Abstract
Distinct lymphocyte populations have been identified that either promote or impede the establishment of chimerism and tolerance through allogeneic bone marrow transplantation (BMT). NKT cells have pleiotropic regulatory properties capable of either augmenting or downmodulating various immune responses. We investigated here whether NKT cells affect outcome in mixed chimerism models employing fully mismatched non-myeloablative BMT with costimulation blockade (CB). The absence of NKT cells had no detectable effect on chimerism or skin graft tolerance after conditioning with 3Gy total body irradiation (TBI), and a limited positive effect with 1Gy TBI. Stimulation of NKT cells with alpha-galactosylceramide (alpha-gal) at the time of BMT prevented chimerism and tolerance. Activation of recipient (as opposed to donor) NKT cells was necessary and sufficient for the alpha-gal effect. The detrimental effect of NKT activation was also observed in the absence of T cells after conditioning with in vivo T cell depletion (TCD). NKT cells triggered rejection of BM via NK cells as chimerism and tolerance were not abrogated when NKT cells were stimulated in the absence of both NK cells and T cells. Thus, activation of NKT cells at the time of BMT overcomes the effects of CB, inhibiting the establishment of chimerism and tolerance.
Keywords: mixed chimerism, tolerance, costimulation blockade, NKT cells
INTRODUCTION
Mixed chimerism established through transplantation of donor BM is an experimental strategy for achieving donor-specific transplantation tolerance whose clinical potential has recently been underscored by results from clinical pilot series (1-3). Previous mixed chimerism protocols relied on the complete and non-specific elimination of the recipient’s T cell repertoire before or at the time allogeneic BMT (e.g. through in vivo T cell depletion) (4-7). The introduction of costimulation blockers as part of non-myeloablative BMT has obviated the need for global recipient TCD (8-11). The mechanisms of tolerance induction in these CB-based protocols are distinct from TCD-based regimens, in particular by involving a more important role for regulatory mechanisms (12;13). More recently, recipient conditioning has been further minimized in CB-based models (14-16). Under limiting conditioning, the remaining immune barrier becomes a critical factor impeding engraftment, as a substantial fraction of transplanted BM is still rejected in vivo (17). Notably, several publications have reported that NK cells impede BM engraftment under such conditions (17;18). These studies, however, did not investigate in detail the role of NKT cells.
NKT cells are required for tolerance induction in a cyclophosphamide-based mixed chimerism model (without CB) employing an MHC-matched, minor antigen-mismatched donor-recipient combination (19). Likewise, NKT cells were critically involved in the induction of chimerism and tolerance following conditioning with TCD plus fractionated lymphoid irradiation (6) and had a protective effect against GVHD in this system (20). By contrast, activation of NKT cells with alpha-gal in another, TBI-based, model exacerbated GVHD (21). The potential role of NKT cells and the effects of NKT activation in CB-based mixed chimerism, however, remain to be defined.
NKT cells have potent and diverse regulatory functions capable of both enhancing and abrogating immune responses (22). Both endogenous and exogenous ligands activating NKT cells have been identified (23-26). NKT cells play an important role in models of autoimmune disease, tumor surveillance and transplantation tolerance (22). Their specific stimulation with the synthetic glycolipid α-galactosylceramide (alpha-gal) can either promote or abrogate immune tolerance depending on the specific model. Alpha-gal induces a massive production of cytokines by NKT cells activating cells of the innate und adaptive immune system. Activation of NKT cells by alpha-gal prevents the onset and recurrence of autoimmune type I diabetes (27) and of experimental autoimmune encephalomyelitis (28), leads to an augmented antitumor response (29), but also causes disease aggravation in arthritis (30) and atherosclerosis models (31).
With regard to organ and tissue transplantation, recipients deficient in NKT cells are resistant to the graft-prolonging effect of anti-B7 mAbs in an allogeneic murine heart transplant model (32) and also resistant to the tolerizing effect of anti-CD4 in a rat xeno-islet model (33). An important role of NKT cells for long-term survival of corneal allografts (34) and the spontaneous acceptance of liver grafts (35) has also been reported. On the other hand, NKT cells contribute to the rejection of murine islet allografts (36) and augment the alloresponse in vitro (37). Thus, NKT cells are capable of promoting tolerance and rejection of grafts, respectively, depending on the specifics of the model investigated (38;39).
In the present study we found that the lack of NKT cells has little effect on the induction of chimerism and tolerance after fully mismatched non-myeloablative BMT with CB, but that the deliberate activation of NKT cells, in contrast, prevents BM engraftment and skin graft tolerance despite CB. This detrimental effect of NKT stimulation is dependent on NK cells but not on T cells.
MATERIALS & METHODS
Animals
Female Balb/c (H-2d), C57BL/6 (B6: H-2b) and C3H/N (H-2k) mice were purchased from the Charles River Laboratories (Sulzfeld, Germany). All mice were kept under specific pathogen-free conditions. NKT knock-out mice (Jα281−/−) on C57BL/6 background and Balb/c background (designated in this manuscript as B6.NKT-KO and Balb/c.NKT-KO, respectively) were generated and provided by Dr. M. Taniguchi (40) and further bred at the Institute for Biomedical Research, Vienna. Absence of Jα281−/− was verified by PCR in breeding pairs. All experiments were approved by the local review board of the Medical University of Vienna and were performed in accordance with national and international guidelines of laboratory animal care.
