Abstract
The histological distinction between bronchioloalveolar carcinoma (BAC) and other adenocarcinomas is tissue invasion. The clinical importance of lung adenocarcinoma invasion is supported by several recent studies indicating that the risk of death in non-mucinous BAC is significantly lower than that of pure invasive tumors and in tumors with greater than 0.6 cm of fibrosis or linear invasion. Using microarray gene expression profiling of human tumors, dysregulation of transforming growth factor-ß (TGF-ß) signaling was identified as an important mediator of tumor invasion. Subsequent studies showed that the CC chemokine RANTES (Regulated on Activation, Normal T-cell Expressed, and presumably Secreted) was upregulated in invasive tumors and was required for invasion in cells with repressed levels of the TGF-ß type II receptor. Taken together, these studies illustrate how information gained from global expression profiling of tumors can be used to identify key pathways and genes mediating tumor growth, invasion, and metastasis.
The World Health Organization subclassifies adenocarcinoma based upon predominant cell morphology and growth pattern.1. The histological distinction between bronchioloalveolar carcinoma (BAC) and other adenocarcinomas is tissue invasion. BAC tumor cells are cuboidal to columnar, with or without mucin, that grow in a noninvasive fashion along alveolar walls. Invasion, defined as tumor disruption of the alveolar basement membrane, is present in other subtypes of adenocarcinoma. Adenocarcinomas with mixed subtypes frequently contain regions of lepidic/noninvasive tumor at the periphery of invasive tumor.
Recent clinical reports suggest that the prognosis and radiographic appearance of BAC is unique and may support modifying the clinical approach to lung adenocarcinomas according to histological subtype. Metastases to lymph nodes and extrathoracic organs are unusual in nonmucinous BAC. The mean five year survival for Stage I BAC and other adenocarcinomas is 81% and 55%, respectively 2. Recent reports suggest that for Stage IA BAC, limited resections rather than lobectomy, which is the current standard resection for Stage IA adenocarcinoma, may be curative3. Notably, low dose chest CT screening detected lung cancer is more likely to be adenocarcinoma than conventionally detected cancer (75% versus 40%)4, 5. In addition, 25% of screen detected cancers are BAC. As a result, the identification of invasion in screen detected malignancy may in the future guide a therapeutic decision of limited versus anatomic resection.
Paralleling malignancies in other organs, such as breast and cervix, where tumors are defined as non-invasive (in-situ carcinoma), micro-invasive (microscopic invasion) or as invasive carcinomas, the extent of the invasive component seen in lung adenocarcinoma is associated with clinical outcomes. The clinical importance of lung adenocarcinoma invasion is supported by several recent studies 2, 6-9 indicating that the risk of death in non-mucinous BAC is significantly lower than that of pure invasive tumors and in tumors with greater than 0.6 cm of fibrosis or linear invasion. In 200 cases of small adenocarcinomas (diameter < 3 cm), Yokose reported no deaths among 66 BAC cases10. In 484 cases of BAC and adenocarcinoma, Terasaki reported that lymph node involvement was absent in all BAC and was present in 20% of adenocarcinomas that had an invasive area greater than 5 mm11. Similarly, among 178 patients with resected lung adenocarcinoma we found five year survival rates of 100% and 90% for patients with BAC or tumors with invasive length less than 6 mm, respectively12. Together, these studies suggest that non-invasive tumors are biologically indolent and that invasion increases the risk of metastatic disease and death in solitary mixed subtype tumors.
Invasion is the first step of carcinoma metastasis, in which epithelial cells lose cell-cell adhesion, gain motility and invade into adjacent stroma. Subsequent steps of metastasis include vascular intravasation and extravasation, establishment of a metastatic niche and angiogenesis.13. Tumor invasiveness, the morphologic characteristic that distinguishes BAC from adenocarcinoma, is determined by the interaction of tumor cells with the surrounding stroma 14, 15.
