Abstract
Allogeneic bone marrow transplantation (BMT) under costimulation blockade allows induction of mixed chimerism and tolerance without global T cell depletion. The mildest such protocols without recipient cytoreduction, however, require clinically impracticable bone marrow (BM) doses. The successful use of mobilized peripheral blood stem cells (PBSC) instead of BM in such regimens would provide a substantial advance, allowing transplantation of higher doses of hematopoietic donor cells. We thus transplanted fully allogeneic murine granulocyte colony-stimulating-factor (G-CSF) mobilized PBSC under costimulation blockade (anti-CD40L and CTLA4Ig). Unexpectedly, PBSC did not engraft, even when very high cell doses together with non-myeloablative total body irradiation (TBI) were used. We show that, paradoxically, T cells contained in the donor PBSC triggered rejection of the transplanted donor cells. Rejection of donor bone marrow was also triggered by the co-transplantation of unmanipulated donor T cells isolated from naïve (non-mobilized) donors. Donor-specific transfusion and transient immunosuppression prevented PBSC-triggered rejection and mixed chimerism and tolerance were achieved, but graft-versus-host disease (GVHD) occurred. The combination of in vivo T cell depletion with costimulation blockade prevented rejection and GVHD. Thus, if allogeneic PBSC are transplanted instead of BM, costimulation blockade alone does not induce chimerism and tolerance without unacceptable GVHD-toxicity, and the addition of T cell depletion is required for success.
Keywords: mixed chimerism, tolerance, costimulation blockade, PBSC
INTRODUCTION
Induction of donor-specific tolerance in organ transplant recipients would substantially improve outcome by preventing graft loss due to acute and chronic rejection and by avoiding side effects of immunosuppressive drug therapy. The induction of mixed chimerism through transplantation of donor hematopoietic stem cells (HSCT) is a promising experimental strategy leading to robust tolerance (1). Its clinical translation, however, has so far been prevented in large part by the toxicity of recipient conditioning.
Mixed chimerism can be successfully induced in rodent and large animal models by global destruction of the recipient T cell repertoire through the use of T cell depleting mAbs, if in addition recipient irradiation is used (2-4). Such protocols relying on in vivo T cell depletion have always required some form of irradiation (TBI or local irradiation to the thymic area) in order to induce lasting chimerism and tolerance. When irradiation was entirely eliminated from these T cell depletion-based protocols, chimerism and tolerance were no longer achieved (5-7). More recently the use of costimulation blockade (anti-CD40L (CD154) with or without CTLA4Ig (8,9)) allowed establishment of mixed chimerism and tolerance without global T cell depletion with either non-myeloablative conditioning (10-15), or entirely without cytoreduction (i.e. without irradiation or cytotoxic drugs) (16-20). Non-cytoreductive protocols, however, usually require transplanting amounts of bone marrow cells (BMC) substantially higher than clinically available. Since non-myeloblative conditioning is regarded as too toxic by many for routine use in organ transplant recipients, non-cytoreductive protocols using numbers of donor hematopoietic cells that are feasible in the clinical setting still need to be developed.
In the clinic, collection of G-CSF-mobilized PBSC allows the harvest of a many-fold higher number of hematopoietic cells (from a living donor). Thus, the clinical development of the mixed chimerism approach for transplantation tolerance should be facilitated if PBSC could be used instead of BMC. While transplantation of mobilized allogeneic PBSC has an established clinical role in the treatment of hematological diseases (21,22), PBSC differ substantially from BMC with respect to several major biological and immunological characteristics (23-27). To the best of our knowledge murine allogeneic mobilized-PBSC have not been investigated so far for the purpose of inducing transplantation tolerance. It is thus unknown whether the distinct properties of PBSC influence the induction of allogeneic mixed chimerism and tolerance.
We have recently shown that transplantation of CD45-congenic PBSC leads to high levels of chimerism after non-myeloablative TBI (28). While roughly 3-4 times as many progenitor cells were found among unseparated BMC as among PBSC, progenitor cells contained in PBSC had similar engraftment capacity on a per-cell basis. These engraftment characteristics in the absence of an immunological barrier encouraged us to investigate transplantation of allogeneic PBSC for the purpose of tolerance induction.
Here we show that allogeneic PBSC behave markedly differently from BMC in murine HSCT protocols as they display a substantially reduced capacity to induce chimerism and tolerance.
MATERIALS AND METHODS
Animals
Mice were purchased from Charles River Laboratories (Sulzfeld, Germany) and were kept under specific pathogen-free conditions. All experiments were approved by the local review board of the Medical University of Vienna, and were performed in accordance with national and international guidelines of laboratory animal care.
BMT and mobilized peripheral blood stem cell transplantation (PBSCT)
Age-matched (6-12-week old) female C57BL/6 (B6: H-2b) mice received TBI (1–3, or 10 Gy, as indicated) 1 day before the cell transplant (d-1). For harvesting murine PBSC a protocol described by Weissman et al. (29) was used with minor modifications. To avoid pooling of PBSC in spleen, donor Balb/c mice were splenectomized at least 14 days prior to the mobilization procedure (29). Thereafter, 5μg of human G-CSF (approx. 250 μg/kg) (Amgen, Netherlands) were injected s.c. for 5 consecutive days. Two hours after the last injection, mice were maximally bled (using tail bleeding and heart puncture [under anesthesia]), and the heparinized blood was pooled and diluted with PBS (1:1). Subsequently the same quantity of 2% dextran T500 solution was added (to give a final concentration of 1% dextran). RBC were separated by sedimentation for 45 minutes at 37°C, before the supernatant fraction containing the mobilized leukocytes (PBSC) was collected and was used without further manipulation (enrichment or depletion), unless indicated otherwise. BM was harvested as described previously (15). BMC and PBSC were filtered through a 70μm filter. Cells were diluted with cold BM media [500ml Medium 199, supplemented with 5ml HEPES, 5mg DNAse and 2mg Gentamycin] and were injected i.v.in a volume of 1ml (d0). In protocol J (DST), 40×106 splenocytes from Balb/c mice were injected on d-6, together with MR1 (1mg on d-6 and 0.5mg on d-4). To rule out that any traces of dextran possibly injected with PBSC might influence outcome, 20×106 BMC were mixed with 2% dextran solution before transplantation in one experiment, without negative effect on chimerism or tolerance after BMT. In protocol K, groups of B6 BMT recipients (20×106 Balb/c BMC, anti-CD40L, CTLA4Ig, 3 Gy TBI) were co-transplanted with 25×106 or 50×106 T cells separated by MACS from naïve, unmanipulated Balb/c spleen and lymph nodes (anti-CD90, Miltenyi Biotec, Germany).
Costimulation blockade
Recipients were treated with a hamster anti-mouse-CD40L mAb (MR1; 1mg; d0) and with human CTLA4Ig (0.5mg; d+2). In two experiments of protocol J, higher doses of costimulation blockade were used (MR1: 1mg on d0, 0.5mg on days 2, 4 and 6; CTLA4Ig: 0.5mg on days 2, 4, 6 and 8). MR1 was purchased from Bioexpress. (New Hampshire), hCTLA4Ig was generously provided by Bristol-Myers Squibb Pharmaceuticals (Princeton, New Jersey),
In vivo and in vitro T cell depletion
Where indicated, recipients were either injected on d-5 and −1 or only on d0 with depleting doses of anti-CD8 mAb (2.43, 1.4mg) and anti-CD4 mAb (GK1.5, 1.8mg), or with PBSC depleted of T cells by MACS separation (anti-CD90, Miltenyi Biotec, Germany). In vitro T cell depletion was typically 80-90% complete for CD4 cells, and 90-100% for CD8 cells.
