-proliferative effects in 5-LO-positive Capan-2 pancreatic cancer cells; BWA4C and CJ-13,610 only caused anti-proliferative effects, while zileuton failed to impair cell viability. Moreover, the concentrations of the 5-LO inhibitors required to induce anti-proliferation and cytotoxicity highly exceeded those for suppression of 5-LO. Supplementation with mitogenic 5-LO products failed to protect Capan-2 cells from the effects of 5-LO inhibitors. Finally, the cytotoxic and anti-proliferative 5-LO inhibitors also potently reduced the viability of 5-LO-deficient tumour cell lines (HeLa, Panc-1 and U937).
CONCLUSIONS AND IMPLICATIONS
Certain 5-LO inhibitors cause cytotoxic and anti-proliferative effects independently of suppression of 5-LO activity. Thus, the role of 5-LO overexpression in tumour cell viability remains unclear and requires further elucidation.
Keywords: leukotriene, apoptosis, cytotoxicity, off-target effect, zileuton, pancreatic cancer
Introduction
5-Lipoxygenase (5-LO), a tightly regulated fatty acid metabolizing enzyme, catalyses the first two steps in leukotriene (LT) biosynthesis by dioxygenation of arachidonic acid (AA). The products of this enzymatic reaction are two chemically unstable intermediates, 5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HPETE) and LTA4. A non-haeme coordinated iron atom, located in the catalytic site of 5-LO, facilitates both chemical reactions. Depending on the cellular enzymes present, LTA4 either can be hydrolysed by LTA4 hydrolase to form LTB4 or can be conjugated with glutathione by LTC4 synthase to generate the cysteinyl LTC4. Further processing of LTC4 produces LTD4 and LTE4, whereas 5-HPETE can be reduced by glutathione peroxidases to form the corresponding 5(S)-hydroxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HETE) (Radmark et al., 2007). In resting cells, 5-LO resides in either the nucleus or the cytosol, depending on the cell type. Upon activation, 5-LO translocates to the nuclear membrane, where the 5-LO-activating protein (FLAP) is thought to facilitate the transfer of phospholipid-derived AA to 5-LO (Abramovitz et al., 1993; Mancini et al., 1993).
Several pharmacological strategies exist to inhibit 5-LO product formation. Non-redox and redox type inhibitors, including CJ-13,610, Rev-5901 and AA-861 compete with fatty acids for binding to the active site cleft(s). Iron-ligand inhibitors such as zileuton and BWA4C suppress enzyme activity through chelation of the central iron-atom and/or by stabilizing the ferrous state. FLAP inhibitors, such as MK-886, act indirectly by interfering with the availability of AA (Ford-Hutchinson et al., 1994).
Leukotrienes primarily mediate inflammatory and allergic reactions (Funk, 2001). However, an increasing body of evidence also suggests a crucial role for 5-LO products in the early stage of pancreatic, prostate and colorectal carcinogenesis (Werz and Steinhilber, 2006). Numerous studies have demonstrated the overexpression of 5-LO in tissue samples of primary tumour cells as well as in established cancer cell lines (Chen et al., 2006). The addition of 5-LO products to cultured tumour cells has also been shown to increase cell proliferation and activation of anti-apoptotic signalling pathways (Ding et al., 2003; Tong et al., 2005). 5-LO antisense technology has been used to demonstrate that impaired tumour cell growth is due to a reduction in the expression of 5-LO (Sveinbjornsson et al., 2008). Finally, pharmacological inhibition of 5-LO potently suppressed tumour cell growth by inducing cell cycle arrest and triggering cell death via the intrinsic apoptotic pathway (Ghosh and Myers, 1998; Ding et al., 1999). Anti-LT drugs therefore are considered a promising and novel pharmacological strategy for cancer prevention and therapy.
However, studies investigating the effects of 5-LO inhibition on tumour cell viability preferentially utilized relatively high concentrations (up to 100 µM) of competitive 5-LO inhibitors, such as AA-861 and Rev-5901 or the FLAP inhibitor MK-886. As the published IC50 values for enzyme inhibition of these drugs are known to be less than 1 µM (Yoshimoto et al., 1982; Musser et al., 1987), we hypothesized that molecular mechanisms independent of 5-LO suppression may contribute to the anti-proliferative and cytotoxic effects mediated by 5-LO inhibitors. In this study, we provide evidence, for the first time, that direct 5-LO inhibitors are able to suppress the viability of various human tumour cells independently of 5-LO inhibition.
Methods
Cell culture
The human pancreatic cancer cell lines Capan-2 and Panc-1 as well as HeLa cervix carcinoma cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The human leukaemic monocyte cell lines U937 and THP-1 were purchased from Deutsche Sammlung für Mikroorgansimen und Zellkulturen (DSMZ, Braunschweig, Germany). Capan-2 cells were cultured in McCoy's 5A medium. Panc-1 and HeLa cells were grown in DMEM (Dulbecco's modified Eagle's medium). U937 and THP-1 cells were maintained in RPMI 1640 medium. Complete growth media contained 10% fetal calf serum (FCS), 100 µg·mL−1 streptomycin and 100 U·mL−1 penicillin. Cells were cultured at 37°C in an atmosphere containing 5% CO2.
In vitro cell viability assay
The WST-1 assay (Roche Diagnostic GmbH, Mannheim, Germany) was used to determine the cell viability after treatment with 5-LO inhibitors. Cells were seeded in 96-well plates at a density of 5 × 103 (Capan-2), 3 × 103 (Panc-1), 1 × 104 (U937 and THP-1) cells per well and treated with inhibitors for 72 h (Capan-2) or 48 h (Panc-1, THP-1 and U937) in presence of 10% FCS. We used a longer incubation period for Capan-2 cells in order to take into account their low division rate (∼50 h). Cell viability was assessed using a microplate reader according to the manufacturer's protocol (infinite M200, Tecan Group Ltd., Crailsheim, Germany). AA-861 potently interfered with the conversion of WST-1 to formazan, apparently due to its redox activity, and was not compatible with this assay. The number of viable cells after AA-861 treatment was therefore assessed using trypan blue staining. All experiments were undertaken at least in triplicate.
Colony forming assay
Capan-2 cells were seeded in 6-well plates at a density of 103 cells per well and incubated for 24 h at 37°C in an atmosphere containing 5% CO2. Cells were then treated with increasing concentrations of 5-LO inhibitors and incubated for 10 days. Inhibitors were diluted in complete growth medium in the presence of 10% FCS. Cells were subsequently fixed with 100% methanol, stained with 0.5% Ponceau red, and single cell colonies were counted. The number of colonies in the dishes devoid of inhibitors was used as an index for a 100% survival rate (control), and this value was used to obtain survival rates, as percentage of control, for the wells containing the inhibitors. The experiments were performed in triplicate.
Bromodeoxyuridine (BrdU) cell proliferation assay
To assess the effects of 5-LO inhibitors on cell proliferation, BrdU (bromodeoxyuridine) incorporation into Capan-2 DNA was measured (BrdU cell proliferation ELISA, colorimetric; Roche Diagnostic GmbH, Mannheim, Germany). Cells (5000 per well; 96-well plate) were treated with inhibitors for 72 h in the presence of 10% FCS. Cell proliferation was assessed using a microplate reader according to the manufacturer's protocol (infinite M200, Tecan Group Ltd.). BrdU incorporation was assessed in triplicate.
