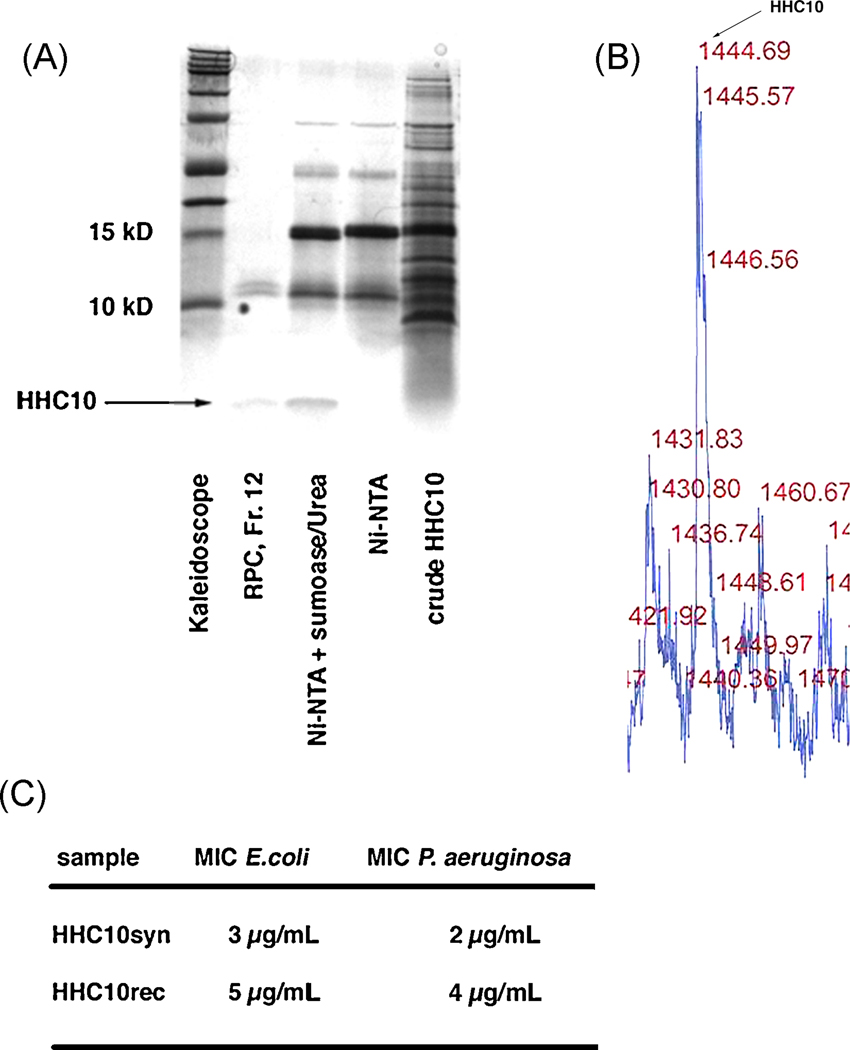
Figure 5.
A. Tricine-SDS-PAGE of the purification steps used to achieve pure HHC-10 from a 1 L shaking flask expression, fraction 12 in lane 2 corresponds to the sample used for mass determination. Again, no larger protein contaminants could be found, indicating that the higher bands visible in the gel are possibly aggregates of HHC-10, which could be avoided in a different solvent.
B. MALDI profile for HHC-10 showing the correct mass of 1444 Da.
C. Direct comparison of MIC for both synthetic and recombinant HHC-10 using E. coli K12 and P. aeruginosa PA014. The recombinant HHC10 showed a slightly higher MIC for E. coli and P. aeruginosa.