In vivo cytokine release
Serum concentration of cytokines was measured using the mouse Th1/Th2 10plex FlowCytomix system (Bender MedSystems, Austria).
Flowcytometric analysis (FCM)
Two-color FCM was used to distinguish donor and recipient cells of particular lineages, by staining with FITC-conjugated antibodies against CD4, CD8, B220, MAC1 and biotinylated H-2Dd (developed with PE) and irrelevant isotype controls. Propidium iodide staining was used to exclude dead cells. Mice were considered chimeric if they showed at least 2% donor cells within the myeloid lineage plus at least one lymphoid lineage. Chimerism levels were calculated as described previously (15).
Skin grafting
Full thickness tail skin was grafted 3-10 weeks after HSCT. Grafts were considered to be rejected when less than 10% of the graft remained viable.
Immunosuppression
Mice were injected daily with immunosuppressive drugs in the indicated groups (d0 to d20 or 27). Drugs were used at following doses: rapamycin: 0.2mg/kg/d; methylprednisolone (MP): 10mg/kg/d; mycophenolate mofetil (MMF): 20mg/kg/d. Drugs were diluted and administered as described previously (15). Rapamycin was kindly provided by Wyeth-Ayerst, New Jersey, and MMF by Roche, Austria.
Mixed lymphocyte reaction (MLR)
MLRs were performed as described previously (15). Briefly, 4×105 responder splenocytes were incubated with 4×105 irradiated (30Gy) stimulator cells of either Balb/c (donor), C3H or SJL (3rd party) and B6 (host) mice or only with medium. After 3 or 4 days, cells were pulsed with 3H-thymidine and incubated for 18 hours. Stimulation indices (SI) were calculated by dividing the mean counts per minute (c.p.m.) from responses against host (B6), donor (Balb/c) or 3rd party (C3H or SJL) by mean background c.p.m. (i.e., c.p.m. with no stimulator population).
GVHD observations
Mice were frequently screened for weight loss, diarrhea, hair loss, skin changes and hunched posture.
Statistics
A two-tailed, unpaired Student’s T test was used for comparing percentages of chimerism and SI values between groups. The chi-square test was used for comparing rates of chimeras, and rates of skin graft acceptance. Skin graft survival was calculated according to the Kaplan-Meier product limit method and compared between groups by using the log-rank test. A P value less than 0.05 was considered to be statistically significant.
RESULTS
PBSC behave differently from BMC in non-myeloablative chimerism protocols relying on costimulation blockade or on recipient T cell depletion
To investigate the use of PBSC for the induction of mixed chimerism and tolerance, we first transplanted escalating doses of fully mismatched G-CSF-mobilized PBSC employing an established protocol that generates chimerism and tolerance in a high proportion of recipients when BMC are transplanted (20×106 fully mismatched, unseparated Balb/c BMC, 2 or 3 Gy TBI, costimulation blockade with anti-CD40L plus CTLA4Ig (10,15,30,31)) (experimental protocols used in this paper are summarized in Table 1).
Table 1. Experimental protocols (A-K).
Groups of B6 mice received TBI one day before being transplanted with the indicated doses of unseparated Balb/c BMC alone, PBSC alone, or BMC together with PBSC (d0 or d94). Costimulation blockade (CB), in vivo or in vitro T cell depletion (TCD), donor-specific transfusion (DST) and transient immunosuppression (IS) were added to the specific HSCT protocols as shown. CB consisted of 1mg anti-CD40L mAb (MR1) on day 0, and 0.5mg CTLA4Ig on day 2. In two (of four) experiments of protocol J (200×106 PBSC) higher doses of anti-CD40L and CTLA4Ig were used, without a significant effect on outcome (MR1: 1mg on day 0, 0.5mg on days 2, 4 and 6; CTLA4Ig: 0.5mg on days 2, 4, 6 and 8). In vivo TCD consisted of a depleting anti-CD8 mAb (2.43, 1.4 mg) and a depleting anti-CD4 mAb (GK1.5, 1.8 mg) injected at d −5 and −1 (protocols C, D) or only d 0 (protocol I). For in vitro TCD of PBSC anti-CD90 MACS separation was employed (protocols E, H). In protocol I additional in vitro TCD was performed which, however, was inefficient for technical reasons. DST in protocol J consisted of 40×106 splenocytes from Balb/c mice injected on d-6, together with anti-CD40L (1mg on d-6 and 0.5mg on d-4). For transient IS rapamycin, mycophenolate mofetil and methylprednisolone were injected in groups of protocol J for 21 or 28 days following HSCT. Donor T cells (group K) were isolated from spleen and lymph nodes of naïve Balb/c mice by MACS separation (CD90+).
| Protocol | TBI (Gy) | HSCT (cells/mouse) | CB | TCD | DST | IS |
|---|---|---|---|---|---|---|
| A | 2, 3 | 20×106 BMC | + | − | − | − |
| B | 0, 1, 1.5, 2, 3 | 20×, 75×, 200×106 PBSC | + | − | − | − |
| C | 3 | 25×106 BMC | − | in vivo d −5, −1 | − | − |
| D | 3 | 75×106 PBSC | − | in vivo d −5, −1 | − | − |
| E | 10 | 40×106 PBSC | − | in vitro | − | − |
| F | 3 | 20×106 BMC (d0) plus 2×, 5×, 10×, 20×, 60× 106 PBSC (d0) |
+ | − | − | − |
| G | 3 | 20×106 BMC (d0) plus 60×106 PBSC (d94) |
+ | − | − | − |
| H | 3 | 20×106 BMC plus 60×106 PBSC (d0) | + | in vitro | − | − |
| I | 3 | 20×106 BMC plus 60×106 PBSC (d0) | + | in vivo d 0 | − | − |
| J | 3 | 75×, 200×106 PBSC | + | − | + | + / − |
| K | 3 | 20×106 BMC plus 25×106 or 50×106 donor T cells (d0) |
+ | − | − | − |
B6 mice receiving 20×106 Balb/c BMC developed long-term macrochimerism (2 Gy TBI: 6/6, 3 Gy: 10/10). In contrast, transplantation of the same, or substantially higher numbers of PBSC after 1 to 3 Gy TBI and costimulation blockade did not lead to long-term macrochimerism (20×106: 2 Gy TBI: 0/6, 3 Gy: 0/6); 75×106: 1 Gy TBI: 0/6, 1.5 Gy: 0/6, 2 Gy: 0/6, 3 Gy: 0/4; 200×106: 3 Gy: 2/9 demonstrated chimerism at 2 weeks, but 0/9 developed long-term chimerism; Table 2). Transplantation of 200×106 BMC under costimulation blockade can induce chimerism and tolerance without any TBI, or other cytoreduction (16,17,19). Since it was shown that under certain circumstances irradiation negatively affects engraftment after BMT and costimulation blockade (19,32), we also transplanted 200×106 Balb/c PBSC without TBI. Again, chimerism was not induced (0/3) (Table 2).
Table 2. PBSC are less tolerogenic compared to BMC in protocols employing costimulation blockade or in vivo T cell depletion.
