Abstract
Methylmercury (MeHg) is a highly neurotoxic pollutant, whose mechanisms of toxicity are related to its pro-oxidative properties. A previous report showed under in vivo conditions the neuroprotective effects of plants of the genus Polygala against MeHg-induced neurotoxicity. Moreover, the flavonoid quercetin, isolated from Polygala sabulosa, displayed beneficial effects against MeHg-induced oxidative damage under in vitro conditions. In this study, we sought for potential beneficial effects of quercetin against the neurotoxicity induced by MeHg in Swiss female mice. Animals were divided into six experimental groups: control, quercetin low dose (5 mg/Kg), quercetin high dose (50 mg/Kg), MeHg (40 mg/L, in tap water), MeHg + quercetin low dose, and MeHg + quercetin high dose. After the treatment (21 days), a significant motor deficit was observed in MeHg + quercetin groups. Biochemical parameters related to oxidative stress showed that the simultaneous treatment with quercetin and MeHg caused a higher cerebellar oxidative damage when compared to the individual exposures. MeHg plus quercetin elicited a higher cerebellar lipid peroxidation than MeHg or quercetin alone. The present results indicate that under in vivo conditions quercetin and MeHg cause additive pro-oxidative effects toward the mice cerebellum and that such phenomenon is associated with the observed motor deficit.
Keywords: Methylmercury, quercetin, neurotoxicity, Polygala
Introduction
Methylmercury (MeHg) is a highly neurotoxic compound that leads to neurological and developmental deficits in both animals and humans (Clarkson et al., 2003). Even though MeHg-induced neurotoxicity is a widely reported phenomenon, the molecular mechanisms related to its toxicity are not completely understood. Mechanisms involved in MeHg neurotoxicity include, but are not limimted to, (1) impairment of intracellular calcium homeostasis (Sirois and Atchison, 2000), (2) oxidative stress (Manfroi et al., 2004; Stringari et al., 2006) and (3) altered glutamate homeostasis (Aschner et al., 2007). Of particular importance, the antioxidant glutathione (GSH) system appears to be an important molecular target of MeHg-induced neurotoxicity (Stringari et al., 2008), corroborated by decreased GSH levels and activities of GSH-related enzymes in the brain of MeHg-exposed animals. Notably, female mice are less sensitive to MeHg-induced pro-oxidative damage when compared to male animals (Rossi et al., 1997; unpublished data from our laboratory).
Despite massive efforts in search of new drugs that counteract mercurial toxicity, there are no effective treatments available that completely abolish its toxic effects. In MeHg poisoning, supportive care is given when necessary to maintain vital functions. In addition, the use of chelating agents assists the body’s ability to eliminate mercury from the tissues (Pingree et al., 2001; Carvalho et al., 2007). However, these drugs appear to be of limited use because of their adverse side effects (Tchounwou et al., 2003) and limited ability to cross the blood-brain barrier (Aposhian et al., 1995).
Some studies have focused their efforts on the protective effects of plants or natural compounds in various neuropathological conditions. Of particular importance, the beneficial effects of plants/natural compounds against metal-induced neurotoxicity have been reported under both in vitro and in vivo conditions (Xu et al., 2005; Gupta & Flora, 2006; Franco et al., 2007). In this regard, a recent study from our group (Farina et al., 2005a) has shown the protective effects of the hydroalcoholic extract of Polygala paniculata - a plant traditionally used in folk medicine as a sedative and an anti-inflammatory (Lee et al., 2004) - against MeHg-induced neurotoxicity in mice. Moreover, we also found that quercetin, a flavonoid isolated from Polygala sabulosa, decreased MeHg-induced hydrogen peroxide generetion in mice brain mitochondria (Franco et al., 2007) and modulated astrocyte metallothionein mRNA expression (Conklin et al., 1998) under in vitro conditions. Although several studies have shown the antioxidant effects of quercetin and related compounds against pro-oxidavive damage (Wagner et al., 2006; Meotti et al., 2007), including metal-induced toxicity (Mishra and Flora, 2008), the potential beneficial effect of quercetin against MeHg-induced neurotoxicity was not yet investigated in in vivo conditions.