We 16 and others 17-19 20 have used microarray gene expression profiling of lung adenocarcinoma to identify signatures associated with histology and invasion. The results of unsupervised analyses, in which the specimens are sorted into groups in a dendogram based upon similarity of gene expression, show lung adenocarcinomas segregate into three major branches comprised predominantly of BAC, AC-Mixed subtype, and pure invasive tumors. These results provide biological plausibility to support the notion that these adenocarcinoma subtypes are distinct entities. Taken together with the clinical prognostic data, these studies have motivated efforts to reinforce the designation of purely noninvasive tumors and to create a designation for minimally invasive tumors in a revision of the WHO lung adenocarcinoma classification scheme.
To identify molecular pathways important for mediating the acquisition of invasion by lung adenocarcinoma, we performed supervised analysis of mRNA microarray data to identify genes differentially expressed in non-invasive BAC and in AC-mixed type tumors. Among the genes differentially expressed in the progression from BAC to invasive tumors was the transforming growth factor-ß (TGF-ß) type II receptor (TβRII), which was less highly expressed by AC-Mixed and solid invasive tumors compared with BAC. This finding, which suggested that TβRII repression was required for lung adenocarcinoma invasion, is supported by genetic models combining targeted deletion of TβRII with other oncogenic events such as Adenomatosis polyposis coli (APC) mutation in colon tumors and KRAS mutations in pancreatic and oropharyngeal carcinomas 21-23. The phenotypes of these TGF-β receptor cancer models clearly demonstrate the importance of TGF-β signaling in tumor invasion.
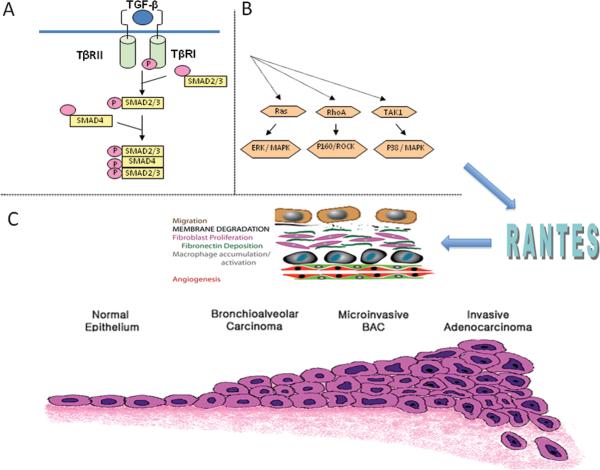
TGF-β, the ligand for the TGF-β type II receptor is a pleiotropic cytokine comprised of family members TGF-β 1, 2, 3 that regulate tissue homeostasis and prevent tumor initiation by inhibiting cellular proliferation, differentiation, and survival 24. It is secreted as a latent molecule and is activated by cleavage by proteases and other molecules25. Signaling primarily occurs through SMAD protein dependent pathways whereby ligand binding to TBRII induces phosphorylation and activation of TGF-β type I receptor (TβRI). After interaction with TβRI, phosphorylated SMAD2 and SMAD3 dissociate to form a heterotrimeric complex with SMAD4 and translocate into the nucleus to regulate gene transcription (Figure 1A). TGF-β signaling may also proceed via less well understood SMAD independent pathways (Figure 1B). These “non-canonical” pathways involve various signaling cascades including Ras/ERK, Rho/ROCK, and TAK1/MAPK, and are likely to have important roles in mediating the pro-tumorigenic effects of TGF-β 26. Depending upon context, TGF-β signaling may alternatively function to suppress tumor growth or to promote tumor cell invasion and metastasis 27-30.
Figure 1.
TGF-β signaling occurs primarily via SMAD dependent pathways. A. Ligand binding to the TGF-β type II receptor (TβRII) induces phosphorylation and activation of type I receptor (TβRI), which phosphorylates and activates the receptor complex SMAD2 and SMAD3. Dissociated SMAD2/3 forms a heterotrimeric complex with SMAD4 that translocates into the nucleus to regulate gene transcription.