Protein extraction and Western blot analysis
Cells treated with 5-LO inhibitors for 72 h in medium containing 10% FCS or untreated control cells were scraped in medium and centrifuged at 1000×g, for 5 min. The cell pellet was washed once with ice-cold PBS (phosphate-buffered saline). For the detection of PARP (poly ADP-ribose polymerase), cells were resuspended in sonification buffer [PBS pH 7.4, protease inhibitor cocktail tablets (Complete, Mini; Roche diagnostics GmbH, Mannheim, Germany)], and disrupted by sonification (3 × 10 s) at 4°C. For the detection of 5-LO, cells were resuspended in extraction buffer [0.25 M sucrose, 30 mM Tris-HCl pH 7.9, protease inhibitor cocktail tablets (CompleteMini; Roche diagnostics GmbH, Mannheim, Germany)] and disrupted by a glass Dounce homogenizer. The resulting cell homogenates were centrifuged at 10 000×g for 10 min at 4°C. Protein concentrations in the supernatant were determined using the Bradford method. Equal quantities of protein extracts were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and proteins were electrophoretically blotted onto a nitrocellulose membrane (Hybond-C Extra, Amersham Biosciences Ltd., Little Chalfont, UK). Membranes were stained with 0.5% ponceau red to confirm equal loading. After being dried, membranes were incubated overnight in Odyssey blocking reagent (LI-COR Biosciences, Bad Homburg, Germany). The next day, membranes were treated with the respective primary antibodies directed against 5-LO (AK-7 rabbit polyclonal, kindly provided by Professor Olof Rådmark, Stockholm, Sweden; BD, mouse monoclonal, BD Biosciences, Franklin Lakes, NJ, USA; 5-LO-122-AP, rabbit polyclonal, kindly provided by Biolipox AB, Stockholm, Sweden), β-Actin (No. I-19, goat, polyclonal, Santa Cruz Biotechnology, Heidelberg, Germany) or PARP-1 (No. F-2, mouse, monoclonal, Santa Cruz Biotechnology, Heidelberg, Germany). All antibodies were diluted in Odyssey blocking reagent. Membranes were washed four times with PBS containing 0.2% Tween 20, and were then incubated with an IRDye680- or IRDye800-conjugated secondary antibody (LI-COR Biosciences, Bad Homburg, Germany) in Odyssey blocking reagent. After extensive rinsing in PBS containing 0.2% Tween 20, protein–antibody complexes were visualized on the Odyssey Infrared Imaging System (LI-COR Biosciences, Bad Homburg, Germany). All Western blot analysis experiments were performed in triplicate.
Annexin V staining
Capan-2 cells were seeded on small glass coverslips and allowed to attach for 24 h at 37°C in an atmosphere containing 5% CO2. Cells were then treated with inhibitors for 24 h in medium containing 10% FCS, followed by washing once with washing buffer. Subsequently, cells were co-stained by covering the slips with a solution containing annexin V labelling reagent and propidium iodide (Annexin-V-FLUOS Staining Kit, Roche Diagnostics GmbH, Basel, Switzerland) for 20 min at 4°C. Coverslips were then washed once with assay buffer, fixed on microscope slides, and cell staining was visualized by fluorescence microscopy (Axio Observer.Z1, Carl Zeiss AG, Jena, Germany). Volocity 4.2 software (Improvision, Tübingen, Germany) was used to quantify the results. The immunocytochemistry experiments were undertaken in quadruplicate.
LDH (lactate dehydrogenase) cytotoxicity assay
Lactate dehydrogenase leakage was used as an index of membrane integrity due to necrosis (in vitro cytotoxicity assay kit, LDH-based; Sigma-Aldrich, Saint Louis, MO, USA). Capan-2 cells were seeded in 96-well plates at a density of 9 × 103 cells per well and incubated for 24 h at 37°C in an atmosphere containing 5% CO2. After treatment with increasing concentrations of 5-LO inhibitors, cells were further incubated for 72 h. Inhibitors were diluted in complete growth medium in the presence of 10% heat-inactivated FCS. Plates were centrifuged (250×g, 4 min) and an aliquot of the supernatant was transferred to a clean microtitre plate. Cell toxicity was measured according to the manufacturer's protocol using a microplate reader (infinite M200, Tecan Group Ltd., Crailsheim, Germany). LDH release induced by a control detergent supplied by the manufacturer was set to 100%. All cytotoxicity experiments were performed in triplicate.
Determination of 5-LO product formation in intact THP-1 cells
To induce 5-LO overexpression, THP-1 cells were differentiated in complete growth medium by addition of transforming growth factor-β1 (TGFβ1, 1 ng·mL−1) and 1,25-dihydroxyvitamin D3 (50 nM) for 4 days at 37°C in an atmosphere containing 6% CO2. Differentiated cells were harvested by centrifugation and washed once in PBS (pH 7.4). Approximately 4 × 106 cells were then resuspended in 1 mL PBS (containing 1 mM Ca2+, 1 g·L−1 glucose, 10% FCS) and preincubated with the 5-LO inhibitors for 15 min at 37°C. The reaction was started by addition of 10 µM AA and 5 µM A23187 (Ca2+ ionophore). After a 10 min incubation at 37°C, the reaction was stopped by the addition of 1 mL of ice-cold methanol. This was followed by addition of 200 ng of prostaglandin B1 (PGB1, internal standard), 30 µL of 1 N HCl and 500 µL of PBS. After centrifugation (10 min, 800×g) the samples were applied to C-18 solid phase extraction columns (100 mg, preconditioned with 1 mL methanol and 1 mL distilled water). After being washed with a solution of 25% methanol in distilled water, the 5-LO metabolites were extracted with 300 µL of pure methanol. The extract was diluted with 120 µL of distilled water. A total of 100 µL of the diluted extract was analysed by HPLC as described previously (Werz and Steinhilber, 1996). 5-LO product formation was expressed relative to the vehicle control (DMSO) and includes 5-HETE and LTB4. 5-LO activity was measured in triplicate.
Reverse transcription polymerase chain reaction (RT-PCR)
For extraction of total cellular RNA, cells were harvested in PBS pH 7.4 and centrifuged at 1000×g for 5 min at 4°C. RNA was isolated with the E.Z.N.A. Total RNA Kit (OMEGA bio.tek, Norcross, GA, USA) according to the manufacturer's protocol. The amount of RNA was determined using a spectrophotometer (NanoPhotometer, Implen GmbH, Munich, Germany). Reverse transcription was performed with 1 µg of isolated RNA using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's protocol. The PCR was performed with an iQ supermix (Biorad Laboratories, Hercules, CA, USA) using specific primers for 5-LO (fwd: 5′-ACCATTGAGCAGATCGTGGACACG-3′; rev: 5′-CCC ATTCTACACAGAGCAGGACTGC-3′) and β-Actin (fwd: 5′-CATTAAGGAGAA-GCTGTGCTAC-3′; rev: 5′-GACTCGTCATACTCCTGCTTG-3′). 5-LO primers have been described in previous publications (Jakobsson et al., 1992). PCR products were separated by gel electrophoresis using 1% agarose. Ethidium bromide (0.5%) was used for visualization, which was recorded using a fluorescence camera (Biovision, Vilber Lourmat, Marne-la-Vallée Cedex, France). PCR experiments were performed in triplicate.
Statistical analysis
All data are presented as mean + SEM. GraphPad Prism version 5.00 (GraphPad Software, San Diego, California, USA) was used for statistical analysis. Data were subjected to one-way anova coupled with Dunnett's post t-test for multiple comparisons. Quantified immunocytochemistry data were subjected to Student's paired t-test. A sigmoidal concentration–response curve fitting model with a variable slope (GraphPad Prism 5.00 software, San Diego California USA) was used to draw the curves following a concentration–inhibition pattern and to calculate IC50 values. Non-sigmoidal data are represented as line drawings from which no IC50 value was calculated.
Drugs, chemical reagents and other materials
Calcium ionophore (A23187), AA, AA-861, BWA4C and 1,25-dihydroxyvitamin D3 were purchased from Sigma-Aldrich (Munich, Germany). 5-HETE, LTB4, LTC4, MK-886, Rev-5901 and zileuton were purchased from Cayman Chemical (Ann Arbor, USA). Glaxo-Smith Kline (London, UK) kindly provided CJ-13,610. WITEGA Laboratorien GmbH (Berlin, Germany) synthesized celecoxib. FCS was purchased from Biochrom AG (Berlin, Germany). DMEM, penicillin and streptomycin were purchased from PAA laboratory GmbH (Pasching, Austria). RPMI 1640 and McCoy's 5A were purchased from Gibco/Invitrogen (Paisley, UK). hTGFβ1 was purified from human platelets (Werz et al., 1996).