Groups of mice were treated as shown in Table 1. In contrast to transplantation of 20×106 BMC (A), the transplantation of up to 200×106 PBSC (B) after non-myeloablative TBI and costimulation blockade did not lead to long-term chimerism or donor-specific hyporesponsiveness in MLR assays. Similarly transplantation of PBSC after global in vivo T cell depletion (without costimulation blockade; D) did not lead to long-term chimerism or tolerance, whereas BMC did (C). Chimerism was measured 1-2 weeks post-HSCT (early), and 2 months post-HSCT (late).
| Protocol | TBI (Gy) |
HSCT (cells/mouse) |
CB | TCD | Rate of chimeras | Rate of skin graft acceptance |
MLR results | |
|---|---|---|---|---|---|---|---|---|
| SI vs. donor | SI vs. 3rd party |
|||||||
| A | 2, 3 | 20×106 BMC | + | − | 6/6, 10/10 | − | 2.3, 1.8 | 1.3, 1.6 |
|
| ||||||||
| B | 2, 3 | 20×106 PBSC | + | − | 0/6, 0/6 | − | − | − |
| 1, 1.5, 2, 3 |
75×106 PBSC | + | − | 0/4, 0/6, 0/6, 0/6 | − | −, −, 6.6, 6.5 | 2.5; 1.5 | |
| 3 | 200×106 PBSC | + | − | 2/9 early, 0/9 late | − | 4.7 | 2.5 | |
| 0 | 200×106 PBSC | + | − | 0/3 | − | 2 | 0.9 | |
|
| ||||||||
| C | 3 | 25×106 BMC | − |
in vivo d -5, -1 |
4/6 | 2/3 | 0.8 | 0.9 |
|
| ||||||||
| D | 3 | 75×106 PBSC | − |
in vivo d -5, -1 |
5/5 early, 0/5 late | 0/4 | 2.9 | 0.9 |
|
| ||||||||
| A vs. B (20×106 PBSC) | p<0.001 | − | − | |||||
| A vs. B (75×106 PBSC) | p<0.001 | − | p<0.005, p=n.s. |
|||||
| A vs. B (200×106 PBSC) | p<0.001 | − | p<0.005 | |||||
| C vs. D | p<0.01 | p=n.s. | p<0.05 | |||||
To explore whether the different properties of PBSC compared to BMC occur only in relation to the effect of costimulation blockade, we transplanted PBSC using a protocol relying on global in vivo recipient T cell depletion (3 Gy TBI, anti-CD4 plus anti-CD8 mAbs on days −5 and −1 (3)). B6 recipients treated with this regimen and 75×106 Balb/c PBSC developed only some early, but no long-term chimerism (0/5 at 7 weeks, Table 2), and did not become tolerant. In contrast, the majority of controls transplanted with 25×106 Balb/c BMC developed long-term chimerism (4/6 at week 7; 2/3 at week 26) and tolerance.
Thus, in contrast to BMC, allogeneic PBSC do not engraft with non-myeloablative conditioning protocols involving either costimulation blockade alone, or in vivo T cell depletion alone.
PBSC are immunogenic and trigger rejection of co-transplanted donor BMC
Two not mutually exclusive factors could be responsible for the failure of PBSC to induce chimerism: (non-immunologic) engraftment failure or rejection. To distinguish between these two possibilities, we transplanted T cell-depleted Balb/c PBSC (40×106) into lethally irradiated (10 Gy TBI) B6 recipients. Although animals shortly after transplantation started to develop signs of GVHD (presumably due to incomplete T cell depletion), this protocol demonstrated that PBSC engrafted successfully, leading to full chimerism (5/5 at week 1, 3/3 at week 6; data not shown). Besides, we have recently shown that transplantation of 20×106 PBSC (harvested with the same technique used in the studies described herein) into CD45-congenic recipients after 1Gy TBI led to substantial levels of stable macrochimerism (28). Furthermore, MLR assays revealed donor-reactivity among allogeneic PBSC recipients, whereas BMC recipients typically demonstrated donor hyporesponsiveness (Table 2). Taken together these lines of evidence show that 20×106 PBSC contain sufficient numbers of hematopoietic progenitors/stem cells to induce lasting macrochimerism in the absence of alloreactivity and that primary engraftment failure is unlikely to be the main factor preventing chimerism induction with allogeneic PBSC. Instead allogeneic PBSC seem to be rejected after non-myeloablative TBI with costimulation blockade or in vivo T cell depletion.
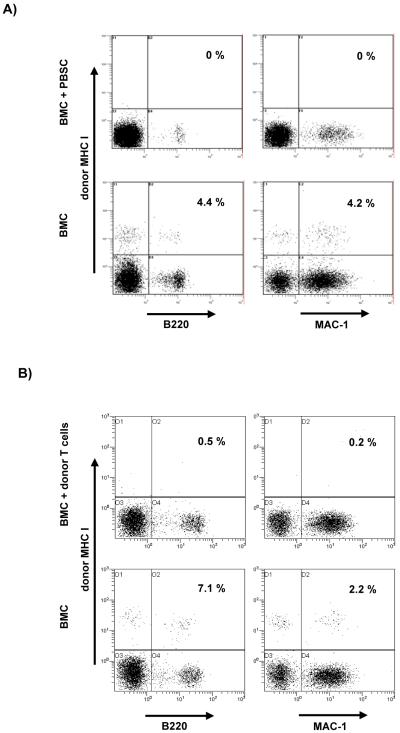
To distinguish whether PBSC are solely less tolerogenic and are rejected because they fail to induce tolerance, or whether they actively trigger rejection, we co-transplanted BMC and PBSC. B6 received 3Gy TBI and costimulation blockade and were injected with 20×106 Balb/c BMC together with 60×106 Balb/c PBSC (n=18; 4 separate experiments). In 3 of 4 experiments chimerism was undetectable in all co-transplanted mice as early as one week after transplantation (0/13) (Figure 1), and tolerance did not ensue (8/8 rejected donor skin [skin grafting was not performed in one experiment with 0/5 chimeric mice], and anti-donor reactivity was observed in MLR: PBSC+BMC vs. BMC; 2.4 (SI) vs. 0.7 p<0.001, Table 3). In one experiment 5/5 co-transplanted mice developed long-term chimerism, but only 1/5 accepted donor skin long-term (MST=39d). Control groups included in each experiment, transplanted with 20×106 BMC alone (without PBSC) developed long-term chimerism (19/22, p<0.001 compared to BMC+PBSC) and tolerance (14/19, p<0.001). Thus, despite some limited variability, taken together these data demonstrate that PBSC did not just fail to induce tolerance, but actively triggered rejection of the co-transplanted donor BMC.
Figure 1. Co-transplantation of PBSC or of donor T cells together with BMC abrogates the induction of chimerism.
A) Groups of mice received either 20×106 BMC alone (lower row) or 20×106 BMC plus 60×106 PBSC (upper row) (with 3 TBI and costimulation blockade, protocols A and F). One week (day 6) after the HSCT two color FCM analysis of WBC revealed approximately 4% chimerism in the B cell and myeloid lineages in a BMC-only recipient, whereas no chimerism was detectable after simultaneous transplantation of BMC plus PBSC. B) Mice receiving 20×106 BMC alone (lower row) developed chimerism whereas mice receiving 20×106 BMC plus 25×106 donor T cells (upper row) (with 3 TBI and costimulation blockade, protocol K) showed no detectable chimerism (day 7 post-BMT). Data from one representative mouse per group are shown.