BMT protocol
Wild-type or NKT knock-out B6 hosts received a non-myeloablative dose (1 or 3 Gy, as indicated; 0.9Gy/min, day −1) of TBI approx. 24 hours prior to being injected with 15-20×106 unseparated bone marrow cells (BMC) harvested from the tibiae, femurs, humeri and pelvis of female wild-type or NKT knock-out Balb/c donors (6 to 12 weeks old), as indicated. Cells were diluted in cold BM media (medium 199 [Sigma, Vienna, Austria]) and were injected in a volume of 0.5 or 1 ml into the tail vein of recipient mice on day 0. In addition recipients were treated with a hamster anti-mouse CD154 (CD40L) mAb (MR1, 1mg injected i.p. on day 0), purchased from BioXCell (New Hampshire, USA), and with human CTLA4Ig (abatacept, 0.5 mg injected i.p. on day 2), generously provided by Bristol-Myers, Squibb Pharmaceuticals (Princeton, NJ), as described previously (12;16;41). In the protocol based on in vivo TCD mice received the same regimen of TBI and BMC with the addition of depleting doses of anti-CD4 mAb (GK1.5, 1.8mg on days −5 and −1) and anti-CD8 mAb (2.43, 1.4mg on days −5 and −1) injected i.p. (5) (purchased from BioXCell).
Additional reagents & in vivo treatments
A synthetic form of alpha-GalCer, KRN7000 was kindly provided by Kirin Brewery Co Ltd (Gunma, Japan). 5μg/mouse of alpha-GalCer or an appropriate amount of control vehicle (polysorbate), both diluted in sterile saline was injected i.p. on days −1, +2, +7, +14 and +21 (early treatment) or days +70 and +77 (late treatment), as indicated. Anti-asialo GM1 (Rabbit) was purchased from Waco Chemicals GmbH (Neuss, Germany). In indicated groups 50μl were injected i.p. on days 0, +4, +8 and +12 (42). Anti-NK1.1 (PK136, BioXCell) was injected i.p. in the indicated groups on days −1, +2, +5, and +8 (0.5mg/mouse/d).
Flow cytometric analysis
Two-color flow cytometric analysis was used to distinguish donor and host cells of particular lineages by staining with fluorescin isothiocyanite-conjugated antibodies against CD4, CD8, B220, MAC1, and biotinylated antibody against H-2Dd (34-2-12, developed with phycoerythrin streptavidin) and isotype controls. To analyze the expression of Vβ subunits, staining was performed with fluorescin isothiocyanite antibodies against Vβ8.1/2, Vβ11, and Vβ5.1/2 (or isotype control) and phycoerythrin-conjugated antibodies against CD4 (all antibodies from Becton Dickinson, San Diego, CA). Propidium iodide staining was used to exclude dead cells. The percentage of donor cells was calculated by subtracting control staining from quadrants containing donor and host cells expressing a particular lineage marker, and by dividing the net percentage of donor cells by total net percentage of donor plus host cells of that lineage. Mice were considered chimeric if they showed at least 2% donor cells within the myeloid lineage and within at least one lymphoid lineage (12;16). An Epics XL_MCL flow cytometer (Beckman Coulter, Fullerton, CA) was used for acquisition and EXPO32 ADC Software (Applied Cytometry Systems, Sheffield, United Kingdom) was used for analysis of flow cytometric data.
Skin grafting
Full thickness tail skin from Balb/c mice (donor-specific) and fully mismatched C3H mice (third-party) was grafted 4-10 weeks after BMT. Grafts were considered rejected when less than 10% of the graft remained viable.
Statistics
The chi-square test was used for comparing rates of chimerism and skin graft acceptance between groups. Skin graft survival was calculated according to the Kaplan-Meier product limit method and was compared between groups with the log-rank test. A 2-tailed Student t test was used for comparing Vβ deletion between groups. A p-value less than 0.05 was considered to be statistically significant.
RESULTS
Absence of NKT cells has limited impact on chimerism and tolerance after non-myeloablative BMT
To investigate the role of NKT cells in tolerance induction through mixed chimerism, we used NKT cell-deficient mice (J281α−/−) as recipient and/or donor in an established non-myeloablative BMT protocol (and in a TCD-based protocol as a control regimen). B6 hosts (WT or NKT-KO) received 3 Gy total body irradiation (TBI, d-1) and 15-20×106 fully allogeneic Balb/c BMC (WT or NKT-KO). Groups of mice (n= 4-8 per group) were conditioned in addition with either CB (1mg anti-CD154 mAb d0, 0.5mg CTLA4Ig d+2) (16) or in vivo TCD (1.8mg/d anti-CD4 and 1.4mg/d anti-CD8 mAb on days −5 and −1) (5).
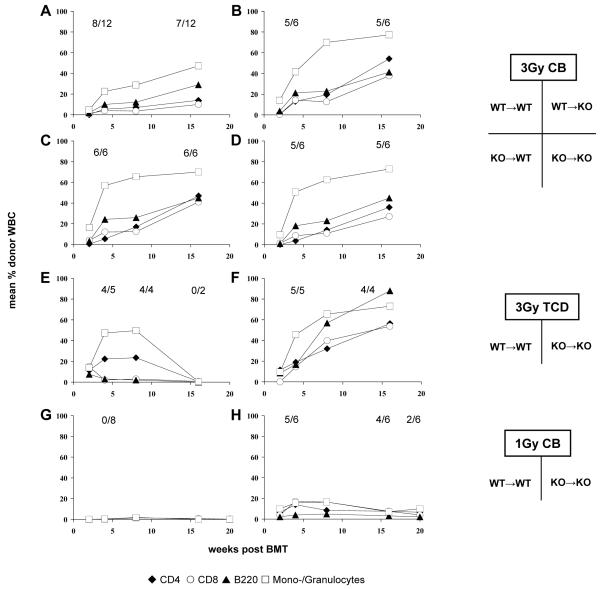
Conditioning with CB and 3 Gy TBI led to high levels of multi-lineage chimerism irrespective of the recipient and/or the donor lacking NKT cells. Rates and levels of chimerism were comparable among all four groups (WT→WT: 8/12 chimeric (a success rate similar to previous experience with this protocol (12;16)); WT→KO: 5/6; KO→WT: 6/6; KO→KO: 5/6, p= n.s.) (Figure 1 A-D). Chimerism levels among chimeras remained mostly stable for the duration of the observation period (~4 months) in all groups suggesting successful engraftment of donor hematopoietic stem cells. To evaluate whether tolerance was achieved, donor and 3rd party (C3H) skin was transplanted four to ten weeks after BMT. Chimeric mice accepted donor grafts for more than 120 days (Table 1). 3rd-party grafts were promptly rejected, indicating that tolerance was donor-specific.