B. TGF-β signaling may also proceed via SMAD-independent pathways that involve various signaling cascades including Ras/ERK, Rho/ROCK, and TAK1/MAPK. These “non-canonical” pathways are likely to have important roles in mediating the pro-tumorigenic effects of TGF-β.
C. The histological distinction between bronchioloalveolar carcinoma (BAC) and other adenocarcinomas is tissue invasion. Invasion requires loss of cell-cell adhesion, migration, membrane degradation with vascular intravasation and extravasation and establishment of the metastatic niche angiogenesis and recruitment of stromal elements (top panel). We have shown that repression of TGF-β type II receptor in lung adenocarcinoma cells increases invasiveness and have used microarray analyses and inhibitor studies to identify the CC chemokine RANTES as an important mediator of lung adenocarcinoma invasion in TβRII deficient tumors. Figure reprinted81 with permission from the American Thoracic Society
TGF-β as a Tumor Suppressor
Although recent research has focused primarily on TGF-β receptor alterations, tumors may employ various mechanisms anywhere along the signaling cascade to circumvent the inhibitory effects of TGF-β31-35. Type II receptor genetic alterations are well characterized in gastrointestinal tumors, in which 25% of colorectal carcinomas have missense mutations associated with microsatellite instability. Animals with targeted deletion of TßRII in the colonic epithelium demonstrate increased tumor progression from adenomas to invasive carcinomas 36 similar to human colorectal tumors with loss of type II receptor 37. In breast carcinoma models, mammary tumors in animals with targeted deletion of TßRII demonstrated increased progression and metastases 38. A recent case control study in human breast tumors indicated that within breast hyperplasia specimens, the proportion of cells with decreased type II receptor immunostaining was associated with increased risk for the development of invasive breast cancer 39. Multiple lung cancer cell lines, both small cell 40-42 and non-small cell 43-46, demonstrate reduced expression TGFβRII. This repression is accompanied by marked reductions in TGF-β mediated growth suppression which is rescued after restoration of the receptor. In human lung tumor specimens, type II receptor repression is evident in ~40% of lung adenocarcinomas overall and in up to 100% of poorly differentiated adenocarcinomas 47. Mechanisms of repression include epigenetic silencing 48, microsatellite instability, and frameshift mutations involving the poly(A) tract 43. For the TGF-β type I receptor, mRNA repression is detectable in non-small cell lung cancer (NSCLC) 49, and recent studies indicate that TβRI SNP variants are associated with an increased risk of lung cancer 50-52.
TGF-β as a Tumor Promoter
Several tumors, including those arising in the lung 53, 54, 55 express high levels of the TGF-β, which correlates with tumor progression and clinical prognosis 34, 56-60. TGF-β signaling promotes epithelial to mesenchymal transition, a characteristic of invasive and metastatic cells 61, 62, with constitutive activation of TGF-β or TβRI leading to increased metastases in animal models of breast cancer 63-65. Likewise, blockade of TGF-β signaling via either dominant negative expression of SMAD3 or defective TβRI leads to decreased lung metastases 66, 67. Systemic inhibition of TGF-β has been shown to suppress metastasis 68-71 and TGF-β overexpression by NSCLC specimens was found by multivariate analysis to be an independent risk factor for pulmonary metastasis 72.
How do we reconcile these findings with those suggesting TGF-β is a tumor suppressor? Context dependency in terms of cell type, tumor stage, and mode of inhibition of TGF-β signaling are important. Other important issues are the degree of repression of TGF-β receptor levels and the stromal response to TGF-β signaling inhibition. Rojas and colleagues have shown that different levels of repression of the TGF-β receptor are associated with differences in the activation of the SMAD and MAPK pathways such that at lower levels of TGF-β receptor activation, the pro-tumorigenic non-SMAD signaling pathways dominate 73. Yang and colleagues showed that targeted deletion of TβRII in the mammary epithelium promoted breast cancer metastases through the CXCL5/CXCR2 chemokine axis mediated recruitment of Gr-1+/CD11b+ myeloid derived suppressor cells. Increased stromal TGF-β levels at the invasive front of tumors was shown to be important for tumor progression and for inhibition of tumor immunosurveillance 74.