Table 3. Co-transplantation of undepleted PBSC or of donor T cells together with BMC prevents chimerism and tolerance.
When 20×106 BMC were transplanted together with 60×106 PBSC (3 Gy TBI and costimulation blockade, protocol F) chimerism and tolerance induction were abrogated in most mice (compared to recipients of BMC alone, protocol A). MLR assays showed reactivity against donor-type stimulator cells in mice co-transplanted with PBSC. When lower doses of PBSC were co-transplanted, the negative effect was diminished or absent, respectively. When injection of 60×106 PBSC was delayed until 94 days post-BMT, no detrimental effect was observed (protocol G). The co-transplantation of in vitro T cell depleted-PBSC (60×106) with 20×106 BMC (both on day 0, protocol H) led to chimerism and donor skin graft acceptance in most recipients. Similarly, co-transplantation of PBSC (60×106) with 20×106 BMC (protocol I) together with in vivo T cell depletion led to chimerism and tolerance. (MLR results against 3rd party stimulators are not available for these experiments due to technical failure.) Co-transplantation of 25×106 or 50×106 donor T cells together with 20×106 BMC (protocol K) prevented chimerism induction.
| Protocol | TBI (Gy) |
HSCT (cells/mouse) | CB | TCD | Rate of chimeras |
Rate of skin graft acceptance |
MLR results |
|---|---|---|---|---|---|---|---|
| SI vs. donor |
|||||||
| A | 3 | 20×106 BMC | + | − | 19/22 | 14/19 | 0.7 |
|
| |||||||
| F | 3 | 20×106 BMC (d0) plus 60×106 PBSC (d0) |
+ | − | 5/18 | 1/13 | 2.4 |
|
| |||||||
| F | 3 | 20×106 BMC (d0) plus 2×, 5×, 10×, 20× 106 PBSC (d0) |
+ | − | 2/3, 2/5, 4/5, 3/4 |
−, −, −, 0/4 | − |
|
| |||||||
| G | 3 | 20×106 BMC (d0) plus 60×106 PBSC (d94) |
+ | − | 5/5 early, 4/4 late |
5/5 early, 4/4 late |
− |
|
| |||||||
| H | 3 | 20×106 BMC plus 60×106 PBSC (d0) |
+ | in vitro | 12/14 | 12/14 | − |
|
| |||||||
| I | 3 | 20×106 BMC plus 60×106 PBSC (d0) |
+ |
in vivo d0 |
7/7 | 7/7 | − |
|
| |||||||
| K | 3 | 20×106 BMC plus 25×106 or 50×106 donor T cells (d0) |
+ | − | 0/5, 0/5 | − | − |
|
|
|||||||
| A vs. I | p=n.s. | p=n.s. | − | ||||
| A vs. H | p=n.s. | p=n.s. | − | ||||
| A vs. G | p=n.s. | p=n.s. | − | ||||
| A vs. F (60×106 PBSC) | p<0.001 | p<0.001 | p<0.001 | ||||
| H vs. F (60×106 PBSC) | p<0.01 | p<0.001 | − | ||||
| G vs. F (60×106 PBSC) | p<0.01 | p<0.001 | − | ||||
To determinate whether the detrimental effect of co-transplanted PBSC is dose-dependent, we transplanted 20×106 BMC together with 2×106, 5×106, 10×106 or 20×106 PBSC (with 3Gy TBI and costimulation blockade). Long-term chimerism was seen in 2/3 (p=n.s.), 2/5 (p=n.s.), 4/5 (p<0.05) and 3/4 (p=n.s.) recipients, respectively (12 weeks post-HSCT, compared to co-transplanting 60×106 PBSC; donor skin was accepted >100 days in 0/3 mice co-transferred with 20×106 PBSC, and 4/5 mice receiving BMC only [p<0.05]) (Table 3). Hence, the rejection-triggering effect of PBSC seems to be dose-dependent to some degree, but the full effect is observed with moderate, clinically relevant doses (60×106/mouse).
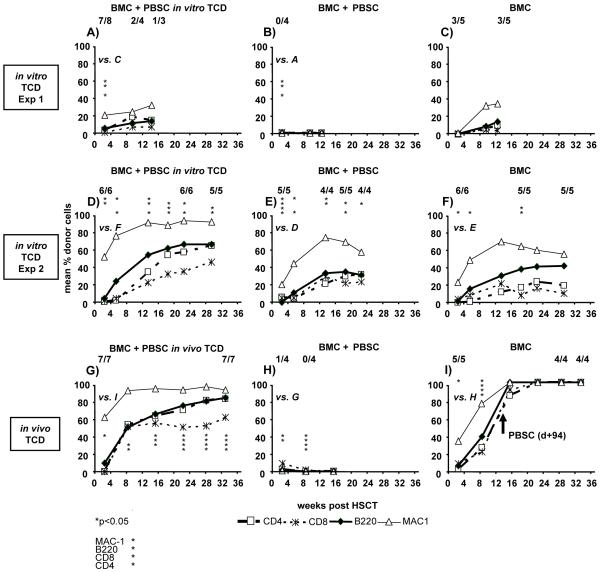
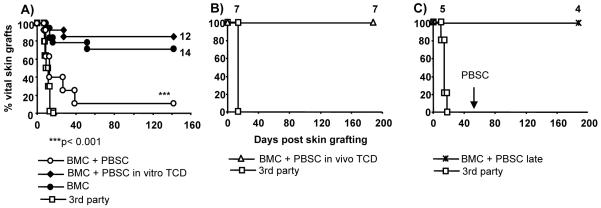
We also determined whether PBSC break tolerance when injected late after BMT into stable chimeras with healed-in donor skin grafts (20×106 BMC, 3Gy TBI plus costimulation blockade; 60×106 PBSC injected 94 days after BMT, protocol G). Injection of PBSC late after BMT led to an increase in chimerism levels which persisted until the end of follow-up (19 weeks post PBSC infusion, Figure 2 I), and did not cause rejection of donor grafts (Figure 3 C). Despite conversion to full chimerism, no signs of GVHD were noticed. Thus PBSC trigger rejection when administered early after conditioning, but do not have a detrimental effect when given at late time points.
Figure 2. Rejection triggered by co-transplantation of PBSC with BMC can be prevented by T cell depletion.
Co-transplantation of 60×106 PBSC with 20×106 BMC prevents chimerism induction in 3 of 4 experiments (3 of them shown in this figure; panels B, E, H; protocol F). When in vitro T cell depleted-PBSC were co-transplanted (panels A, D; protocol H), chimerism levels were observed which were higher than in the corresponding groups transplanted with BMC alone (panels C, F; protocol A). Likewise, in vivo T cell depletion prevented rejection (panel G, protocol I). Late administration of PBSC into BMT chimeras augments chimerism (panel I, protocol G). All groups received 3 Gy TBI and costimulation blockade. Two-color FCM was used to determine chimerism among WBC at multiple time points post-HSCT. Chimerism levels are shown as mean. The fractions displayed in each panel indicate the fraction of analyzed mice showing chimerism at the time point below. Details of experimental protocols are shown in Table 1. Each row shows groups of one particular experiment to allow direct comparisons. *p<0.05 indicates a significant difference in chimerism levels of a particular lineage between the indicated groups. Pooled results from these experiments are shown in Table 3. Skin graft results from these experiments are shown in Figure 3.