Results
Assessment of the effects of different classes of 5-LO inhibitors on the viability of Capan-2 pancreatic cancer cells using the WST-1 assay
Numerous studies have described 5-LO inhibitor-mediated anti-proliferative and cytotoxic effects in cultured tumour cells, with a preferred use of AA-competitive inhibitors, such as AA-861 and Rev-5901. Given that these effects relate to inhibition of 5-LO product formation, all types of 5-LO inhibitors should have the ability to suppress the viability of tumour cells. Representatives for each type of 5-LO inhibitor were used: the redox-type inhibitor AA-861, the non-redox type inhibitors Rev-5901 and CJ-13,610, the iron-ligand inhibitors zileuton and BWA4C as well as the FLAP inhibitor MK-886. Human Capan-2 pancreatic adenocarcinoma cells, which possess a well-documented 5-LO overexpression and susceptibility to 5-LO inhibitors, were employed (Ding et al., 1999). Cells were treated with increasing concentrations of 5-LO inhibitors for 72 h and overall cellular viability was assessed by measuring the metabolic activity of the mitochondria using the WST-1 assay. As expected, Rev-5901, AA-861 and MK-886 concentration-dependently reduced overall viability of the tumour cells with IC50 values of 76 µM, 57 µM and 37 µM respectively (Figure 1A). Surprisingly, the potent 5-LO inhibitors CJ-13,610, BWA4C and zileuton failed to induce similar effects, even at concentrations as high as 100 µM (Figure 1B). Time-dependent experiments using AA-861 and Rev-5901 (100 µM each) further demonstrated that incubation periods of ≥36 h were required to achieve a significant reduction in cell viability (Figure 1C). Notably, a reduction of the overall cellular viability may derive from an anti-proliferative effect of the compounds, which would reduce the total number of viable cells. Alternatively, compounds may directly impair cellular viability by inducing cytotoxic effects via induction of apoptosis or necrosis. Thus, the WST-1 assay does not distinguish between these different types of growth-modulatory effects. IC50 values including confidence intervals can be found in Table 1.
Figure 1.
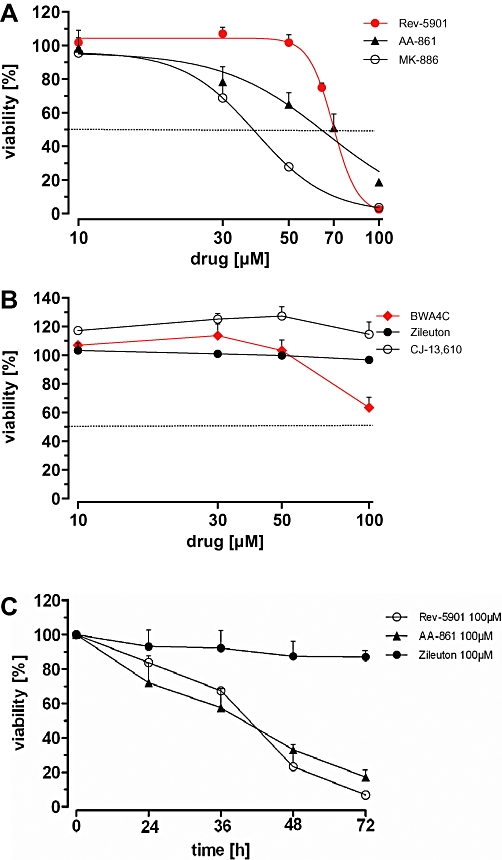
Capan-2 cell viability after treatment with various 5-lipoxygenase inhibitors using the WST-1 proliferation assay. Cells were treated with increasing concentrations (10–100 µM) of Rev-5901, AA-861, MK-886 (A) and BWA4C, CJ-13,610 and zileuton (B) for 72 h and the viability was assessed. (C) Time-dependent effect of Rev-5901, AA-861 and zileuton (100 µM each) on the viability of Capan-2 cells. Values represent mean + SEM of three independent experiments.
Table 1.
IC50 values and confidence intervals of the 5-lipoxygenase inhibitors in the different assay types
| [µM] | AA-861 | BWA4C | CJ-13,610 | MK-886 | Rev-5901 | |
|---|---|---|---|---|---|---|
| Capan-2a | Mean | 57 | n.d. | n.d. | 37 | 76 |
| CI | 32–81 | n.d. | n.d. | 22–52 | 74–78 | |
| Panc-1a | Mean | 27 | n.d. | n.d. | n.d. | 42 |
| CI | 13–41 | n.d. | n.d. | n.d. | 37–47 | |
| Helaa | Mean | 34 | 10 | 43 | 24 | 19 |
| CI | ≤71d | 5–15 | 21–66 | 15–33 | 2–37 | |
| THP-1a | Mean | 40 | n.d. | n.d. | 32 | 46 |
| CI | 0.2–79 | n.d. | n.d. | 2–61 | 34–58 | |
| U937a | Mean | 12 | 29 | 24 | 10 | 17 |
| CI | 5–18 | 19–40 | ≤69d | 6–15 | ≤35d | |
| Capan-2b | Mean | 25 | 13 | n.d. | 15 | 10 |
| CI | 19–31 | 9–18 | n.d. | 11–18 | 8–12 | |
| Capan-2c | Mean | 76 | 23 | n.d. | 24 | 56 |
| CI | 52–100 | 19–26 | n.d. | ≤63d | 36–77 |
IC50 values represent mean of the IC50 values obtained from the three independent experiments (n = 3).
WST-1 assay.
Colony forming assay.
Cell proliferation elisa.
Lower limit of the confidence interval not quoted as below zero.
CI, 95% confidence interval; n.d., not detectable (IC50 > 100 µM).
Investigation of the ability of the 5-LO inhibitors to induce cytotoxic effects in Capan-2 cells
The reduction of tumour cell viability mediated by 5-LO inhibitors has previously been attributed to their ability to induce cytotoxic effects by triggering cell death via the intrinsic pathway of apoptosis (Ghosh and Myers, 1998). Alternatively, 5-LO inhibitors may induce cytotoxic effects by triggering passive cell death (necrosis). To determine whether the disparate effects of the various 5-LO inhibitors in the WST-1 assay (Figure 1) might also be reflected in different pro-apoptotic activities, early stage apoptosis in Capan-2 cells was examined using annexin V staining, which indicates intracellular cytochrome C release. Preliminary experiments revealed an incubation period of 24 h as most applicable, because treatment of the cells for ≥48 h led to severe loss of cell integrity and partial displacement of the cells from the glass cover slips (data not shown). Moreover, the shorter incubation period of 24 h takes into account that cytochrome C release precedes caspase activation and cell death, which may essentially contribute to the reduction in cell viability after 48 and 72 h (Figure 1C). Capan-2 cells were incubated with the different 5-LO inhibitors for 24 h and the cells were then stained with annexin V in the presence of propidium iodide. Annexin V staining was visible for cells treated with AA-861 and Rev-5901, but negative for cells treated with zileuton and CJ-13,610 (Figure 2A). Notably, annexin V staining of cells treated with AA-861 and Rev-5901 was accompanied by intracellular propidium iodide staining, indicating disruption of the cell membrane through necrosis. The right panel of Figure 2A shows the quantification of the fluorescence signals obtained with the different compounds. To confirm the different pro-apoptotic effects of the 5-LO inhibitors, PARP-cleavage was assessed as a specific marker for late apoptosis. PARP is a 112 kDa nuclear protein that is specifically cleaved by activated caspase-3 and caspase-6 into an 89 kDa and 29 kDa fragment. Treatment of Capan-2 cells with MK-886, AA-861 and Rev-5901 for 72 h led to the concentration-dependent cleavage of full-length PARP and an augmented appearance of the 89 kDa cleavage product (Figure 2B), as compared with untreated control cells. Notably, BWA4C, zileuton and CJ-13,610 at 100 µM exerted an opposite effect, by not only failing to enhance PARP cleavage relative to the untreated control, but to preserve the intact PARP (Figure 2B, lower panel). To obtain unequivocal evidence for an induction of necrosis by the 5-LO inhibitors, Capan-2 cells were treated with the compounds for 72 h and the LDH release into the medium was assessed as an index of disturbed membrane integrity (Figure 2C). Treatment with Rev-5901 and MK-886 led to a clear and significant increase in LDH release. BWA4C produced only a weak effect at a high concentration. Zileuton and CJ-13,610 were ineffective. The COX-2 inhibitor celecoxib was used as a cytotoxic control agent due to its documented ability to disrupt cell membrane integrity (Maier et al., 2009). AA-861 potently interfered with the LDH assay, apparently due to its redox activity, and was therefore excluded from this experiment.
Figure 2.