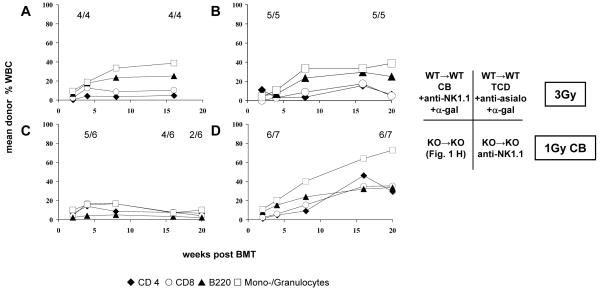
Figure 1. Absence of NKT cells has limited impact on chimerism and tolerance after non-myeloablative BMT.
Groups of B6 WT and NKT KO mice received TBI (3 Gy or 1 Gy, as indicated) and 15-20×106 Balb/c BMC (either from WT or KO). In addition either costimulation blockade (anti-CD154 mAb and CTLA4Ig) or in vivo TCD (anti-CD4 and anti-CD8) was administered. (A-D) Groups received 3 Gy TBI and costimulation blockade; (A) WT→WT; (B) WT→KO, (C) KO→WT; (D) KO→KO. (E-F) Groups received 3 Gy TBI and in vivo T cell depletion; (E) WT→WT; (F) KO→KO. (G-H) Groups received 1 Gy TBI and costimulation blockade; (G) WT→WT; (H) KO→KO. Following non-myeloablative conditioning with 3 Gy TBI and either costimulation blockade (A-D) or TCD (E-F), rates and levels of chimerism were similar whether or not recipients and/or donors lacked NKT cells. Under limiting conditioning with 1 Gy TBI (and costimulation blockade), the absence of NKT cells in donor and recipient promoted early chimerism, while WT mice failed to develop chimerism (G-H). Note: in the group depicted in panel E three mice died before the end of follow-up, the remaining two lost their chimerism. Mean percentages of net donor chimerism among CD4+ cells (◆), CD8+ cells (○), B cells (▲) and monocytes/granulocytes (□) are shown, as measured by FCM; rates of chimeras are depicted for the indicated time points (also in figures 3 and 5).
Table 1. Summary of chimerism and skin graft survival after non-myeloablative and limited conditioning in WT and NKT KO mice.
For detailed group descriptions please refer to the result section and to figure 1. Rates of chimerism and tolerance are shown 90 days post BMT. Mice were considered chimeric if they had at least 2% donor blood cells in two cell lines. Median survival time (MST) is shown for donor and third party skin grafts.
| donor | recipient | conditioning | chimerism rate |
tolerance rate |
MST donor |
MST 3rd party |
|---|---|---|---|---|---|---|
| WT | WT | 3 Gy/CoBl | 8/12 | 7/12 | >120 | 13 |
| WT | KO | 3 Gy/CoBl | 5/6 | 5/6 | >120 | 10 |
| KO | WT | 3 Gy/CoBl | 6/6 | 6/6 | >120 | 13 |
| KO | KO | 3 Gy/CoBl | 5/6 | 5/6 | >120 | 13 |
| WT | WT | 3 Gy/TCD | 4/4 | 4/4 | 90 | 15 |
| KO | KO | 3 Gy/TCD | 4/4 | 3/4 | >120 | 14 |
| WT | WT | 1 Gy/CoBl | 0/8 | 0/8 | 13 | 12 |
| KO | KO | 1 Gy/CoBl | 5/6 | 5/6 | >100 | 13 |
Likewise, mice conditioned with TCD and 3 Gy TBI developed similar rates of early chimerism whether NKT cells were present or not (WT→WT 4/5 early chimeras; KO→KO 5/5 chimeras, Figure 1, E-F). Rates of donor skin graft acceptance were also comparable within the first weeks after BMT (4/5 vs. 5/5). (Three mice in the control group and one in the KO group died of non-treatment related causes during follow-up, precluding conclusions on long-term chimerism).
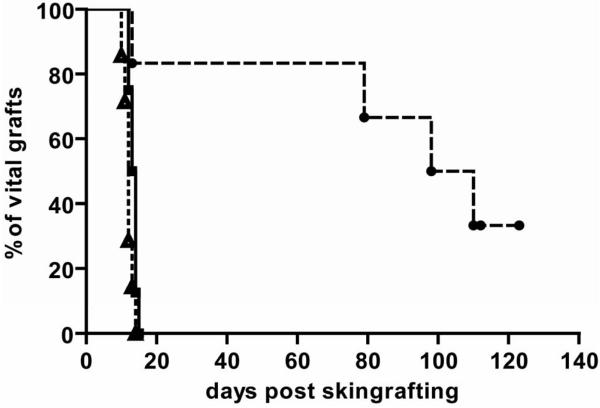
Next, we evaluated if the absence of NKT cells has a positive effect on BM engraftment and tolerance induction under limiting recipient conditioning (1Gy TBI with CB) (16) (Figure 1 G-H). The absence of NKT cells led to the induction of early multi-lineage chimerism with this otherwise unsuccessful conditioning protocol (5/6 chimeric KO→KO vs. 0/8 WT→WT, p<0.01). Chimeric mice of the KO→KO showed substantial levels of multi-lineage chimerism in all tested lineages (CD4 6.6%; CD8 3.9%; B cells 2.1%; myeloid cells 10.0%, 17 weeks post-BMT). Late after BMT, a difference between groups was no longer detectable. Donor skin graft survival was significantly prolonged in the KO group (MST >100 days vs 13 days in the WT group; p= 0.003 [log rank test]; 3rd party grafts were rapidly rejected in both groups) (Figure 2). Thus, employing limiting recipient conditioning with low dose TBI, the lack of NKT has a transient positive effect on early BM engraftment associated with improved donor skin graft survival.