Figure 3. In vitro or in vivo T cell depletion allows induction of skin graft tolerance.
After co-transplantation of 20×106 BMC plus 60×106 PBSC donor skin grafts were rejected in almost all recipients (pooled results of four experiments, A), whereas co-transplantation of in vitro T cell depleted PBSC, and transplantation of BMC alone led to long-term acceptance of donor grafts (pooled results of three experiments). Likewise, co-transplantation of in vivo T cell depleted PBSC allowed donor-specific skin graft tolerance (B). Late administration of PBSC into BMT chimeras did not lead to rejection of healed-in donor skin (C). Donor and 3rd party skin was grafted 3-6 weeks after HSCT. *p<0.001 for comparison of BMC+PBSC vs. BMC+PBSC in vitro TCD or vs. BMC.
Donor T cells contained in PBSC trigger rejection
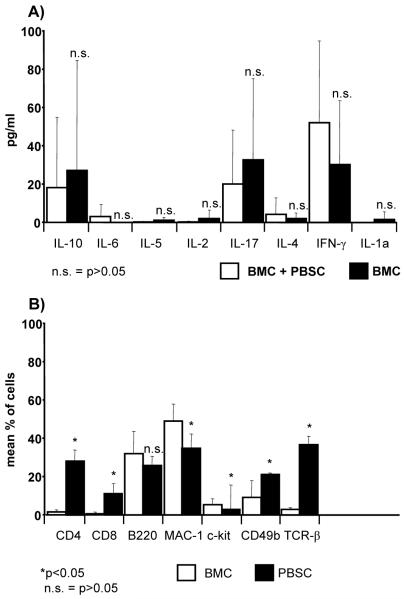
Transplantation of PBSC has been reported to induce a Th2-shift in the cytokine response of recipients (23) and some evidence suggests that costimulation blockade affects Th1 and Th2 responses differently (33,34). We therefore measured serum levels of prototypical Th1 and Th2 cytokines 5 days after HSCT in groups of mice transplanted with either 20×106 BMC alone or 20×106 BMC plus 60×106 PBSC (3 Gy TBI plus costimulation blockade). As shown in Figure 4A, cytokine levels varied considerably among individual mice within the same group, but no statistically significant differences between the two groups were evident. While we cannot rule out that local or intracellular shifts in cytokine levels might be missed by analyzing serum, our limited cytokine analysis does not provide evidence that a Th2 shift is the main cause of the failure of the PBSC protocols.
Figure 4. PBSC contain significantly more T cells and do not trigger an evident cytokine shift.
A) Cytokine levels were measured in serum 5 days after transplantation of BMC alone or BMC together with PBSC (n=4-6). Wide variations of cytokine levels among individual mice of the same group were observed, without clear evidence for a major shift in cytokine response between the groups. B) Percentages of cell populations contained in either BMC or PBSC were determined by FCM. Pooled results from 13 experiments are shown as mean plus standard deviation.
The cell compositions of PBSC and BMC differ considerably (Figure 4B). In particular, consistent with the published literature (23,35), PBSC contained markedly higher percentages of CD4+ and CD8+ cells [mean value 28.1% vs. 1.6% for CD4+ (p<0.0001) and 10.8.% vs. 0.7% for CD8+ cells (p<0.0001), pooled results of 13 experiments]. To assess whether it is the T cells contained in PBSC that trigger rejection, we co-transplanted Balb/c PBSC that had been T cell-depleted in vitro by MACS separation, with 20×106 BMC (3 Gy TBI and costimulation blockade). While chimerism was undetectable in all mice receiving BMC with un-depleted PBSC (Figure 2B) (MST for donor skin =7d, n=4), chimerism and tolerance were induced in 7/8 mice co-transplanted with T cell-depleted PBSC (MST>31d) (Figure 2A). Despite T cell depletion, however, signs of GVHD developed from day 48 on, with only 4 mice (2/4 chimeric) alive on day 59. In a separate experiment, chimerism and tolerance were again observed after co-transplantation of T cell-depleted PBSC together with BMC (6/6 Figure 2 D, MST>142d for donor skin). No signs of GVHD were observed, possibly because depletion was more complete in this particular experiment. Unexpectedly, however, long-term chimerism was seen after co-transplantation of un-depleted PBSC as well (5/5 mice Figure 2E, but 4/5 mice lost the donor graft [MST=39d]) (as mentioned above, this was the only of four experiments were such an outcome occurred, Figure 2). GVHD was observed in these recipients co-transplanted with un-depleted PBSC, which was never seen in BMC-only recipients. Since the levels of chimerism were lower when un-depleted PBSC were transplanted compared to depleted PBSC (e.g. 66% vs. 34% B cell chimerism p<0.05 at week 22, Figure 2 D+E) it is suggested that the transplanted donor cells were partially rejected in the group without T cell depletion. Furthermore, chimerism levels were higher in the group co-transplanted with T cell-depleted PBSC than in the BMC-only group (e.g. 57% vs. 24% CD4 p<0.05, 66% vs. 41% B cell p<0.05 at week 22, Figure 2 D+F), indicating that the PBSC have successfully engrafted and contributed to chimerism.
When in vivo T cell depletion was used in recipients co-transplanted with BMC and PBSC (3 Gy TBI, 20×106 BMC plus 60×106 PBSC, costimulation blockade, anti-CD4 + anti-CD8 [d0], protocol I) high levels of multilineage chimerism developed. Chimerism persisted for the length of follow-up (7/7; Figure 2G), and chimerism levels were significantly higher than in recipients of BMC alone [e.g. 53% vs. 23% CD4 p<0.05 at week 8, Figure 2 G+I (BMC alone till d+94)], indicating that the PBSC have successfully engrafted. All recipients accepted donor skin (MST>188d; Figure 3B), without signs of GVHD.
T cells contained in PBSC might trigger rejection because they are qualitatively distinct (following G-CSF mobilization) or because of the quantity of co-transplanted T cells. To distinguish between these two possibilities, we co-transplanted 25×106 or 50×106 donor T cells isolated from naïve Balb/c spleen and lymph nodes (the approximate number of T cells contained in 60×106 and 120×106 PBSC, respectively) together with 20×106 Balb/c BMC (costimulation blockade and 3 Gy TBI). Already 7 days post-BMT none of the mice receiving additional donor T cells demonstrated chimerism (0/5 and 0/5 with 25×106 and 50×106 T cells, respectively) whereas a control group receiving just BMC developed chimerism (Figure 1B and Table 3). No anti-donor antibodies were detectable in sera of mice co-transplanted with donor T cells (data not shown). Thus unmanipulated donor T cells trigger rapid rejection of co-transplanted donor BMC, without inducing a humoral anti-donor response.
Thus, donor T cells contained in PBSC trigger rejection of donor cells. This rejection is related to the quantity rather than the quality of T cells contained in PBSC and can be prevented by T cell depletion (in vitro or in vivo) (summarized in Table 3). The development of GVHD is prevented after PBSC transplantation through adequate T cell depletion.