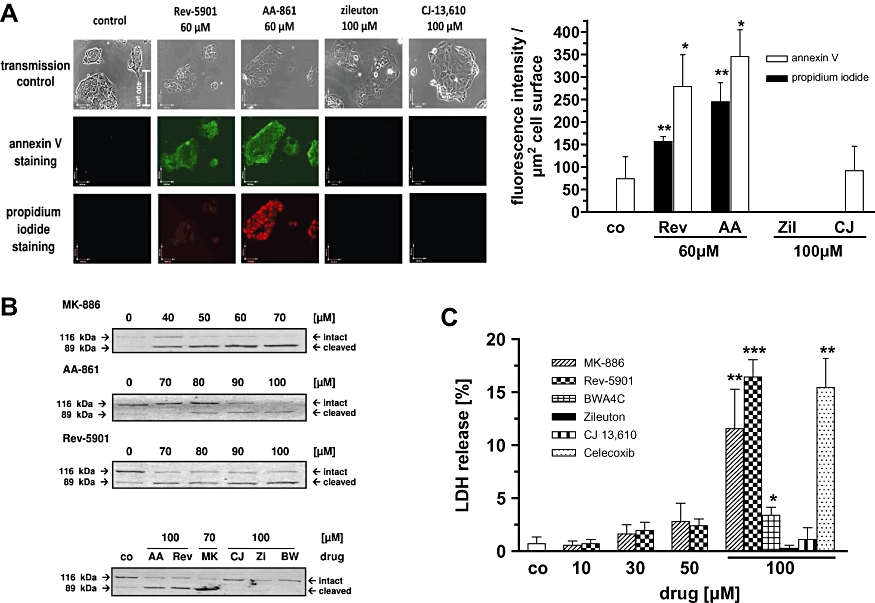
Assessment of the potency of the 5-LO inhibitors to induce cytotoxic effects. (A) Annexin V/propidium iodide co-staining after treatment of Capan-2 cells with various 5-LO inhibitors for 24 h. Capan-2 cells were grown on coverslips and incubated with 5-LO inhibitors or a vehicle control (DMSO) for 24 h. Cells were then incubated for 20 min with a staining solution containing annexin-V-fluorescein and propidium iodide. Coverslips were then fixed on microscope slides and analysed by fluorescence microscopy. One of four independent experiments is shown. The right panel shows the quantification of the fluorescence signals (mean + SEM) obtained from the four experiments. (B) Western blot analysis of PARP cleavage after treatment of Capan-2 cells with increasing concentrations of various 5-LO inhibitors. Cells were treated with MK-886, AA-861, Rev-5901, CJ-13,610, zileuton and BWA4C at the indicated concentrations for 72 h. Thirty micrograms of total protein extract were separated on a 10% SDS-polyacrylamide gel and electroblotted onto a nitrocellulose membrane. Cleaved and intact PARP were detected using a specific antibody. One of four independent experiments is shown. (C) Effect of the 5-LO inhibitors on the release of LDH by Capan-2 cells. Cells were treated with MK-886, Rev-5901, CJ-13,610, zileuton, BWA4C and celecoxib at the indicated concentrations for 72 h and LDH release into the medium was assessed by a commercially available cytotoxicity assay. LDH release induced by a control detergent supplied by the manufacturer was set to 100%. Values represent mean + SEM of three independent experiments. Significant changes versus untreated controls (DMSO) are indicated with an asterisk. 5-LO, 5-lipoxygenase; AA, AA-861; BW, BWA4C; CJ, CJ-13,610; co, control; LDH, lactate dehydrogenase; MK, MK-886; PARP, poly ADP-ribose polymerase; Rev, Rev-5901; Zi, zileuton. *P≤ 0.05; **P≤ 0.01; ***P≤ 0.001.
Assessment of the ability of the 5-LO inhibitors to induce anti-proliferative effects in Capan-2 cells using the BrdU incorporation assay
To evaluate whether drug-related anti-proliferative effects contribute to the reduction of overall cellular viability (Figure 1), a well-established proliferation assay was applied that measures the incorporation of BrdU, a thymidine analogue, into newly synthesized DNA strands of actively proliferating cells during the S phase. Capan-2 cells were treated with increasing concentrations of 5-LO inhibitors for 72 h, and incorporated BrdU was detected immunochemically (Figure 3). Treatment with MK-886 and BWA4C concentration-dependently decreased BrdU incorporation with IC50 values of 24 and 23 µM respectively (Figure 3A). AA-861 and Rev-5901 induced anti-proliferative effects only at higher concentrations (IC50 values >50 µM). CJ-13,610 only had weak effects on DNA synthesis (∼40% reduction at 100 µM). Notably, BrdU incorporation and thus DNA synthesis was not affected by zileuton (Figure 3B). The respective IC50 values including confidence intervals can be found in Table 1.
Figure 3.
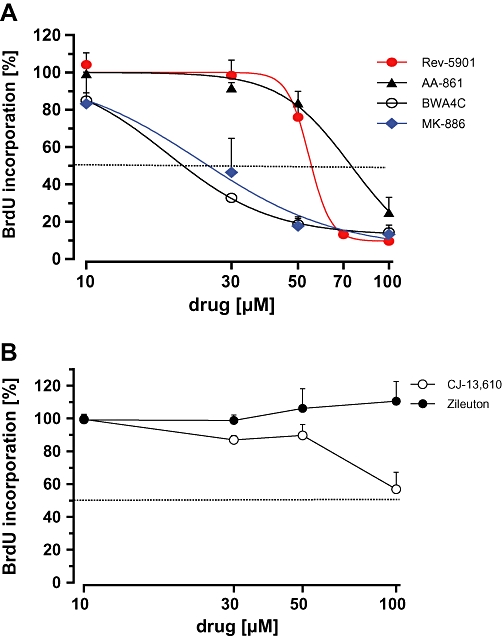
Effect of the 5-lipoxygenase inhibitors on the incorporation of bromodeoxyuridine (BrdU) into Capan-2 DNA. Cells were treated with (A) BWA4C, MK-886, Rev-5901 and AA-861 or (B) CJ-13,610 and zileuton at the indicated concentrations for 72 h and BrdU incorporation was assessed by a commercially available assay. Values represent mean + SEM of three independent experiments.
Effects of the 5-LO inhibitors on Capan-2 colony formation
As the short incubation period of 72 h may account for the lacking or weak cytotoxic and anti-proliferative effects with zileuton and CJ-13,610, Capan-2 cells were treated with increasing concentrations of 5-LO inhibitors for 10 days, followed by assessment of the numbers of colonies formed (Figure 4). Importantly, various drug-related effects may affect colony formation, such as induction of apoptosis, necrosis or cell cycle arrest. Discrimination between these effects is not possible. However, the lack of an effect on colony formation after treatment with a drug strongly points to the absence of a cytotoxic and anti-proliferative activity. Long-term treatment did increase the growth-inhibitory potency of AA-861 (IC50 = 25 µM), Rev-5901 (IC50 = 10 µM) and MK-886 (IC50 = 15 µM), as expected. Under the same conditions, BWA4C suppressed the number of Capan-2 colonies formed (IC50 = 13 µM). However, CJ-13,610 reduced colony formation by ∼50% while zileuton, at concentrations up to 50 µM, failed to induce any effects. The IC50 values for the inhibition of colony formation, including confidence intervals, are summarized in Table 1.
Figure 4.