Figure 2. Donor skin graft survival is significantly prolonged in the absence of NKT cells.
Groups of mice received 1 Gy TBI with costimulation blockade and were grafted with donor and third party skin; donor skin graft survival for WT→WT (■ solid line; n= 8); donor skin graft survival for KO→KO (● slashed line; n= 6). Absence of NKT cells was associated with significantly prolonged survival of donor skin (p= 0.004 by log-rank test). Percent graft survival is shown for third-party (△ dotted line; pooled data from both groups; n= 14) and donor skin as estimated by the Kaplan-Meier product limit method.
From these experiments we conclude that the presence of NKT cells has no essential role in promoting or inhibiting the induction of mixed chimerism and skin graft tolerance after non-myeloablative recipient conditioning (3 Gy TBI) involving CB and has a limited beneficial role after recipient conditioning with 1 Gy.
Stimulation of NKT cells prevents induction of chimerism and tolerance
Next, we tested whether active stimulation of NKT cells affects chimerism and tolerance. Groups of mice (n= 4-8) were treated with alpha-galactosylceramide (alpha-gal) during the immediate post-BMT period (five doses of 5μg alpha-gal or vehicle i.p. on days −1, +2, +7, +14, +21, as described by (27)). Alpha-gal is a synthetic glycosphingolipid originally isolated from marine sponges which specifically activates NKT cells (43).
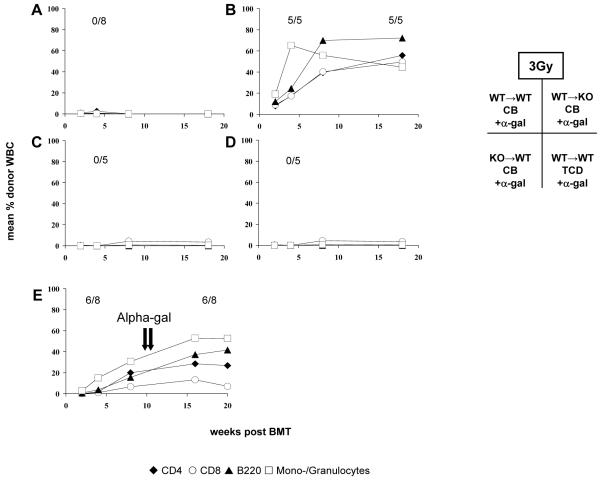
First NKT cells were activated with alpha-gal at the time of BMT in WT mice conditioned with CB and 3 or 1 Gy TBI, respectively. An additional group received vehicle only. Using limiting conditioning with 1 Gy TBI, we observed no (beneficial) effect of alpha-gal treatment on chimerism and tolerance (0/8 chimeric with alpha-Gal vs. 0/8 without, data not shown). Notably, alpha-Gal prevented BM engraftment (0/8 chimeric) (Figure 3A) and tolerance induction after 3 Gy TBI (Table 2). All mice receiving the vehicle developed chimerism and long-term donor skin graft survival. Hence, NKT activation leads to costimulation blockade-resistant BM rejection. To evaluate whether donor and/or recipient NKT cells are responsible for the detrimental effect of alpha-gal treatment after non-myeloablative BMT with 3 Gy TBI we administered alpha-gal after BMT with CB employing donor or recipient NKT KO mice (n= 5). All NKT KO recipients transplanted with WT BM became chimeric despite alpha-gal treatment (5/5, Figure 3B) and did not reject their donor skin grafts (MST >120 days) (Table 2). In contrast, NKT KO BM failed to engraft in WT recipients stimulated with alpha-Gal (0/5) (Figure 3C). Thus stimulation of recipient NKT cells is critical and sufficient for preventing BM engraftment.
Figure 3. Stimulation of NKT cells prevents induction but not maintenance of chimerism and tolerance.
Groups of BMT recipients received 3 Gy TBI with costimulation blockade (A-C, E) or in vivo T cell depletion (D), and in addition were treated with alpha-gal at the time of BMT (5μg i.p. on days −1, +2, +7, +14, +21) (A-D) or several months after BMT (5μg i.p. alpha-gal on days +70 and +77 post-BMT) (E). (A) WT→WT + CB + alpha-gal; (B) WT→KO + CB + alpha-gal; (C) KO→WT + CB + alpha-gal; (D) WT→WT + TCD + alpha-gal; (E) WT→WT + CB + late alpha-gal. After non-myeloablative TBI and CB, alpha-gal treatment at the time of BMT abrogated chimerism in recipients having NKT cells (A,C), but not in recipients deficient in NKT cells (B), indicating that the activation of recipient NKT cells is critical and sufficient for preventing BM engraftment. BM engraftment was also abrogated in recipients lacking T cells (D), suggesting that T cells are not required for BM rejection triggered by NKT cell activation. Late administration of alpha-gal did not affect chimerism (E).
Table 2. Summary of chimerism and skin graft survival after NKT stimulation with alpha-gal.