Additional immunosuppression and DST allow induction of chimerism and tolerance after transplantation of PBSC with costimulation blockade
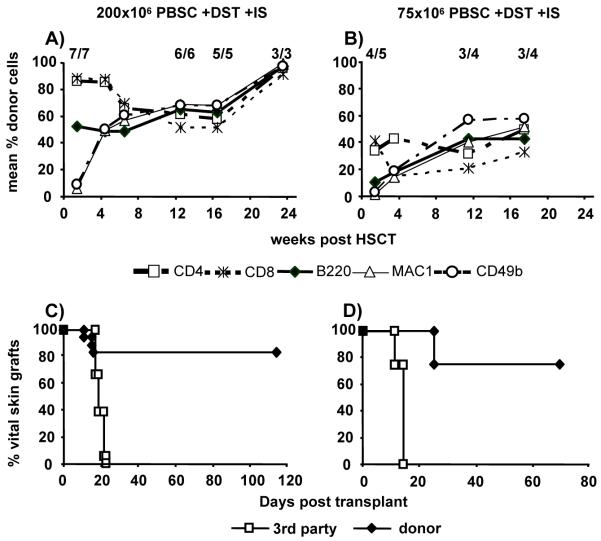
Both in vitro T cell depletion (36-38), and in vivo recipient T cell depletion are problematic in the clinical setting. Thus, protocols without T cell depletion would be desirable (39). We, and others, have recently shown that rapamycin improves engraftment after BMT (15,40) and DST reduces recipient donor-reactivity in several protocols (32,41). Therefore, we investigated whether the rejection triggered by PBSC is prevented by the addition of transient immunosuppression and DST to the non-myeloablative costimulation-based protocol (40×106 Balb/c SPL d-6 plus anti-CD40L; 3Gy TBI d-1; 200×106 or 75×106 undepleted Balb/c PBSC; costimulation blockade; rapamycin+MMF+methylprednisolone d0-27). The majority of mice developed full chimerism when transplanted with 200×106 PBSC (18/19, pooled data from four separate experiments) and skin graft tolerance (15/18; MST>99d, third party grafts were promptly rejected) (Figure 5A+C). MLR assays revealed donor-specific hyporesponsiveness (SI against donor 0.6; 3rd party 3.3, data from one experiment). However, approximately 2 months post-HSCT chimeric PBSC recipients developed clinical signs of chronic GVHD including weight loss, skin changes, and hunched posture, without evident diarrhea.
Figure 5. Additional immunosuppression and DST allow induction of chimerism and tolerance after transplantation of PBSC with costimulation blockade.
Transplantation of 200×106 (A, C) or 75×106 (B, D) PBSC leads to high levels of chimerism and donor-specific skin graft tolerance when DST and transient immunosuppression are added to costimulation blockade and 3 Gy TBI (protocol J). However, GVHD occurred in a large fraction of mice that had to be sacrificed. Panel A shows representative chimerism data from one of four separate experiments, panel C shows pooled skin graft data from all four experiments (n=18). Two-color FCM was used to determine chimerism among WBC at multiple time points post-HSCT. Chimerism levels are shown as mean. Donor and 3rd party skin were grafted 7-8 weeks after HSCT. IS denotes immunosuppression which was administered from d0 to 27.
When only 75×106 PBSC (which is a clinically more relevant dose on a per KG basis) were transplanted with the regimen including DST and immunosuppression, 4/5 recipients developed mixed chimerism (at week 3), with 3/4 long-term chimeric mice surviving more than 100 days after HSCT (Figure 5B). Chimeras permanently accepted donor skin (MST>69d), while promptly rejecting 3rd party grafts (Figure 5D). However, also these chimeras developed GVHD (starting from day 62). When 75×106 PBSC were transplanted with additional DST, but without immunosuppression, 3/5 (p=n.s.) mice showed chimerism at 1 week post-HSCT), and 0/3 at 11 weeks (two chimeras died before week 11) (not shown). No mouse treated with additional immunosuppression but without DST showed chimerism at 1 week post-HSCT (0/5, p<0.01) (not shown). Taken together, these results suggest that DST is critical for allowing the induction of chimerism and tolerance, and that the best results might be achieved when both DST and immunosuppression are given. Lasting chimerism and tolerance are thus achieved after transplantation of a moderate dose of PBSC under costimulation blockade and non-myeloablative TBI. However, severe GVHD uniformly develops in such chimeras.
DISCUSSION
Unless minimally toxic mixed chimerism regimens are developed, the translation of this tolerance strategy from a laboratory solution into routine clinical use remains unlikely (42). Thus we investigated whether the transplantation of PBSC, instead of BMC, would allow the development of such clinically feasible and acceptable mixed chimerism protocols. However, we found that the use of PBSC not only failed to allow minimization of recipient conditioning, but on the contrary that PBSC are less tolerogenic and trigger their own rejection, hence requiring intensified conditioning.
Surprisingly, PBSC uniformly failed to engraft in well-established costimulation blockade-based and in in vivo T cell depletion-based models. Chimerism was not achieved even when the10-fold dose of PBSC was transplanted which led to chimerism in a CD45-congenic model (28). Co-transplantation of PBSC together with BMC provided direct evidence that PBSC not merely fail to engraft or fail to induce tolerance but that they actively trigger rejection. Hence allogeneic PBSC are not just less tolerogenic but they are distinctly immunogenic. The quantity of donor T cells contained in PBSC was pinpointed as the decisive factor in triggering rejection. As co-transplantation of unmanipulated, naïve donor T cells had the same effect, a critical role of G-CSF in this effect can be ruled out. As a consequence, profound (in vitro) depletion of T cells provides a clinically relevant way to successfully transplant allogeneic PBSC.
Co-transplantation of the same dose of PBSC late after HSCT did not break tolerance, but led to conversion into full chimerism without inducing GVHD. This time-dependent effect of donor T cells contained in PBSC is reminiscent of a cyclophosphamide-based murine non-myeloablative mixed chimerism model employing in vivo T cell depletion, in which donor lymphocyte infusions were shown to have different effects depending on the time of injection (43). While infusion of donor T cells early after BMT paradoxically triggered T cell-mediated rejection of donor BM, late donor lymphocyte infusions (>35 days post-BMT) converted mixed to full donor chimerism without causing GVHD. In this model donor CD4 cells triggered rejection that was mediated by residual recipient T cells (44). To the best of our knowledge, a similar effect for donor T cells has not been previously described in costimulation blockade-based BMT regimens. The precise effector mechanism by which in this system donor T cells induce rejection of donor hematopoietic cells by the recipient immune system remains to be delineated. As no anti-donor antibodies were detectable a humoral response can be ruled out as the main cause of rejection. Conceivably cytokine-mediated by-stander activation of recipient T cells might be involved, as has been suggested in the cyclophosphamide-based model (44). Recipient NK cells have recently been shown to be an important impediment to BM engraftment in costimulation blockade-based protocols (12)(18). Possibly, transplantation of large numbers of donor T cells leads to cytokine-mediated activation of recipient NK cells which - being relatively costimulation-blockade resistant - then rapidly reject donor BM.
We and others have previously shown that short-term rapamycin-based immunosuppression promotes engraftment of allogeneic BMC in non-myeloablative, costimulation blockade-based protocols (15,40,45). DST enhances chimerism after treatment with anti-CD40L mainly by overcoming host CD8 reactivity (32,41). The additional use of immunosuppression and DST prevented the PBSC-triggered rejection when costimulation blockade and non-myeloablative TBI were given. GVHD, though slightly delayed, was however not prevented.