There is evidence that cerebellar cells are selectively targeted by mercurials in vivo (Sanfeliu et al., 2003) and that MeHg neurotoxicity affects the motor system (Grandjean et al., 1997). The relationship between MeHg-induced motor deficit and MeHg-induced cerebellar damage is a well-described phenomenon (Sakamoto et al., 1993). In this regard, we have reported motor alterations in animals exposed to MeHg during adulthood (Dietrich et al., 2005; Farina et al., 2005b) and the postnatal period (Manfroi et al., 2004; Stringari et al., 2006). From a molecular point of view, it is proposed that the observed harmful effects of MeHg on motor performance are related, at least in part, to its deleterious pro-oxidative effects in the cerebellum (Franco et al., 2006). (Manfroi et al., 2004; Stringari et al., 2006).
Taking into account that (i) there are no effective treatments for MeHg poisoning; (ii) plants of the genus Polygala have presented beneficial effects against MeHg-induced neurotoxicity in experimental in vivo conditions; (iii) Polygala sabulosa-derived quercetin presents protective effects against mercurial-induced oxidative stress under experimental in vitro conditions; (iv) the cerebellar antioxidant GSH system represents an important molecular target involved in MeHg-induced neurotoxicity, the present study was aimed at the investigation of potential neuroprotective effects of quercetin in MeHg-exposed mice. Considering the relationship between motor impairment and cerebellar oxidative damage after MeHg exposure, both phenomena were used for evaluating neurotoxicity and neuroprotection.
Materials and methods
Chemicals and plant material
Methylmercury(II) chloride was from Aldrich Chemical Co. (Milwaukee, WI, USA). β-Nicotinamide adenine dinucleotide phosphate sodium salt, reduced form, 5,5′-dithio-bis (2-nitrobenzoic) acid, GR from Baker's yeast, and reduced GSH were obtained from Sigma (St. Louis, MO, USA). All other chemicals were of the highest grade available commercially.
Plant Material, Extraction and Isolation
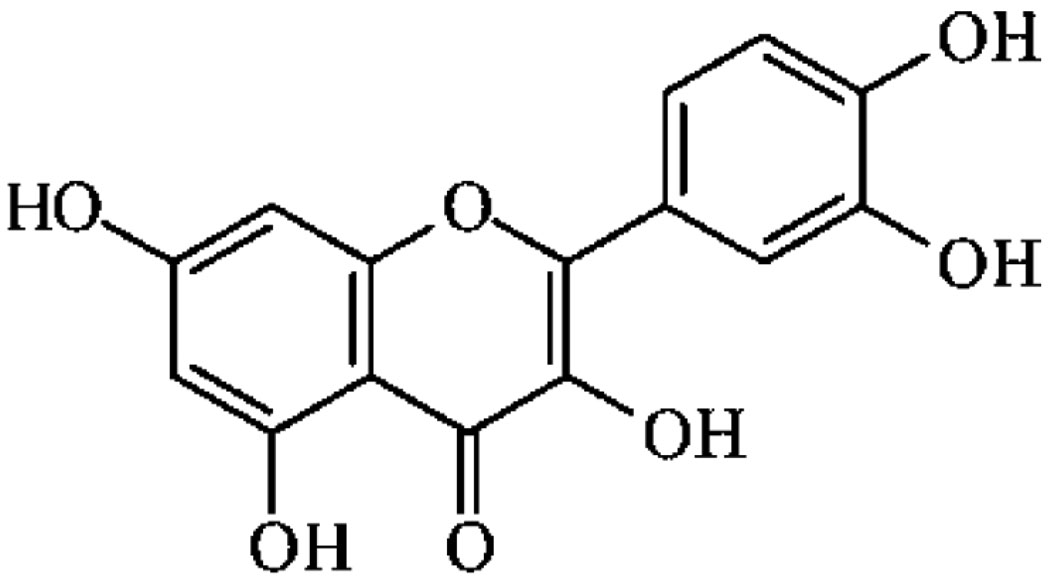
Polygala sabulosa A.W. Bennett was collected in Rancho Queimado (SC, Brazil) and identified by comparison with the voucher UPCNB19640 by Prof. Dr. Olavo de Araújo Guimarães of the Botany Department of the Universidade Federal do Paraná, Brazil. The dried and powdered whole plant (500 g) was extracted exhaustively with ethanol (96%) at room temperature. The crude extract (135 g) obtained was partitioned into hexane, CH2Cl2, ethyl acetate (EtOAc), and water to give four distinct hexane-soluble (16.1 g), CH2Cl2-soluble (28 g), EtOAc soluble (16.8 g), and aqueous (64.6 g) fractions. The EtOAc-soluble fraction (10 g) was subjected to column chromatography over silica gel eluted with a hexan/EtOAc/EtOH gradient system to give 72 fractions of 50 mL each. The combined fr. 30–36 eluted with hexan/EtOAc/EtOH (15:80:5) was further chromatographed on silica gel (3 × 25 cm) eluting with a hexane-acetone gradient from 90:10 to 0:100 (28 fr., 30 mL each) to obtain quercetin as pure yellow crystals (indicating purity higher than 99%), m.p. 312–314 °C (25 mg). The compound isolated was identified by comparison of their physical and spectral (IV, 1H, and 13C NMR) data with authentic samples, and their chemical structure are depicted in Figure 1.