For detailed group descriptions please refer to the result section and to figures 3 and 5. Rates of chimerism and tolerance are shown 90 days post BMT. Mice were considered chimeric if they had at least 2% donor blood cells in two cell lines. Median survival time (MST) is shown for donor and third party skin grafts.
| donor | recipient | conditioning + additional treatment |
chimerism rate |
tolerance rate |
MST donor |
MST 3rd party |
|
|---|---|---|---|---|---|---|---|
| WT | WT | 3 Gy/CoBl | alpha-gal | 0/8 | 0/8 | 18 | 10 |
| WT | WT | 3 Gy/CoBl | vehicle | 4/4 | 4/4 | >120 | 11 |
| WT | WT | 3 Gy/TCD | alpha-gal | 0/5 | 0/5 | 16 | 14 |
| WT | KO | 3 Gy/CoBl | alpha-gal | 5/5 | 5/5 | >120 | 14 |
| KO | WT | 3 Gy/CoBl | alpha-gal | 0/5 | 0/5 | 16 | 14 |
| WT | WT | 3 Gy/CoBl | alpha-gal anti-NK1.1 |
4/4 | 4/4 | >120 | 12 |
| WT | WT | 3 Gy/TCD | alpha-gal anti-asialo GM1.1 |
5/5 | 5/5 | >120 | 12 |
| WT | WT | 3 Gy/CoBl | alpha-Gal late (d 70 and 77) |
6/8 | 6/8 | >112 | 13 |
| KO | KO | 1 Gy/CoBl | anti-NK1.1 | 6/7 | 6/7 | >120 | 13 |
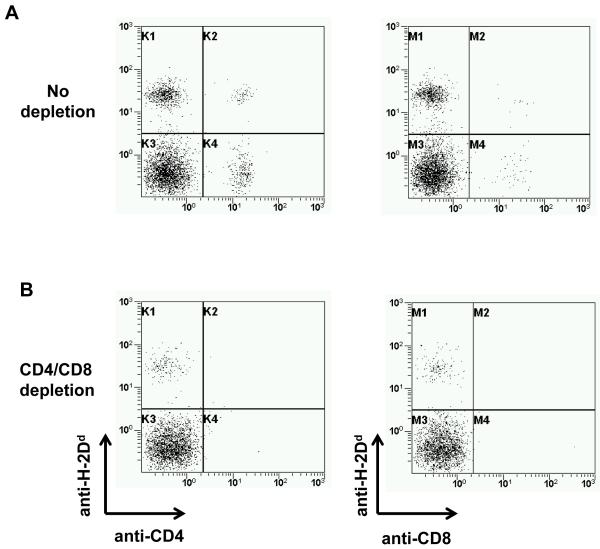
Next, we stimulated NKT cells in WT BMT recipients conditioned with high doses of in vivo T cell depleting mAbs (n= 5, 3 Gy). In vivo TCD was virtually complete as assessed by flow cytometry (Figure 4). None of the BMT recipients became chimeric after alpha-gal treatment and all mice rapidly rejected their donor skin grafts (Figure 3D, Table 2). Thus, activation of NKT cells by alpha-gal abrogates BM engraftment also after in vivo TCD, indicating that recipient T cells are not required to mediate this effect. Besides, these data demonstrate that the detrimental effect of alpha-gal is not limited to abolishing the effect of CB.
Figure 4. CD4 and CD8 T cells after in vivo T cell depletion.
Donor and recipient CD4 and CD8 cells were analyzed by FCM two weeks post-BMT in mice conditioned with (B) or without (A) in vivo T cell depletion. Treatment with anti-CD4 mAb (GK1.5, 1.8mg on days −5 and −1) and anti-CD8 mAb (GK2.43, 1.4mg on days −5 and −1) led to virtual complete absence of donor and recipient CD4 and CD8 T cells. Data from representative mice are shown.
Taken together, these results reveal that stimulation of recipient NKT cells with alpha-gal prevents BM engraftment and tolerance induction in non-myeloablative BMT regimens employing either CB or in vivo TCD.
Stimulation of NKT cells does not break maintenance of established chimerism and tolerance
Mechanisms responsible for the induction of tolerance early after CB-based BMT differ substantially from those for the maintenance of tolerance late after BMT (12). We thus investigated the effect of alpha-gal when administered several months after BMT and CB (3 Gy TBI) to mice with stable levels of mixed chimerism and viable donor skin grafts. Administration of two doses of alpha-gal (5μg i.p. at days +70 and +77 after BMT) to established chimeras (n= 6) did not cause a detectable decline in chimerism levels (Figure 3E) and had no effect on skin graft survival (Table 2). Thus activation of NKT cells at a late time point – when established tolerance is maintained primarily through central deletion in this model (12) – does not break tolerance.
Absence of NKT cells does not interfere with deletion of donor reactive T cells
Deletion of donor reactive T cells was shown to be a critical mechanism during the early period of tolerance induction immediately after non-myeloablative BMT with CB (8;12;16). We therefore evaluated the deletion of donor reactive T cells by following Vβ11+ and Vβ5+CD4+ cells that recognize endogenous superantigens presented by donor but not recipient MHC and that serve as a surrogate population of donor-reactive T cells (8;16). Deletion correlated with chimerism, as observed previously (8;16). Deletion of donor-reactive T cells was also observed in NKT KO recipients (KO→KO, 3 Gy TBI, CB)(e.g. 2.67% Vβ11+ CD4 vs. 3.93% for naïve B6, p<0.01, 3 weeks post-BMT, data not shown). Thus, the lack of recipient NKT cells has no obvious influence on the deletion of donor-reactive T cells.