GVHD does not occur after BMT with costimulation blockade, even when very high doses of (undepleted) BMC are transplanted (16,17). Thus costimulation blockade injected at the time of BMT is evidently capable of tolerizing certain quantities of injected donor T cells towards recipient antigens, thereby preventing GVHD. In sharp contrast, costimulation blockade (together with DST and transient immunosuppression) did not prevent GVHD after transplantation of PBSC. This might be a purely quantitative phenomenon, with some donor T cells escaping tolerization, going on to cause GVHD. Alternatively, T cells contained in PBSC might differ qualitatively in a way that makes them costimulation blockade-resistant. T cells in G-CSF mobilized PBSC are known to be skewed towards a Th2-phenotype. The role of cytokines in the induction of graft acceptance through costimulation blockade is complex and remains incompletely understood (34), but IFNγ, a prototypic Th1 cytokine, was shown to be critical for the graft-prolonging effect of anti-CD40L plus CTLA4Ig in skin and heart graft models (46). Thus, a different cytokine profile might render donor T cells relatively resistant to costimulation blockade.
PBSC have been used for the induction of allo-tolerance (4,7,47) and xeno-tolerance (48) in only a limited number of large animal models. In a pig model in which haplo-identical PBSC were transplanted at a dose of 1-2×1010/kg after profound in vivo T cell depletion with an immunotoxin-conjugated anti-CD3 mAb, the best results in terms of stable long-term chimerism and allograft tolerance required irradiation to the thymic area of 10 Gy (7,49). Very recently Larsen and colleagues succeeded in inducing transient mixed chimerism in rhesus monkeys (50). BM and PBSC yielded apparently comparable results with a costimulation blockade-based regimen that was supplemented with anti-CD25 mAb and rapamycin treatment. Overall, the limited experience in large animals shows that under certain circumstances PBSC are able to induce chimerism. Whether PBSC from large animals (and humans) indeed have a similar capacity as BM to establish chimerism and tolerance, or whether the lower capacity of PBSC did not yet become evident in the preliminary studies that have been performed to date, remains to be seen.
Hence, lasting chimerism and tolerance can be achieved after transplantation of a clinically relevant dose of PBSC under costimulation blockade and non-myeloablative TBI, if recipient conditioning is intensified by the addition of DST and short-course immunosuppression. However, severe GVHD uniformly develops in such chimeras. Thus, some form of T cell depletion (in vivo or in vitro) seems to be a critical part of experimental protocols inducing chimerism and tolerance through the transplantation of PBSC. The distinct properties of PBSC delineated herein warrant consideration when pre-clinical large animal tolerance protocols are developed.
ACKNOWLEDGMENTS
We thank Maria Weiss, Helga Bergmeister; Martin Ploder and Franz Winkler, for technical assistance, and Andreas Heitger for critical review of the manuscript.
Funding sources: This work was supported by grants from the Roche Organ Transplantation Research Foundation (ROTRF, #110578928), from the Jubilee Fund of the Austrian National Bank (#10809), and from the Austrian Science Fund (FWF, F2310).
ABBREVIATIONS
- BM
bone marrow
- BMC
bone marrow cells
- BMT
bone marrow transplantation
- CFU
colony forming unit
- CFU-GM
colony forming unit-granulocyte/monocyte progenitor
- DST
donor-specific transfusion
- G-CSF
granulocyte colony stimulating factor
- Gy
Gray
- HSC
hematopoietic stem cells
- HSCT
hematopoietic stem cell transplantation
- PBSC
mobilized peripheral blood stem cells
- PBSCT
mobilized peripheral blood stem cells transplantations
- PB
peripheral blood
- SPL
spleen
- TBI
total body irradiation
Footnotes
The authors declare no competing financial interests.
REFERENCES
- 1.Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353–370. doi: 10.1146/annurev.med.52.1.353. [DOI] [PubMed] [Google Scholar]
- 2.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomita Y, Sachs DH, Khan A, Sykes M. Additional mAb injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti-T cell mAbs and 3 Gy whole body irradiation. Transplantation. 1996;61:469–477. doi: 10.1097/00007890-199602150-00027. [DOI] [PubMed] [Google Scholar]
- 4.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wekerle T, Nikolic B, Pearson DA, Swenson KG, Sykes M. Minimal conditioning required in a murine model of T cell depletion, thymic irradiation and high-dose bone marrow transplantation for the induction of mixed chimerism and tolerance. Transpl Int. 2002;15:248–253. doi: 10.1007/s00147-002-0411-3. [DOI] [PubMed] [Google Scholar]
- 6.Sykes M, Szot GL, Swenson K, Pearson DA. Induction of high levels of allogeneic hematopoietic reconstitution and donor-specific tolerance without myelosuppressive conditioning. Nature Med. 1997;3:783–787. doi: 10.1038/nm0797-783. [DOI] [PubMed] [Google Scholar]
- 7.Fuchimoto Y, Huang CA, Shimizu A, et al. Mixed chimerism without whole body irradiation in a large animal model. J Clin Invest. 2000;105:1779–1789. doi: 10.1172/JCI8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pree I, Wekerle T. New appproaches to prevent transplant rejection: Co-stimulation blockers anti-CD40L and CTLA4Ig. Drug Discovery Today: Therapeutic Strategies. 2006;3:41–47. [Google Scholar]
- 9.Snanoudj R, de Preneuf H, Creput C, et al. Costimulation blockade and its possible future use in clinical transplantation. Transpl Int. 2006;19:693–704. doi: 10.1111/j.1432-2277.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 10.Wekerle T, Sayegh MH, Hill J, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams AB, Durham MM, Kean L, et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol. 2001;167:1103–1111. doi: 10.4049/jimmunol.167.2.1103. [DOI] [PubMed] [Google Scholar]
- 12.Westerhuis G, Maas WGE, Willemze R, Toes REM, Fibbe WE. Long-term mixed chimerism after immunologic conditioning and MHC-mismatched stem-cell transplantation is dependent on NK-cell tolerance. Blood. 2005;106:2215–2220. doi: 10.1182/blood-2005-04-1391. [DOI] [PubMed] [Google Scholar]
- 13.Seung E, Iwakoshi N, Woda BA, et al. Allogeneic hematopoietic chimerism in mice treated with sublethal myeloablation and anti-CD154 antibody: absence of graft-versus-host disease, induction of skin allograft tolerance, and prevention of recurrent autoimmunity in islet-allografted NOD/Lt mice. Blood. 2000;95:2175–2182. [PubMed] [Google Scholar]
- 14.Quesenberry PJ, Zhong S, Wang H, Stewart M. Allogeneic chimerism with low-dose irradiation, antigen presensitization, and costimulator blockade in H-2 mismatched mice. Blood. 2001;97:557–564. doi: 10.1182/blood.v97.2.557. [DOI] [PubMed] [Google Scholar]
- 15.Blaha P, Bigenzahn S, Koporc Z, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101:2886–2893. doi: 10.1182/blood-2002-10-3014. [DOI] [PubMed] [Google Scholar]