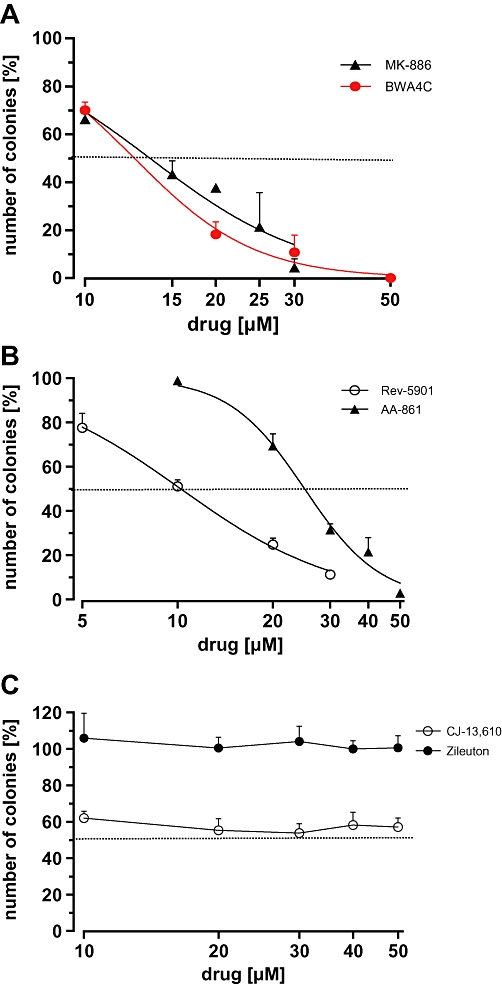
Determination of colony formation of Capan-2 cells after treatment with various 5-lipoxygenase inhibitors. Cells were seeded at a density of 103 cells per dish and incubated with increasing concentrations of (A) MK-886 and BWA4C, (B) Rev-5901 and AA-861 or (C) CJ-13,610 and zileuton for 10 days. The number of colonies formed was assessed and expressed relative to the number of colonies in the vehicle control (100%). Values represent mean + SEM of three independent experiments.
Determination of the potency of the 5-LO inhibitors to suppress 5-LO activity
The contrasting cytotoxic and anti-proliferative activities of the 5-LO inhibitors could potentially be due to different inhibitory potencies for suppression of 5-LO activity. This possibility was addressed by measuring 5-LO enzyme activity in intact Capan-2 cells after treatment with the 5-LO inhibitors, which would allow a direct comparison of 5-LO-inhibitory potency and anti-proliferative and cytotoxic potential. Unfortunately, 5-LO product concentrations in the Capan-2 medium were below the detection limit (<50 pg·mL−1 for LTB4 and 5-HETE) for LC/MS-MS (liquid chromatography coupled with tandem mass spectrometry) analysis (data not shown, for description of LC/MS-MS see Maier et al., 2008). Stimulation of the cells with the Ca2+-ionophore A23187 in the presence of AA (a standard stimulus of 5-LO) also failed to trigger 5-LO product formation (data not shown). Leukaemic THP-1 cells, with a documented 5-LO expression and inducible 5-LO activity, were therefore tested as an alternative (Riddick et al., 1997). Cells were preincubated with the 5-LO inhibitors for 10 min. and 5-LO product formation was initiated by treatment with 5 µM A23187 in presence of 10 µM AA. All incubations received 10% FCS to allow a direct comparison with the IC50 values of the viability and colony forming assays (Figures 1 and 4). With the exception of Rev-5901, all of the 5-LO inhibitors potently suppressed 5-LO product formation by THP-1 cells with IC50 values below 1 µM (Figure 5 and Table 2). To allow direct comparisons, the IC50 values of the various 5-LO inhibitors for reduction of THP-1 viability were determined (Table 1).
Figure 5.
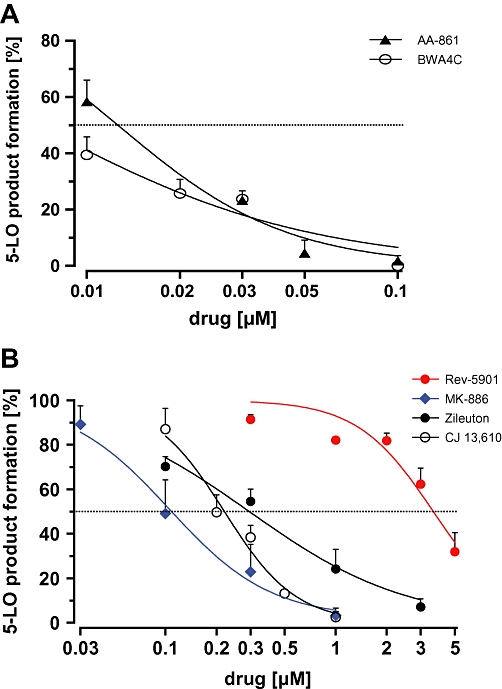
Effect of the 5-LO inhibitors on 5-LO product formation in THP-1 leucocytes. Cells were preincubated with (A) AA-861 and BWA4C or (B) Rev-5901, MK-886, zileuton and CJ-13,610 for 15 min in the presence of 10% FCS. 5-LO product formation was triggered by addition of A23187 (final 5 µM) and AA (final 10 µM). Downstream 5-LO products (LTB4, 5-HETE) were analysed via reverse phase HPLC. Values represent mean + SEM of three independent experiments. 5-LO product formation in untreated control was 50.21 ± 6 ng per 106 cells. 5-HETE, 5(S)-hydroxy-6-trans-8,11,14-cis-eicosatetraenoic acid; 5-LO, 5-lipoxygenase; AA, arachidonic acid; FCS, fetal calf serum.
Table 2.
IC50 values and confidence intervals for enzyme inhibition of the different 5-lipoxygenase inhibitors in intact THP-1 cells
| [µM] | AA-861 | BWA4C | CJ-13,610 | MK-886 | Rev-5901 | Zileuton |
|---|---|---|---|---|---|---|
| Mean | 0.014 | 0.0064 | 0.21 | 0.15 | 4.2 | 0.3 |
| CI | 0.0044–0.024 | ≤0.014a | 0.05–0.36 | 0.018–0.29 | 1.1–7.2 | 0.24–0.35 |
IC50 values represent mean of the IC50 values obtained from the three independent experiments (n = 3).
Lower limit of the confidence interval not quoted as below zero.
CI, 95% confidence interval.
Effect of addition of 5-LO products on the 5-LO inhibitor induced reduction of tumour cell viability
Due to the difference in 5-LO inhibitor concentrations required to induce cell death and anti-proliferative effects compared with the concentrations needed to suppress 5-LO enzyme activity, 5-LO-independent molecular mechanisms were considered as possible causes of the observed cytotoxic and anti-proliferative effects. Increasing concentrations of the 5-LO products 5-HETE and LTB4 were therefore added to 5-LO inhibitor-treated Capan-2 cells, as these metabolites were reported to be the primary determinants of the 5-LO-mediated mitogenicity in pancreatic cancer cells (Ding et al., 2003; Tong et al., 2005). To exclude the involvement of CysLTs, we supplemented with high concentrations of LTC4 (Figure 6). However, the reduction of overall cellular viability induced by Rev-5901 (Figure 6A) or AA-861 (Figure 6B) was not significantly attenuated by the 5-LO products, despite the use of rather high concentrations (500 ng·mL−1 each). Notably, the addition of 5-LO products partially restored the inhibition of cellular viability observed in the presence of 100 µM MK-886 (Figure 6A). To clarify this potential effect, MK-886 was used at a lower concentration of 40 µM, close to the drug's IC50 for reduction of cell viability (Figure 1) and 5-LO products were again added. However, addition of the products still produced no significant effects on the MK-886-induced impairment of cell viability (Figure 6C).
Figure 6.
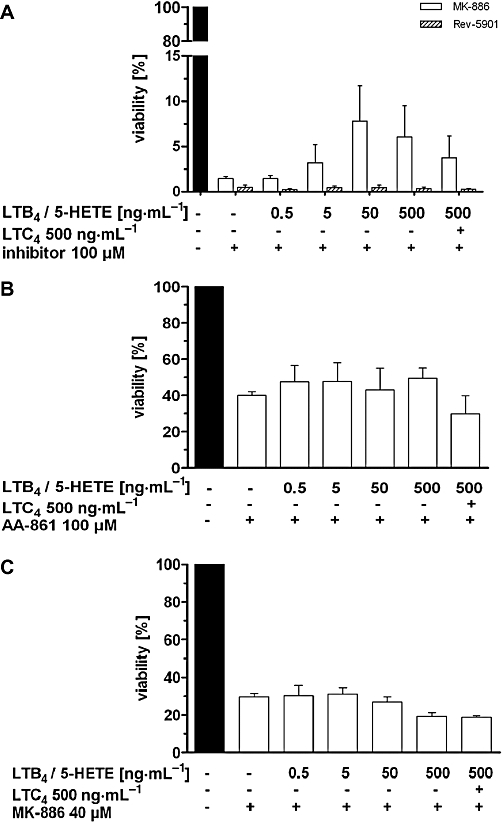
Effect of exogenously added 5-LO products (5-HETE, LTB4 and LTC4) on the viability of Capan-2 cells treated with different 5-LO inhibitors. Cells were co-incubated with (A, C) MK-886, Rev-5901 or (B) AA-861 at the concentrations indicated and increasing concentrations of mitogenic 5-LO products for 72 h. Media were replaced daily by fresh medium containing the inhibitors and 5-LO products due to chemical instability of the leukotrienes and 5-HETE, as determined by LC/MS-MS. Cell viability was measured using the WST-1 assay (Rev-5901, MK-886) or by assessing the number of viable cells using trypan blue staining (AA-861). Values represent mean + SEM of four independent experiments. 5-HETE, 5(S)-hydroxy-6-trans-8,11,14-cis-eicosatetraenoic acid; 5-LO, 5-lipoxygenase; LC/MS-MS, liquid chromatography coupled with tandem mass spectrometry.