Abrogation of BM engraftment through stimulation of NKT cells is mediated by NK cells
NKT cells mediate their numerous effects through several mechanisms. Direct cytotoxic effects of NKT cells have been described (44). Importantly, however, activation of NKT cells leads to massive production and secretion of several cytokines (especially IFN-γ and IL-4) which then activate different effector cell populations, in particular T cells (45) and NK cells (46). Our results with alpha-gal treatment in T cell-depleted recipients (described above) demonstrate that the detrimental effect of NKT stimulation at the time of BMT occurs also in the absence of recipient T cells, and thus rule out T cells as the critical cell population mediating BM rejection. To investigate whether stimulation of NKT cells prevents chimerism and tolerance via activation of NK cells, we administered alpha-gal to recipients depleted of NK cells and T cells (TCD + anti-asialo GM1 on days 0, 4, 8, 12, 3 Gy TBI). Anti-asialo GM1 has been shown to deplete NK but not NKT cells (47;48). As control alpha-gal was given to recipients depleted of both NK and NKT cells (CB+anti-NK1.1 mAb, 3 Gy).
Mice depleted of NK and NKT cells and treated with alpha-gal developed stable chimerism for the length of follow-up (4/4) and accepted donor skin long-term (MST >120 days) (Figure 5A and Table 2). Thus, as expected alpha-gal has no effect on outcome in the absence of NKT cells (and NK cells). Mice depleted of NK cells and T cells (but not NKT cells) and treated with alpha-gal also developed chimerism and tolerance (5/5 chimeric; MST >120 days; Figure 5B and Table 2). These data provide evidence that BM rejection through NKT stimulation is not mediated directly by NKT cells themselves, nor by T cells, but is mediated indirectly by activation of NK cells.
Figure 5. Abrogation of BM engraftment through stimulation of NKT cells is mediated by NK cells.
Groups of mice were treated with anti-NK1.1 (depleting NK and NKT cells) plus alpha-gal (A), or in vivo TCD plus anti-asiolo GM1 (depleting NK cells) (B) (3 Gy TBI with costimulation blockade). One group of NKT KO mice received NKT KO BMC and was treated with CB plus anti-NK1.1 (D) (1 Gy TBI). In panel (C), chimerism is shown for mice receiving the same protocol as mice in (D), except that they were not treated with anti-NK1.1. Panel (C) is identical with figure 1 H and is shown here again for reasons of better clarity.
NK cells have been previously shown to inhibit BM engraftment under limiting conditioning (17;18). To dissect the possible contribution of indirect NK activation via NKT to this effect, we investigated the effect of NK depletion in the absence of NKT cells. NKT-KO recipients of NKT-KO donor BM were depleted of NK cells under limiting conditioning (1Gy TBI, CB plus anti-NK1.1). Additional NK depletion did not have a detectable effect on early chimerism rates (6/7 with anti-NK1.1 vs. 5/6 without, p=n.s.) and early chimerism levels in NKT deficient mice (compared to KO → KO, Figure 5D). There was a clear trend towards improved chimerism late after BMT (6/7 with anti-NK1.1 vs. 2/6 without, p=n.s.). Thus, the beneficial effect of NK depletion on BM engraftment is somewhat diminished but apparently not completely abolished in the absence of NKT cells.
DISCUSSION
The presented investigations reveal that pharmacological NKT cell activation at the time of fully mismatched BMT (but not late after BMT) overcomes the pro-tolerogenic effects of CB, abrogating chimerism and tolerance. Thus, we have identified NKT cells as a hurdle impeding tolerance induction through CB-based mixed chimerism.
Mixed chimerism is an appealing tolerance strategy, but the myelosuppressive recipient conditioning necessary to allow engraftment of MHC-mismatched BM is a major impediment preventing widespread use of such protocols (1;49). As a consequence, a lot of effort has been put into the development of experimental BMT regimens devoid of such prohibiting toxicities, with most of the recent strategies relying on the use of costimulation blockers. In the course of such research, cell populations whose elimination promotes BM engraftment and tolerance induction have been identified, such as CD8 cells (50) and NK cells (18). We found here that NKT cells are an additional, so far unrecognized, population that impedes establishment of mixed chimerism. Activation of recipient – but not donor – NKT cells at the time of BMT completely abrogated chimerism and tolerance despite CB. Thus, NKT activation is an additional mechanism by which CB-resistant rejection can be triggered.
NK cells are critically required for the detrimental effect of NKT cell activation in the BMT models investigated herein as NK depletion abrogated the negative consequences of alpha-gal administration. The important role of NK cells in resisting engraftment of allogeneic BM has been recognized for a long time (51), and has also been demonstrated in CB-based BMT models (17;18). Our results suggest that NKT cell activation can be the upstream event that is in part responsible for triggering NK activation and rejection in this setting.
NKT activation several months post-BMT did not break tolerance, suggesting that NKT cell activation interferes with non-deletional mechanisms of tolerance induction which are critical in the early induction phase in this BMT model but not in the late phase when tolerance is mainly maintained through deletional mechanisms (12). The mere presence of NKT cells – without deliberate activation – had no detectable effect under more pronounced conditioning with 3 Gy TBI but somewhat impeded early BM engraftment under limiting conditioning with 1 Gy TBI. Under such reduced intensity conditioning subtle immune barriers become more clearly visible and critical (16). Conceivably, NKT cells become activated in this setting through the BMT regimen, possibly as a consequence of irradiation-induced tissue damage.
Little is known about the state of activation of NKT cells in organ or BM transplant recipients (38;39). Tissue damage, e.g. induced through irradiation, ischemia-reperfusion or T cell-mediated acute rejection, can lead to NKT cell activation through endogenous ligands, such as the lysosomal glycosphingolipid iGb3 (23). Of particular relevance to transplantation, NKT cells are known to be activated through numerous pathogens, including CMV (24) and certain bacterial strains (25;26). Thus, the tolerance-abrogating effect of certain infections at the time of transplant could in part be mediated through NKT cell activation (52). Thus, endogenous and exogenous ligands relevant to the transplant setting trigger NKT activation and are thus of concern in patients receiving a BMT-based tolerance regimen.