Chemokine Signaling in Human Tumors with Repressed TβRII expression- CCL5
Our results in lung adenocarcinoma and in in vitro systems indicate that repression of the TGF-β type II receptor increases invasiveness. We have shown that activation of SMAD2 and Akt are lower in TβRII knock-down cells while p38 activation is slightly increased 16. We expect that TGF-β signaling in cells with moderately reduced type II receptor levels persists in the invasive tumors and in the knock-down cells and that SMAD independent pathways modulate this effect 73, 75. We used a tumor cell invasion system and microarray analysis to identify and characterize downstream mediators of TGF-ß signaling important for lung adenocarcinoma invasion 16. Among potential mediators identified was the CC (or β-chemokine) family member CCL5 (RANTES), which was upregulated by invasive tumors and TßRII knockdown cells. RANTES is involved in immunoregulatory and inflammatory processes and is secreted by T cells and other inflammatory cells, stromal cells, as well as tumor cells and normal bronchial epithelium. RANTES is a ligand for chemokine receptors CCR1, CCR3, CCR4, and CCR5, which are expressed on epithelial cells, macrophages, lymphocytes, dendritic cells and stromal cells 76-79. Inhibition of RANTES signaling significantly abrogates tumor invasion, suggesting that RANTES is required for invasion in TGF-β type II receptor repressed lung adenocarcinoma cells (Figure 1C). The clinical significance of this pathway is further supported by the finding that tumor expression of RANTES and CCR5 in lung adenocarcinoma is associated with patient survival80. Small molecule inhibitors of CCR5 may have the potential to treat and prevent lung adenocarcinoma. Taken together, these studies illustrate how information gained from global expression profiling of tumors can be used to identify key pathways and genes mediating tumor growth, invasion, and metastasis.
References
- 1.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–68. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 2.Rena O, Papalia E, Ruffini E, et al. Stage I pure bronchioloalveolar carcinoma: recurrences, survival and comparison with adenocarcinoma of the lung. Eur J Cardiothorac Surg. 2003;23:409–14. doi: 10.1016/s1010-7940(02)00830-8. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama H, Yamada K, Saito H, et al. Sublobar resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg. 2007;84:1675–9. doi: 10.1016/j.athoracsur.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 5.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med. 2002;165:508–13. doi: 10.1164/ajrccm.165.4.2107006. [DOI] [PubMed] [Google Scholar]
- 6.Maeshima AM, Niki T, Maeshima A, Yamada T, Kondo H, Matsuno Y. Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546–54. doi: 10.1002/cncr.11006. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol. 2004;28:198–206. doi: 10.1097/00000478-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Yokose T, Yoshida J, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000;69:893–7. doi: 10.1016/s0003-4975(99)01331-4. [DOI] [PubMed] [Google Scholar]
- 9.Yim J, Zhu LC, Chiriboga L, Watson HN, Goldberg JD, Moreira AL. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol. 2007;20:233–41. doi: 10.1038/modpathol.3800734. [DOI] [PubMed] [Google Scholar]
- 10.Yokose T, Suzuki K, Nagai K, Nishiwaki Y, Sasaki S, Ochiai A. Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer. 2000;29:179–88. doi: 10.1016/s0169-5002(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 11.Terasaki H, Niki T, Matsuno Y, et al. Lung adenocarcinoma with mixed bronchioloalveolar and invasive components: clinicopathological features, subclassification by extent of invasive foci, and immunohistochemical characterization. Am J Surg Pathol. 2003;27:937–51. doi: 10.1097/00000478-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol. 2009;33:462–9. doi: 10.1097/PAS.0b013e318190157c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 14.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–84. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 16.Borczuk AC, Kim HK, Yegen HA, Friedman RA, Powell CA. Lung adenocarcinoma global profiling identifies type II transforming growth factor-beta receptor as a repressor of invasiveness. Am J Respir Crit Care Med. 2005;172:729–37. doi: 10.1164/rccm.200504-615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi T, Tomida S, Yatabe Y, et al. Expression Profile-Defined Classification of Lung Adenocarcinoma Shows Close Relationship With Underlying Major Genetic Changes and Clinicopathologic Behaviors. Journal of Clinical Oncology. 2006;24:1679–88. doi: 10.1200/JCO.2005.03.8224. [DOI] [PubMed] [Google Scholar]