Effect of the 5-LO inhibitors on the viability of 5-LO-negative tumour cells using the WST assay
To obtain unequivocal evidence that 5-LO inhibitors reduce tumour cell viability independently of suppression of 5-LO product formation, the effects of 5-LO inhibitors in tumour cell lines lacking 5-LO were tested. The different 5-LO expression levels were confirmed by Western blot analysis (Figure 7A) and RT-PCR (Figure 7B). The myeloid lineage U937 is well documented as being deficient in 5-LO, due to hypermethylation of the 5-LO promoter (Kargman et al., 1993; Uhl et al., 2002), and is morphologically comparable with the 5-LO-positive THP-1 leucocytes. The pancreatic adenocarcinoma cell line Panc-1 has previously been described as expressing 5-LO (Hennig et al., 2005), but when grown under the current cell culture conditions and analysed by Western blot using three different 5-LO antibodies (Figure 7A shows the experiment with the rabbit polyclonal Biolipox antibody, other blots were not shown) and with RT-PCR, no expression could be detected. Additionally, we utilized HeLa cervix carcinoma cells, which possess a well-documented deficiency in 5-LO expression (Fischer et al., 2003; Sabirsh et al., 2005).
Figure 7.
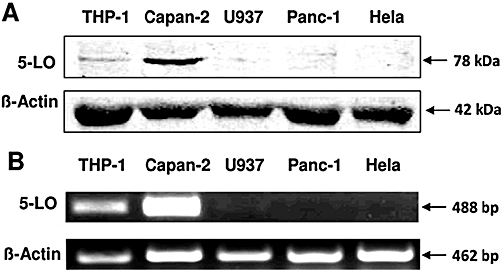
Analysis of 5-lipoxygenase (5-LO) expression in Capan-2, THP-1, Panc-1, U937 and HeLa cells. (A) Thirty micrograms of total protein extract was loaded onto a 12% SDS-polyacrylamide gel and electroblotted onto a nitrocellulose membrane. 5-LO protein expression was determined using a specific polyclonal antibody raised against the N-terminus of 5-LO. Equal loading of the gel was checked by reprobing the membrane with a β-actin antibody. (B) For RT-PCR, RNA from the cell lines was extracted using a purification kit and reverse transcription was performed with an iScript™ cDNA synthesis kit. PCR reaction was carried out with an iQ™ supermix kit and specific primers for 5-LO and β-actin. PCR products were separated via 1% agarose gel electrophoresis, and ethidiumbromide-labelled DNA bands were visualized using a fluorescence camera. The results from one of three independent experiments are shown.
Similar to the results obtained with Capan-2 cells (Figure 1A), Rev-5901 and AA-861 caused a concentration-dependent suppression of Panc-1 viability (Figure 8A). By contrast, zileuton, CJ-13,610 and MK-886 produced no or only weak effects. BWA4C, surprisingly, slightly increased the overall viability of the tumour cells. Furthermore, all 5-LO inhibitors, except zileuton, potently suppressed cell viability in U937 cells (Figure 8B). The IC50 values of the 5-LO inhibitors for the reduction of tumour cell viability in all tumour cell lines tested are summarized in Table 1.
Figure 8.
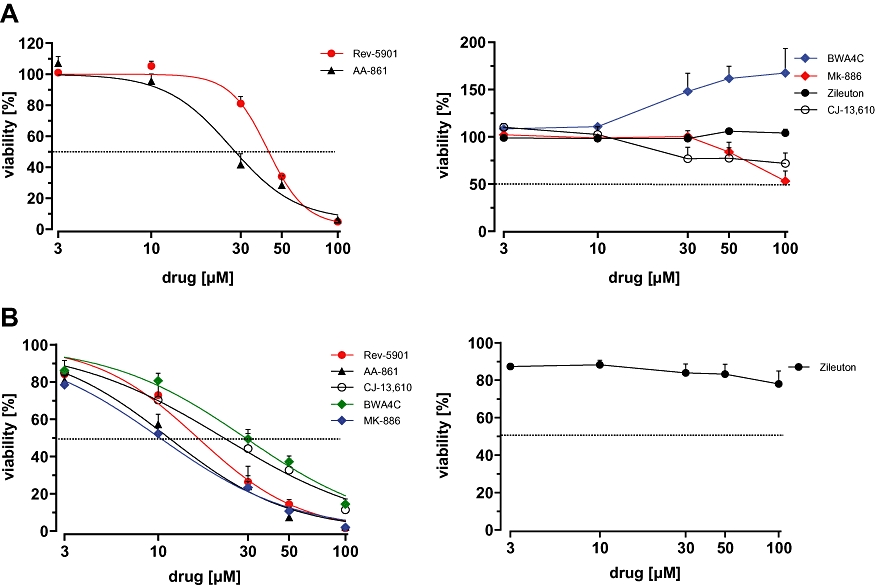
Determination of Panc-1 and U937 cell viability using the WST-1 assay. Panc-1 (A) and U937 (B) cells were treated for 48 h with increasing concentrations of 5-lipoxygenase inhibitors as indicated and the viabilities were assessed. Values represent mean + SEM of three independent experiments.
Discussion and conclusions
Numerous studies have described 5-LO inhibitor-induced cytotoxic and anti-proliferative effects in cultured tumour cells as an important basis for the involvement of 5-LO in tumourigenesis. This report demonstrates, for the first time, that the frequently used 5-LO inhibitors AA-861, Rev-5901, BWA4C and CJ-13,610 can reduce the viability of different tumour cells independently of suppression of 5-LO product formation.
The hypothesis of a 5-LO-independent mechanism for cytotoxicity and anti-proliferation was substantiated using several independent experimental approaches. First, the various 5-LO inhibitors were shown to possess different abilities to reduce the viability of cultured 5-LO-positive Capan-2 pancreas carcinoma cells. We found that treatment of the tumour cells with AA-861, MK-886 and Rev-5901 led to an induction of both cytotoxic and anti-proliferative effects. By contrast, CJ-13,610 and BWA4C produced no or only marginal cytotoxic effects, but impaired DNA synthesis, which may explain the drug's suppressive potency in the long-term colony forming assay (10 days) and the lacking, or weak effects, in assays with shorter incubation periods (e.g. WST-1 assay, 72 h). Notably, zileuton, the only commercially available 5-LO inhibitor, failed to induce any anti-proliferative or cytotoxic effects in all types of tumour cells employed in this study. Interestingly, in the PARP cleavage experiment (Figure 2B), CJ-13,610, BWA4C and zileuton preserved the intact PARP protein suggesting that these compounds could have anti-apoptotic effects in this context, although the molecular mechanism remains unclear and needs further investigation. Second, the IC50 values of the inhibitors for induction of cytotoxic and anti-proliferative effects in Capan-2 and THP-1 cells exceeded the respective IC50 values for inhibition of 5-LO enzyme activity by more than 20-fold (Rev-5901) and up to 5000-fold (AA-861) (Table 2). Third, the addition of mitogenic 5-LO products failed to abolish the 5-LO inhibitor-induced reduction of Capan-2 cell viability. Furthermore, the rather low amounts of 5-LO products found (<50 pg·mL−1 5-HETE and LTB4 and below the LC/MS-MS detection limit) in the Capan-2 supernatant suggested that these metabolites would probably not regulate tumour cell viability to a significant degree. Lastly, three 5-LO-negative tumour cell lines (Panc-1, HeLa and U937) exhibited a higher susceptibility towards the 5-LO inhibitors than their morphologically related 5-LO-positive counterparts (Capan-2, THP-1). Collectively, the present findings strongly support the notion that 5-LO-independent mechanisms constitute the basis for the cytotoxic and anti-proliferative effects of the 5-LO inhibitors used in this study.