A markedly different role of NKT cells was found in another murine mixed chimerism model. Iwai et al reported that the absence of NKT cells in the recipient prevented induction of skin graft tolerance in a cyclophosphamide-based protocol (19). This well-studied model – in which donor splenocytes are administered to naïve recipients two days before cyclophosphamide is given – differs in several aspects from the two systems reported upon in our present study. Most importantly it works only across minor histocompatibility barriers, but not across MHC barriers, whereas our models use fully allogeneic donor – recipient combinations (crossing full MHC plus multiple minor antigen barriers). Consequently, the tolerance mechanisms also differ fundamentally between these models. The results of Iwai et al suggest that NKT cells play a more important role in tolerance induction towards minor antigens (at least in their specific experimental setting), whereas our results indicate that in fully mismatched models employing either TCD or CB NKT cells are not critically required. As the deliberate stimulation of NKT cells has not been investigated in the cyclophosphamide-based model, it is unclear whether NKT activation would be detrimental in this minor mismatch system.
The effects of immunosuppressive drugs on NKT cell activation remain largely undefined, with limited data suggesting the cyclosporine has an inhibitory effect on murine NKT cells (53). As NKT cells share a significant number of surface molecules with T cells and NK cells, it appears likely that they are targets of polyclonal anti-T cell globulin preparations (ATG), but it is unclear how effective their depletion/inactivation might be (54). Hence, therapeutics that have a an inhibitory effect on NKT cell activation in the clinical setting still need to be defined.
In conclusion, our studies have identified a relevant role for NKT cells in influencing the outcome of CB-based BMT protocols for the induction of mixed chimerism and tolerance with activation of NKT cells at the time of BMT abrogating BM engraftment. Thus, the control of NKT cells deserves attention when reduced intensity conditioning protocols are developed for the induction of mixed chimerism and tolerance.
ACKNOWLEDGMENTS
We thank Franz Winkler for helpful technical assistance, the staff of the Institute of Biomedical Research for expert animal care.
Funding: This work was supported by a grant from the Austrian Science Fund (FWF, SFB F2310 to TW).
ABBREVIATIONS
- alpha-gal
alpha-galactosylceramide
- BM
bone marrow
- BMC
bone marrow cells
- BMT
bone marrow transplantation
- CB
costimulation blockade
- KO
knock-out
- TBI
total body irradiation
- TCD
in vivo T cell depletion
- WT
wild-type
REFERENCES
- 1.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–8. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 3.Fehr T, Sykes M. Clinical experience with mixed chimerism to induce transplantation tolerance. Transpl Int. 2008;21:1118–35. doi: 10.1111/j.1432-2277.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomita Y, Sachs DH, Khan A, Sykes M. Additional mAb injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti-T cell mAbs and 3 Gy whole body irradiation. Transplantation. 1996;61:469–477. doi: 10.1097/00007890-199602150-00027. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi M, Zeng D, Shizuru J, et al. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 7.Wekerle T, Nikolic B, Pearson DA, Swenson KG, Sykes M. Minimal conditioning required in a murine model of T cell depletion, thymic irradiation and high-dose bone marrow transplantation for the induction of mixed chimerism and tolerance. Transpl Int. 2002;15:248–253. doi: 10.1007/s00147-002-0411-3. [DOI] [PubMed] [Google Scholar]
- 8.Wekerle T, Sayegh MH, Hill J, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams AB, Durham MM, Kean L, et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol. 2001;167:1103–1111. doi: 10.4049/jimmunol.167.2.1103. [DOI] [PubMed] [Google Scholar]
- 10.Taylor PA, Lees CJ, Waldmann H, Noelle RJ, Blazar BR. Requirements for the promotion of allogeneic engraftment by anti-CD154 (anti-CD40L) monoclonal antibody under nonmyeloablative conditions. Blood. 2001;98:467–474. doi: 10.1182/blood.v98.2.467. [DOI] [PubMed] [Google Scholar]
- 11.Wekerle T, Blaha P, Langer F, Schmid M, Muehlbacher F. Tolerance through bone marrow transplantation with costimulation blockade. Transpl Immunol. 2002;9:125–133. doi: 10.1016/s0966-3274(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 12.Bigenzahn S, Blaha P, Koporc Z, et al. The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant. 2005;5:1237–1247. doi: 10.1111/j.1600-6143.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 13.Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002;14:592–600. doi: 10.1016/s0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 14.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nature Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 15.Durham MM, Bingaman AW, Adams AB, et al. Administration of anti-CD40 ligand and donor bone marrow leads to hematopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J Immunol. 2000;165:1–4. doi: 10.4049/jimmunol.165.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Blaha P, Bigenzahn S, Koporc Z, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101:2886–2893. doi: 10.1182/blood-2002-10-3014. [DOI] [PubMed] [Google Scholar]
- 17.Westerhuis G, Maas WGE, Willemze R, Toes REM, Fibbe WE. Long-term mixed chimerism after immunologic conditioning and MHC-mismatched stem-cell transplantation is dependent on NK-cell tolerance. Blood. 2005;106:2215–2220. doi: 10.1182/blood-2005-04-1391. [DOI] [PubMed] [Google Scholar]
- 18.Kean LS, Hamby K, Koehn B, et al. NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am J Transplant. 2006;6:292–304. doi: 10.1111/j.1600-6143.2005.01172.x. [DOI] [PubMed] [Google Scholar]