- 16.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nature Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 17.Durham MM, Bingaman AW, Adams AB, et al. Administration of anti-CD40 ligand and donor bone marrow leads to hematopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J Immunol. 2000;165:1–4. doi: 10.4049/jimmunol.165.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Kean LS, Hamby K, Koehn B, et al. NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am J Transplant. 2006;6:292–304. doi: 10.1111/j.1600-6143.2005.01172.x. [DOI] [PubMed] [Google Scholar]
- 19.Blaha P, Bigenzahn S, Koporc Z, Sykes M, Muehlbacher F, Wekerle T. Short-term immunosuppression facilitates induction of mixed chimerism and tolerance after bone marrow transplantation without cytoreductive conditioning. Transplantation. 2005;80:237–243. doi: 10.1097/01.tp.0000164510.25625.70. [DOI] [PubMed] [Google Scholar]
- 20.Graca L, Daley S, Fairchild P, Cobbold S, Waldmann H. Co-receptor and co-stimulation blockade for mixed chimerism and tolerance without myelosuppressive conditioning. BMC Immunology. 2006;7:9. doi: 10.1186/1471-2172-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;345:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 22.Korbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98:2900–2908. doi: 10.1182/blood.v98.10.2900. [DOI] [PubMed] [Google Scholar]
- 23.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–90. [PubMed] [Google Scholar]
- 24.Cutler C, Antin JH. Peripheral blood stem cells for allogeneic transplantation: A Review. Stem Cells. 2001;19:108–117. doi: 10.1634/stemcells.19-2-108. [DOI] [PubMed] [Google Scholar]
- 25.Gyger M, Stuart RK, Perreault C. Immunobiology of allogeneic peripheral blood mononuclear cells mobilized with granulocyte-colony stimulating factor. Bone Marrow Transplant. 2000;26:1–16. doi: 10.1038/sj.bmt.1702464. [DOI] [PubMed] [Google Scholar]
- 26.Pan L, Delmonte J, Jalonen CK, Ferrara JLM. Pretreatment of donor mice with granulocytes colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-vs-host disease. Blood. 1996;86:4422–4429. [PubMed] [Google Scholar]
- 27.Sloand EM, Kim S, Maciejewski JP, et al. Pharmacologic doses of granulocyte colony-stimulating factor affect cytokine production by lymphocytes in vitro and in vivo. Blood. 2000;95:2269–74. [PubMed] [Google Scholar]
- 28.Koporc Z, Bigenzahn S, Blaha P, et al. Induction of mixed chimerism through transplantation of CD45-congenic mobilized peripheral blood stem cells after nonmyeloablative irradiation. Biol Blood Marrow Transplant. 2006;12:284–92. doi: 10.1016/j.bbmt.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigenzahn S, Blaha P, Koporc Z, et al. The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant. 2005;5:1237–1247. doi: 10.1111/j.1600-6143.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 31.Pree I, Bigenzahn S, Fuchs D, et al. CTLA4Ig promotes the induction of hematopoietic chimerism and tolerance independently of indoleamine-2,3-dioxygenase (IDO) Transplantation. 2007;83:663–667. doi: 10.1097/01.tp.0000255594.23445.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi Y, Ito H, Kurtz J, Wekerle T, Ho L, Sykes M. Earlier low-dose TBI or DST overcomes CD8+ T-cell-mediated alloresistance to allogeneic marrow in recipients of anti-CD40L. Am J Transplant. 2004;4:31–40. doi: 10.1046/j.1600-6135.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 33.Kishimoto K, Dong VM, Issazadeh S, et al. The role of CD154-CD40 versus CD28-B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Invest. 2000;106:63–72. doi: 10.1172/JCI9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002;14:592–600. doi: 10.1016/s0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 35.Ringden O, Remberger M, Runde V, et al. Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation. Blood. 1999;94:455–64. [PubMed] [Google Scholar]
- 36.Martin PJ, Hansen JA, Buckner CD, et al. Effects of in vitro depletion of T cells in HLA-identical allogeneic marrow grafts. Blood. 1985;66:664–672. [PubMed] [Google Scholar]
- 37.Martin PJ, Hansen JA, Torok-Storb B, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant. 1988;3:445–456. [PubMed] [Google Scholar]
- 38.Pirsch JD, Maki DG. Infectious complications in adults with bone marrow transplantation and T-cell depletion of donor marrow. Increased susceptibility to fungal infections. Ann Intern Med. 1986;104:619–31. doi: 10.7326/0003-4819-104-5-619. [DOI] [PubMed] [Google Scholar]
- 39.Seidl MG, Fritsch G, Matthes-Martin S, et al. In vitro an in vivo T-cell depletion with myeloablative or reduced-intensity conditioning in pediatric hematopoietic stem cell transplantation. Haematologica. 2005;90:1405–14. [PubMed] [Google Scholar]
- 40.Taylor PA, Lees CJ, Wilson JM, et al. Combined effects of calcineurin inhibitors or sirolimus with anti-CD40L mAb on alloengraftment under nonmyeloablative conditions. Blood. 2002;100:3400–3407. doi: 10.1182/blood-2002-03-0872. [DOI] [PubMed] [Google Scholar]
- 41.Seung E, Mordes JP, Rossini AA, Greiner DL. Hematopoietic chimerism and central tolerance created by peripheral-tolerance induction without myeloablative conditioning. J Clin Invest. 2003;112:795–808. doi: 10.1172/JCI18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashton-Chess J, Brouard S, Soulillou J-P. Is clinical tolerance realistic in the next decade? Transpl Int. 2006;19:539–548. doi: 10.1111/j.1432-2277.2006.00339.x. [DOI] [PubMed] [Google Scholar]
- 43.Pelot MR, Pearson DA, Swenson K, et al. Lymphohematopoietic graft-vs-host reactions can be induced without graft-vs-host disease in murine mixed chimeras established with a cyclophosphamide-based non-myeloablative conditioning regimen. Biol.Blood Marrow Transplant. 1999;5:133–143. doi: 10.1053/bbmt.1999.v5.pm10392959. [DOI] [PubMed] [Google Scholar]
- 44.Kim Y-M, Mapara MY, Down JD, et al. Graft-versus-host-reactive donor CD4 cells can induce T cell-mediated rejection of the donor marrow in mixed allogeneic chimeras prepared with nonmyeloablative conditioning. Blood. 2004;103:732–739. doi: 10.1182/blood-2003-02-0643. [DOI] [PubMed] [Google Scholar]
- 45.Heitger A, Blaha P, Bigenzahn S, Muehlbacher F, Wekerle T. The influence of immunosuppressive drugs on cell-induced graft tolerance. Curr Opin Organ Transplant. 2004;9:307–313. [Google Scholar]
- 46.Konieczny BT, Dai Z, Elwood ET, et al. IFN-γ is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J.Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 47.Colby C, Chang Q, Fuchimoto Y, et al. Cytokine-mobilized peripheral blood progenitor cells for allogeneic reconstitution of miniature swine. Transplantation. 2000;69:135–140. doi: 10.1097/00007890-200001150-00023. [DOI] [PubMed] [Google Scholar]
- 48.Buhler L, Awwad M, Treter S, et al. Pig hematopoietic cell chimerism in baboons conditioned with a nonmyeloablative regiment and CD154 blockade. Transplantation. 2002;73:12–22. doi: 10.1097/00007890-200201150-00004. [DOI] [PubMed] [Google Scholar]
- 49.Gleit ZL, Fuchimoto Y, Yamada K, et al. Variable relationship between chimerism and tolerance after hematopoietic cell transplantation without myelosuppressive conditioning. Transplantation. 2002;74:1535–1544. doi: 10.1097/00007890-200212150-00010. [DOI] [PubMed] [Google Scholar]
- 50.Kean LS, Adams AB, Strobert E, et al. Induction of chimerism in rhesus macaques through stem cell transplant and costimulation blockade-based immunosuppression. Am J Transplant. 2007;7:320–335. doi: 10.1111/j.1600-6143.2006.01622.x. [DOI] [PubMed] [Google Scholar]