Ghosh and Myers were among the first to demonstrate that 60 µM of AA-861 abolishes the AA stimulated increase of prostate cancer cell growth (Ghosh and Myers, 1997) and that inhibition of 5-LO by 10 µM MK-886 triggers severe apoptosis in human prostate cancer cells (Ghosh and Myers, 1998). Subsequent studies showed that AA-861 is capable of suppressing the growth of oesophageal cancer cells in vitro with an IC50 value of 30–60 µM (Hoque et al., 2005) and the proliferation of MCF-7 breast cancer cells with an IC50 value of approximately 20 µM (Hammamieh et al., 2007). AA-861 has been shown to have similar effects in colorectal cancer cells (Ihara et al., 2007). A reduction of serum in the growth medium or the use of serum-free conditions understandably led to higher anti-proliferative and cytotoxic potencies for AA-861 (IC50 of 8–61 µM) and Rev-5901 (IC50 of 5–50 µM) in these studies (Ding et al., 1999; Tong et al., 2002; Titos et al., 2003; Hayashi et al., 2006; Melstrom et al., 2008; Sveinbjornsson et al., 2008). Together, the anti-proliferative and cytotoxic potencies of AA-861, Rev-5901 and the other 5-LO inhibitors determined in this study are in agreement with the effects of the drugs reported by others. Thus, the data indicating that anti-proliferation and cytotoxicity may derive, at least partially, from 5-LO-independent mechanisms should be broadly applicable.
Several previous studies support the findings of the present work. Datta et al. (1999) were the first to provide evidence that MK-886, a FLAP inhibitor, may induce apoptosis independently of FLAP, which is in line with the reactivity of MK-886 observed in our assays. More recently, Sabirsh et al. (2005) described 5-LO-independent effects of various LT synthesis inhibitors on Ca2+ signalling in 5-LO-deficient HeLa carcinoma cells. Finally, Hennig et al. (2005) observed similar growth-inhibitory effects of Rev-5901 that were independent of whether the NIH-3T3 cells expressed 5-LO or not.
Given that some 5-LO inhibitors induce cytotoxic and anti-proliferative effects independently of suppression of 5-LO product formation, addition of exogenous 5-LO products should fail to protect tumour cells from 5-LO inhibitor-induced reduction in cell viability, as demonstrated in the present study. However, several studies reported attenuated anti-proliferation through the addition of 5-LO products. The extreme concentrations required to observe the effect stand as one caveat to these experiments, as the final concentration of 5-LO products had to be up to 10 000-fold greater than those in the medium of untreated cells (Ghosh and Myers, 1998; Hoque et al., 2005; Sveinbjornsson et al., 2008). The cytoprotective effects of high concentrations of supplemented 5-LO products may be due to the ability of 5-HETE and LTB4 to increase the cell proliferation and viability by activating the mitogenic and anti-apoptotic MAPK and Akt kinase signalling pathways (Ding et al., 2003; Tong et al., 2005). It is therefore reasonable to postulate that high concentrations of 5-LO products could counteract diverse pro-apoptotic stimuli by mechanisms not directly related to inhibition of 5-LO.
The primary goal of this study was to address the question of indirect mechanisms associated with 5-LO inhibitors, and, importantly, not to question the role of 5-LO in the proliferation of malignant or non-tumour cells in general. For example, the growth of freshly isolated murine neuronal stem cells, which produce considerable basal amounts of LTB4 (∼7 ng per 106 cells), was suppressed by AA-861 at concentrations of less than 1 µM (Wada et al., 2006). Furthermore, zileuton, the only drug devoid of anti-proliferative and cytotoxic off-target effects in the present study, was capable of inhibiting the growth of RAW macrophages at concentrations below 1 µM. The macrophages synthesized the 5-LO product LTB4 and addition of physiologically relevant concentrations of LTB4 reversed the growth-inhibitory effects of zileuton (Wada et al., 2006). Several studies have shown moderately reduced cell proliferation rates due to down-regulation of 5-LO expression by antisense approaches (Ding et al., 1999; Uz et al., 2001; Walker et al., 2002; Sveinbjornsson et al., 2008). As stated by the authors, the extent of mitogenic effects of 5-LO that originate from its enzymatic activity remains unclear. Interestingly, non-enzymatic functions, including an interaction with cytoskeleton proteins or with the adaptor protein GRb2, involved in growth–factor signalling, have been reported (Lepley and Fitzpatrick, 1994) and may contribute to the decrease in cell proliferation through down-regulation of 5-LO expression. Together, this evidence suggests that 5-LO products have mitogenic functions in tumour as well as non-tumour cells, but this effect appears to be restricted to certain cell types.
Several conclusions with relevance to future research on 5-LO can be drawn from the present study: (i) the anti-proliferative and cytotoxic effects of 5-LO inhibitors are substance-specific and may include 5-LO-independent mechanisms. The molecular non-5-LO targets are of interest and warrant further elucidation as novel pharmacological strategies for potent suppression of tumour cell growth. However, results from experiments on the role of 5-LO in proliferation of tumour cells using high concentrations (>1 µM) of 5-LO inhibitors may be misleading; (ii) Zileuton appears to be the most applicable drug for studying anti-proliferative and cytotoxic effects deriving from 5-LO inhibition, because it is the only agent devoid of interfering off-target effects. However, growth-inhibitory activities of zileuton in cell culture assays will require a certain susceptibility of the cells towards 5-LO products as already observed with macrophages (Wada et al., 2006); and (iii) several tumour cells, such as Capan-2, overexpress 5-LO, but suppression of enzymatic activity by zileuton or other selective 5-LO inhibitors, such as CJ-13,610, produced no or only marginal effects on cell viability. Thus, the role of 5-LO overexpression in these types of tumour cells remains unclear. Further studies are therefore required that address both indirect tumourigenic effects, such as promotion of angiogenesis in vivo as well as possible non-enzymatic functions of 5-LO, such as interactions of the protein with components of cellular signalling cascades or the cytoskeleton.
Acknowledgments
The authors thank Dr Wesley McGinn-Straub for the linguistic revision of the manuscript, Professor Dr Rolf Marschalek and Irina Eberle (Goethe-University, Frankfurt) for technical support with the fluorescence microscope and Professor Dr Heiko Mühl (Goethe-University, Frankfurt) for editing the manuscript. Furthermore, the authors thank Glaxo-Smith Kline (London, UK) for kindly providing CJ-13,610. This work was supported by the German Graduate School ‘Biologicals’ (DFG GRK 1172); by Merck KGaA, Darmstadt, Germany; by the ‘Landes Offensive zur Entwicklung Wissenschaftlich Ökonomischer Exzellenz (LOEWE)/Lipid Signaling Forschungszentrum Frankfurt’ (LiFF), and by the German Excellence Cluster ‘Cardio-Pulmonary System’ (ECCPS).
Glossary
Abbreviations
- 5-HETE
5(S)-hydroxy-6-trans-8,11,14-cis-eicosatetraenoic acid
- 5-HPETE
5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid
- 5-LO
5-lipoxygenase
- AA
arachidonic acid
- BrdU
bromodeoxyuridine
- FCS
fetal calf serum
- FLAP
5-lipoxygenase-activating protein
- LC/MS-MS
liquid chromatography coupled with tandem mass spectrometry
- LDH
lactate dehydrogenase
- LT
leukotriene
- PARP
poly ADP-ribose polymerase
- PGB1
prostaglandin B1
Conflict of interest
The authors state no conflict of interest.