- 18.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–27. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 19.Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–24. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 20.Shedden K, Taylor JM, Enkemann SA, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med. 2008;14:822–7. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ijichi H, Chytil A, Gorska AE, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–60. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu SL, Herrington H, Reh D, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–42. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz NM, Upton M, Rojas A, et al. Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Res. 2006;66:9837–44. doi: 10.1158/0008-5472.CAN-06-0890. [DOI] [PubMed] [Google Scholar]
- 24.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–21. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 25.Yoshinaga K, Obata H, Jurukovski V, et al. Perturbation of transforming growth factor (TGF)-beta1 association with latent TGF-beta binding protein yields inflammation and tumors. Proc Natl Acad Sci U S A. 2008;105:18758–63. doi: 10.1073/pnas.0805411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 27.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 28.Elliott RL, Blobe GC. Role of Transforming Growth Factor Beta in Human Cancer. J Clin Oncol. 2005;23:2078–93. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 29.Tang B, Vu M, Booker T, et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–24. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markowitz S, Wang J, Myeroff L, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–8. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 32.Kang SH, Bang YJ, Im YH, et al. Transcriptional repression of the transforming growth factor-beta type I receptor gene by DNA methylation results in the development of TGF-beta resistance in human gastric cancer. Oncogene. 1999;18:7280–6. doi: 10.1038/sj.onc.1203146. [DOI] [PubMed] [Google Scholar]
- 33.Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Im YH, Markowitz SD, Bang YJ. Molecular mechanisms of inactivation of TGF-beta receptors during carcinogenesis. Cytokine Growth Factor Rev. 2000;11:159–68. doi: 10.1016/s1359-6101(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 36.Biswas S, Chytil A, Washington K, et al. Transforming growth factor beta receptor type II inactivation promotes the establishment and progression of colon cancer. Cancer Res. 2004;64:4687–92. doi: 10.1158/0008-5472.CAN-03-3255. [DOI] [PubMed] [Google Scholar]
- 37.Grady WM, Rajput A, Myeroff L, et al. Mutation of the type II transforming growth factor-beta receptor is coincident with the transformation of human colon adenomas to malignant carcinomas. Cancer Res. 1998;58:3101–4. [PubMed] [Google Scholar]
- 38.Forrester E, Chytil A, Bierie B, et al. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 39.Gobbi H, Dupont WD, Simpson JF, et al. Transforming growth factor-beta and breast cancer risk in women with mammary epithelial hyperplasia. J Natl Cancer Inst. 1999;91:2096–101. doi: 10.1093/jnci/91.24.2096. [DOI] [PubMed] [Google Scholar]