- 19.Iwai T, Tomita Y, Okano S, et al. Regulatory roles of NKT cells in the induction and maintenance of cyclophosphamide-induced tolerance. J Immunol. 2006;177:8400–8409. doi: 10.4049/jimmunol.177.12.8400. [DOI] [PubMed] [Google Scholar]
- 20.Pillai A, George T, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113:4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuns RD, Morris ES, MacDonald KPA, et al. Invariant natural killer T cell-natural killer cell interactions dictate transplantation outcome after {alpha}-galactosylceramide administration. Blood. 2009;113:5999–6010. doi: 10.1182/blood-2008-10-183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou D, Mattner J, Ill C, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 24.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattner J, DeBord K, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 26.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nature Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 27.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nature Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 28.Singh AK, Wilson MT, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong C, Park SH. Application of natural killer T cells in antitumor immunotherapy. Crit Rev Immunol. 2007;27:511–525. doi: 10.1615/critrevimmunol.v27.i6.20. [DOI] [PubMed] [Google Scholar]
- 30.Kim HY, Kim HJ, Min HS, et al. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor {beta}1 production. J Exp Med. 2005;201:41–47. doi: 10.1084/jem.20041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tupin E, Nicoletti A, Elhage R, et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199:417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seino KI, Fukao K, Muramoto K, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci USA. 2001;98:2577–2581. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikehara Y, Yasunami Y, Kodama S, et al. CD4+ Vα14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest. 2000;105:1761–1767. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonoda K-H, Taniguchi M, Stein-Streilein J. Long-term survival of corneal allografts is dependent on intact CD1d-reactive NKT cells. J Immunol. 2002;168:2028–2034. doi: 10.4049/jimmunol.168.4.2028. [DOI] [PubMed] [Google Scholar]
- 35.Kiyomoto T, Ito T, Uchikoshi F, et al. The potent role of graft-derived NKR-P1+ TCRαβ+T (NKT) cells in the spontaneous acceptance of rat liver allografts. Transplantation. 2005;80:1749–1755. doi: 10.1097/01.tp.0000185306.40150.28. [DOI] [PubMed] [Google Scholar]
- 36.Toyofuku A, Yasunami Y, Nabeyama K, et al. Natural killer T-cells participate in rejection of islet allografts in the liver of mice. Diabetes. 2006;55:34–39. [PubMed] [Google Scholar]
- 37.Patterson S, Kotsianidis I, Almeida A, et al. Human invariant NKT cells are required for effective in vitro alloresponses. J Immunol. 2005;175:5087–5094. doi: 10.4049/jimmunol.175.8.5087. [DOI] [PubMed] [Google Scholar]
- 38.Jukes J, Wood K, Jones N. Natural killer T cells: a bridge to tolerance or a pathway to rejection? Transplantation. 2007;84:679–681. doi: 10.1097/01.tp.0000280551.78156.ac. [DOI] [PubMed] [Google Scholar]
- 39.Pratschke J, Stauch D, Kotsch K. Role of NK and NKT cells in solid organ transplantation. Transpl Int. 2009;22:859–868. doi: 10.1111/j.1432-2277.2009.00884.x. [DOI] [PubMed] [Google Scholar]
- 40.Cui J, Shin T, Kawano T, et al. Requirement for V{alpha}14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 41.Pree I, Bigenzahn S, Fuchs D, et al. CTLA4Ig promotes the induction of hematopoietic chimerism and tolerance independently of indoleamine-2,3-dioxygenase (IDO) Transplantation. 2007;83:663–667. doi: 10.1097/01.tp.0000255594.23445.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trambley J, Bingaman AW, Lin A, et al. Asialo GM1(+) CD8 (+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 44.Kawano T, Cui J, Koezuka Y, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura T, Kitamura H, Iwakabe K, et al. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int Immunol. 2000;12:987–994. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 46.Carnaud C, Lee D, Donnars O, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 47.Smyth MJ, Crowe NY, Godfrey DI. Nk cells and NKT cells collaborate in host protection from methylcholantrene-induced fibrosarcoma. Int Immunol. 2001;13:459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 48.Haraguchi K, Takahashi T, Matsumoto A, et al. Host-residual invariant NK T cells attenuate graft-versus-host immunity. J Immunol. 2005;175:1320–1328. doi: 10.4049/jimmunol.175.2.1320. [DOI] [PubMed] [Google Scholar]
- 49.Pilat N, Klaus C, Schwaiger E, Wekerle T. Hurdles to the induction of tolerogenic mixed chimerism. Transplantation. 2009;87:S79–84. doi: 10.1097/TP.0b013e3181a2b9cc. [DOI] [PubMed] [Google Scholar]
- 50.Kurtz J, Ito H, Wekerle T, Shaffer J, Sykes M. Mechanisms involved in the establishment of tolerance through costimulatory blockade and BMT: Lack of requirement for CD40L-mediated signaling for tolerance or deletion of donor-reactive CD4+ cells. Am J Transplant. 2001;1:339–349. doi: 10.1034/j.1600-6143.2001.10409.x. [DOI] [PubMed] [Google Scholar]
- 51.Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F1 hybrid mice. J Exp Med. 1971;134:1513–1528. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams MA, Tan JT, Adams AB, et al. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J Immunol. 2001;167:4987–95. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 53.Kajiwara T, Tomita Y, Okano S, et al. Effects of cyclosporin A on the activation of natural killer T cells induced by α–galactosylceramide. Transplantation. 2007;83:184–192. doi: 10.1097/01.tp.0000250573.50046.89. [DOI] [PubMed] [Google Scholar]
- 54.Dalle J, Dardari R, Menezes J, Cordeiro P, Champagne M, Duval M. Binding of thymoglobulin to natural killer cells leads to cell activation and interferon-gamma production. Transplantation. 2009;87:473–481. doi: 10.1097/TP.0b013e3181949c57. [DOI] [PubMed] [Google Scholar]