Supplemental material
References
- Abramovitz M, Wong E, Cox ME, Richardson CD, Li C, Vickers PJ. 5-lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur J Biochem. 1993;215:105–111. doi: 10.1111/j.1432-1033.1993.tb18012.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Sood S, Yang CS, Li N, Sun Z. Five-lipoxygenase pathway of arachidonic acid metabolism in carcino-genesis and cancer chemoprevention. Curr Cancer Drug Targets. 2006;6:613–622. doi: 10.2174/156800906778742451. [DOI] [PubMed] [Google Scholar]
- Datta K, Biswal SS, Kehrer JP. The 5-lipoxygenase-activating protein (FLAP) inhibitor, MK886, induces apoptosis independently of FLAP. Biochem J. 1999;340(Pt 2):371–375. [PMC free article] [PubMed] [Google Scholar]
- Ding XZ, Iversen P, Cluck MW, Knezetic JA, Adrian TE. Lipoxygenase inhibitors abolish proliferation of human pancreatic cancer cells. Biochem Biophys Res Commun. 1999;261:218–223. doi: 10.1006/bbrc.1999.1012. [DOI] [PubMed] [Google Scholar]
- Ding XZ, Tong WG, Adrian TE. Multiple signal pathways are involved in the mitogenic effect of 5(S)-HETE in human pancreatic cancer. Oncology. 2003;65:285–294. doi: 10.1159/000074640. [DOI] [PubMed] [Google Scholar]
- Fischer L, Szellas D, Radmark O, Steinhilber D, Werz O. Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 2003;17:949–951. doi: 10.1096/fj.02-0815fje. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson AW, Gresser M, Young RN. 5-Lipoxygenase. Annu Rev Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Myers CE. Arachidonic acid stimulates prostate cancer cell growth: critical role of 5-lipoxygenase. Biochem Biophys Res Commun. 1997;235:418–423. doi: 10.1006/bbrc.1997.6799. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammamieh R, Sumaida D, Zhang X, Das R, Jett M. Control of the growth of human breast cancer cells in culture by manipulation of arachidonate metabolism. BMC Cancer. 2007;7:138. doi: 10.1186/1471-2407-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Nishiyama K, Shirahama T. Inhibition of 5-lipoxygenase pathway suppresses the growth of bladder cancer cells. Int J Urol. 2006;13:1086–1091. doi: 10.1111/j.1442-2042.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- Hennig R, Grippo P, Ding XZ, Rao SM, Buchler MW, Friess H, et al. 5-Lipoxygenase, a marker for early pancreatic intraepithelial neoplastic lesions. Cancer Res. 2005;65:6011–6016. doi: 10.1158/0008-5472.CAN-04-4090. [DOI] [PubMed] [Google Scholar]
- Hoque A, Lippman SM, Wu TT, Xu Y, Liang ZD, Swisher S, et al. Increased 5-lipoxygenase expression and induction of apoptosis by its inhibitors in esophageal cancer: a potential target for prevention. Carcinogenesis. 2005;26:785–791. doi: 10.1093/carcin/bgi026. [DOI] [PubMed] [Google Scholar]
- Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- Jakobsson PJ, Steinhilber D, Odlander B, Radmark O, Claesson HE, Samuelsson B. On the expression and regulation of 5-lipoxygenase in human lymphocytes. Proc Natl Acad Sci USA. 1992;89:3521–3525. doi: 10.1073/pnas.89.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargman S, Rousseau P, Reid GK, Rouzer CA, Mancini JA, Rands E, et al. Leukotriene synthesis in U937 cells expressing recombinant 5-lipoxygenase. J Lipid Mediat. 1993;7:31–45. [PubMed] [Google Scholar]
- Lepley RA, Fitzpatrick FA. 5-Lipoxygenase contains a functional Src homology 3-binding motif that interacts with the Src homology 3 domain of Grb2 and cytoskeletal proteins. J Biol Chem. 1994;269:24163–24168. [PubMed] [Google Scholar]
- Maier TJ, Tausch L, Hoernig M, Coste O, Schmidt R, Angioni C, et al. Celecoxib inhibits 5-lipoxygenase. Biochem Pharmacol. 2008;76:862–872. doi: 10.1016/j.bcp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Maier TJ, Schiffmann S, Wobst I, Birod K, Angioni C, Hoffmann M, et al. Cellular membranes function as a storage compartment for celecoxib. J Mol Med. 2009;87:981–993. doi: 10.1007/s00109-009-0506-8. [DOI] [PubMed] [Google Scholar]
- Mancini JA, Abramovitz M, Cox ME, Wong E, Charleson S, Perrier H, et al. 5-lipoxygenase-activating protein is an arachidonate binding protein. FEBS Lett. 1993;318:277–281. doi: 10.1016/0014-5793(93)80528-3. [DOI] [PubMed] [Google Scholar]
- Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, Strouch M, et al. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14:6525–6530. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- Musser JH, Chakraborty UR, Sciortino S, Gordon RJ, Khandwala A, Neiss ES, et al. Substituted arylmethyl phenyl ethers. 1. A novel series of 5-lipoxygenase inhibitors and leukotriene antagonists. J Med Chem. 1987;30:96–104. doi: 10.1021/jm00384a017. [DOI] [PubMed] [Google Scholar]
- Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Riddick CA, Ring WL, Baker JR, Hodulik CR, Bigby TD. Dexamethasone increases expression of 5-lipoxygenase and its activating protein in human monocytes and THP-1 cells. Eur J Biochem. 1997;246:112–118. doi: 10.1111/j.1432-1033.1997.00112.x. [DOI] [PubMed] [Google Scholar]
- Sabirsh A, Bristulf J, Karlsson U, Owman C, Haeggstrom JZ. Non-specific effects of leukotriene synthesis inhibitors on HeLa cell physiology. Prostaglandins Leukot Essent Fatty Acids. 2005;73:431–440. doi: 10.1016/j.plefa.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsson B, Rasmuson A, Baryawno N, Wan M, Pettersen I, Ponthan F, et al. Expression of enzymes and receptors of the leukotriene pathway in human neuroblastoma promotes tumor survival and provides a target for therapy. FASEB J. 2008;22:3525–3536. doi: 10.1096/fj.07-103457. [DOI] [PubMed] [Google Scholar]
- Titos E, Claria J, Planaguma A, Lopez-Parra M, Villamor N, Parrizas M, et al. Inhibition of 5-lipoxygenase induces cell growth arrest and apoptosis in rat Kupffer cells: implications for liver fibrosis. FASEB J. 2003;17:1745–1747. doi: 10.1096/fj.02-1157fje. [DOI] [PubMed] [Google Scholar]
- Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2002;1:929–935. [PubMed] [Google Scholar]
- Tong WG, Ding XZ, Talamonti MS, Bell RH, Adrian TE. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res Commun. 2005;335:949–956. doi: 10.1016/j.bbrc.2005.07.166. [DOI] [PubMed] [Google Scholar]
- Uhl J, Klan N, Rose M, Entian KD, Werz O, Steinhilber D. The 5-lipoxygenase promoter is regulated by DNA methylation. J Biol Chem. 2002;277:4374–4379. doi: 10.1074/jbc.M107665200. [DOI] [PubMed] [Google Scholar]
- Uz T, Manev R, Manev H. 5-Lipoxygenase is required for proliferation of immature cerebellar granule neurons in vitro. Eur J Pharmacol. 2001;418:15–22. doi: 10.1016/s0014-2999(01)00924-4. [DOI] [PubMed] [Google Scholar]
- Wada K, Arita M, Nakajima A, Katayama K, Kudo C, Kamisaki Y, et al. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- Walker JL, Loscalzo J, Zhang YY. 5-Lipoxygenase and human pulmonary artery endothelial cell proliferation. Am J Physiol Heart Circ Physiol. 2002;282:H585–H593. doi: 10.1152/ajpheart.00003.2001. [DOI] [PubMed] [Google Scholar]
- Werz O, Steinhilber D. Selenium-dependent peroxidases suppress 5-lipoxygenase activity in B-lymphocytes and immature myeloid cells. The presence of peroxidase-insensitive 5-lipoxygenase activity in differentiated myeloid cells. Eur J Biochem. 1996;242:90–97. doi: 10.1111/j.1432-1033.1996.0090r.x. [DOI] [PubMed] [Google Scholar]
- Werz O, Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Werz O, Brungs M, Steinhilber D. Purification of transforming growth factor beta 1 from human platelets. Pharmazie. 1996;51:893–896. [PubMed] [Google Scholar]
- Yoshimoto T, Yokoyama C, Ochi K, Yamamoto S, Maki Y, Ashida Y, et al. 2,3,5-Trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone (AA861), a selective inhibitor of the 5-lipoxygenase reaction and the biosynthesis of slow-reacting substance of anaphylaxis. Biochim Biophys Acta. 1982;713:470–473. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