- 40.Hougaard S, Norgaard P, Abrahamsen N, Moses HL, Spang-Thomsen M, Skovgaard Poulsen H. Inactivation of the transforming growth factor beta type II receptor in human small cell lung cancer cell lines. Br J Cancer. 1999;79:1005–11. doi: 10.1038/sj.bjc.6690161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damstrup L, Rygaard K, Spang-Thomsen M, Skovgaard Poulsen H. Expression of transforming growth factor beta (TGF beta) receptors and expression of TGF beta 1, TGF beta 2 and TGF beta 3 in human small cell lung cancer cell lines. Br J Cancer. 1993;67:1015–21. doi: 10.1038/bjc.1993.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jonge RR, Garrigue-Antar L, Vellucci VF, Reiss M. Frequent inactivation of the transforming growth factor beta type II receptor in small-cell lung carcinoma cells. Oncol Res. 1997;9:89–98. [PubMed] [Google Scholar]
- 43.Kim WS, Park C, Hong SK, Park BK, Kim HS, Park K. Microsatellite instability(MSI) in non-small cell lung cancer(NSCLC) is highly associated with transforming growth factor-beta type II receptor(TGF-beta RII) frameshift mutation. Anticancer Res. 2000;20:1499–502. [PubMed] [Google Scholar]
- 44.Kim TK, Mo EK, Yoo CG, et al. Alteration of cell growth and morphology by overexpression of transforming growth factor beta type II receptor in human lung adenocarcinoma cells. Lung Cancer. 2001;31:181–91. doi: 10.1016/s0169-5002(00)00169-0. [DOI] [PubMed] [Google Scholar]
- 45.Park C, Kim WS, Choi Y, Kim H, Park K. Effects of transforming growth factor beta (TGF-beta) receptor on lung carcinogenesis. Lung Cancer. 2002;38:143–7. doi: 10.1016/s0169-5002(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 46.Anumanthan G, Halder SK, Osada H, et al. Restoration of TGF-beta signalling reduces tumorigenicity in human lung cancer cells. Br J Cancer. 2005;93:1157–67. doi: 10.1038/sj.bjc.6602831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang Y, Prentice MA, Mariano JM, et al. Transforming growth factor-beta 1 and its receptors in human lung cancer and mouse lung carcinogenesis. Exp Lung Res. 2000;26:685–707. doi: 10.1080/01902140150216765. [DOI] [PubMed] [Google Scholar]
- 48.Zhang HT, Chen XF, Wang MH, et al. Defective expression of transforming growth factor beta receptor type II is associated with CpG methylated promoter in primary non-small cell lung cancer. Clin Cancer Res. 2004;10:2359–67. doi: 10.1158/1078-0432.ccr-0959-3. [DOI] [PubMed] [Google Scholar]
- 49.Zhao J, Liu Z, Li W, Liu X, Chen XF, Zhang HT. Infrequently methylated event at sites -362 to -142 in the promoter of TGF beta R1 gene in non-small cell lung cancer. J Cancer Res Clin Oncol. 2008;134:919–25. doi: 10.1007/s00432-008-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang HT, Fei QY, Chen F, et al. Mutational analysis of the transforming growth factor beta receptor type I gene in primary non-small cell lung cancer. Lung Cancer. 2003;40:281–7. doi: 10.1016/s0169-5002(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 51.Kang HG, Chae MH, Park JM, et al. Polymorphisms in TGF-beta1 gene and the risk of lung cancer. Lung Cancer. 2006;52:1–7. doi: 10.1016/j.lungcan.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Park KH, Lo Han SG, Whang YM, et al. Single nucleotide polymorphisms of the TGFB1 gene and lung cancer risk in a Korean population. Cancer Genet Cytogenet. 2006;169:39–44. doi: 10.1016/j.cancergencyto.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Barthelemy-Brichant N, David JL, Bosquee L, et al. Increased TGFbeta1 plasma level in patients with lung cancer: potential mechanisms. Eur J Clin Invest. 2002;32:193–8. doi: 10.1046/j.1365-2362.2002.00956.x. [DOI] [PubMed] [Google Scholar]
- 54.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 55.Domagala-Kulawik J, Hoser G, Safianowska A, Grubek-Jaworska H, Chazan R. Elevated TGF-beta1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Arch Immunol Ther Exp (Warsz) 2006;54:143–7. doi: 10.1007/s00005-006-0016-0. [DOI] [PubMed] [Google Scholar]
- 56.Bruna A, Darken RS, Rojo F, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–60. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer. 2001;91:964–71. [PubMed] [Google Scholar]
- 58.Saito H, Tsujitani S, Oka S, et al. The expression of transforming growth factor-beta1 is significantly correlated with the expression of vascular endothelial growth factor and poor prognosis of patients with advanced gastric carcinoma. Cancer. 1999;86:1455–62. doi: 10.1002/(sici)1097-0142(19991015)86:8<1455::aid-cncr11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 59.Tsushima H, Kawata S, Tamura S, et al. High levels of transforming growth factor beta 1 in patients with colorectal cancer: association with disease progression. Gastroenterology. 1996;110:375–82. doi: 10.1053/gast.1996.v110.pm8566583. [DOI] [PubMed] [Google Scholar]
- 60.Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37:19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–52. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- 62.Deckers M, van Dinther M, Buijs J, et al. The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006;66:2202–9. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- 63.Muraoka-Cook RS, Kurokawa H, Koh Y, et al. Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004;64:9002–11. doi: 10.1158/0008-5472.CAN-04-2111. [DOI] [PubMed] [Google Scholar]
- 64.Muraoka-Cook RS, Shin I, Yi JY, et al. Activated type I TGFbeta receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene. 2006;25:3408–23. doi: 10.1038/sj.onc.1208964. [DOI] [PubMed] [Google Scholar]
- 65.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A. 2003;100:8430–5. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian F, Byfield SD, Parks WT, et al. Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res. 2004;64:4523–30. doi: 10.1158/0008-5472.CAN-04-0030. [DOI] [PubMed] [Google Scholar]
- 67.Tian F, DaCosta Byfield S, Parks WT, et al. Reduction in Smad2/3 signaling enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res. 2003;63:8284–92. [PubMed] [Google Scholar]
- 68.Bandyopadhyay A, Agyin JK, Wang L, et al. Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. Cancer Res. 2006;66:6714–21. doi: 10.1158/0008-5472.CAN-05-3565. [DOI] [PubMed] [Google Scholar]
- 69.Yang YA, Dukhanina O, Tang B, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–15. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biswas S, Guix M, Rinehart C, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305–13. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nam JS, Terabe M, Mamura M, et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–43. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saji H, Nakamura H, Awut I, et al. Significance of expression of TGF-beta in pulmonary metastasis in non-small cell lung cancer tissues. Ann Thorac Cardiovasc Surg. 2003;9:295–300. [PubMed] [Google Scholar]
- 73.Rojas A, Padidam M, Cress D, Grady WM. TGF-beta receptor levels regulate the specificity of signaling pathway activation and biological effects of TGF-beta. Biochim Biophys Acta. 2009;1793:1165–73. doi: 10.1016/j.bbamcr.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 Signaling Is Necessary for Transforming Growth Factor-beta Activation of p38MAPK and Epithelial Plasticity. J Biol Chem. 2001;276:46707–13. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 76.Fraziano M, Cappelli G, Santucci M, et al. Expression of CCR5 is increased in human monocyte-derived macrophages and alveolar macrophages in the course of in vivo and in vitro Mycobacterium tuberculosis infection. AIDS Res Hum Retroviruses. 1999;15:869–74. doi: 10.1089/088922299310575. [DOI] [PubMed] [Google Scholar]
- 77.Kunkel EJ, Boisvert J, Murphy K, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002;160:347–55. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma B, Liu W, Homer RJ, et al. Role of CCR5 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2006;176:4968–78. doi: 10.4049/jimmunol.176.8.4968. [DOI] [PubMed] [Google Scholar]
- 79.van Deventer HW, O'Connor W, Jr., Brickey WJ, Aris RM, Ting JP, Serody JS. C-C chemokine receptor 5 on stromal cells promotes pulmonary metastasis. Cancer Res. 2005;65:3374–9. doi: 10.1158/0008-5472.CAN-04-2616. [DOI] [PubMed] [Google Scholar]
- 80.Borczuk AC, Papanikolaou N, Toonkel RL, et al. Lung adenocarcinoma invasion in TGFbetaRII-deficient cells is mediated by CCL5/RANTES. Oncogene. 2008;27:557–64. doi: 10.1038/sj.onc.1210662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borczuk AC, Toonkel RL, Powell CA. Genomics of lung cancer. Proc Am Thorac Soc. 2009;6:152–8. doi: 10.1513/pats.200807-076LC. [DOI] [PMC free article] [PubMed] [Google Scholar]