Figure 1.
Chemical structure of Quercetin
Animals and Treatment
Adult (2-months-old) female mice (Swiss albino) obtained from the Central Biotery (UFSC, Florianópolis, Brazil) were maintained at 25 °C on a 12:12 h light/dark cycle, with free access to food. Animals were weighed daily. All experiments were conducted in accordance with the Guiding Principles in the Use of Animals in Toxicology, adopted by the Society of Toxicology in July 1989, and all experiments were approved by our ethics committee for animal use (313/CEUA). Thirty-six animals were divided into six experimental groups: control, quercetin low dose (5 mg/Kg), quercetin high dose (50 mg/Kg), MeHg, MeHg + quercetin low dose, and MeHg + quercetin high dose, with six animals each. The flavonoid was administrated daily by subcutaneous injections and MeHg was diluted in tap water (40 mg/L, ad libitum). A daily mercury dose of 5.4 ± 0.5 mg/kg was calculated based on the daily liquid ingestion (7.2 ± 0.5 mL/animal), which was not statistically different among groups. The flavonoid was dissolved in dimethylsulfoxide and the control animals received just vehicle injections (1 mL/kg body weight). Quercetin and MeHg doses were based in previous studies (Lu et al., 2006; Dietrich et al., 2005). The use of dimethylsulfoxide as injection medium was due to the relative high hydrophobicity of quercetin and due to the absence of significant pro-oxidative effects of the vehicle when administered once a day at 1 mL/kg body weight (Farina et al., 2003).
Behavioral tests
At the end of the treatment (3 weeks), animals were subjected to behavioral/functional tests for evaluating locomotor activity and coordination (open-field and rotarod tasks). Open-field tests were performed in an isolated facility with no interference noise or human activity. The locomotor activity was assessed during the treatment in 6 min sessions using an open-field box [56 (long) × 42 (wide) × 40 cm (high)] with the floor divided into 12 squares. The duration of the trials (6 min) was based on well-standardized protocols (Rosa et al., 2003; Farina et al., 2005b). The number of squares crossed with the four paws was used as a measure of locomotor activity. After the open-field test, mice were subjected to the rotarod task, which was based on the study of Dunham and Miya (1957), with minor modifications. In short, the homemade apparatus consisted of a bar with a diameter of 2.5 cm subdivided into four compartments by disks of, 25 cm with diameters. The bar rotated at a constant speed of 21 rpm and the durations (s) that the animals remained on the apparatus were recorded. Each mouse was subjected to three trials and the interval between trials was 60 sec. The mean of their values was used in the statistical analysis as dependent variable. The maximal time for a trial was 60 sec, after which the animal w as removed if it was still in the device. The investigator that performed the behavioral tests was blind to the treatment assignments.
Tissue preparation for biochemical analyses
After the behavioral tests, animals were killed by decapitation and the cerebella were homogenized (1:5 w/v) in [N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)], 25 mM, pH 7.4, buffer. Tissue homogenates were centrifuged at 20,000 × g at 4 °C for 30 min. The supernatants obtained were used for determining enzymatic activities (glutathione peroxidase and glutathione reductase), as well as glutathione and thiobarbituric acid reactive substance (TBARS) levels.
Biochemical analyses
Antioxidant enzymes
Glutathione peroxidase (GPx) activity was measured by the Wendel (1981) method, using tert-butylhydroperoxide as a substrate. NADPH disappearance was monitored by a spectrophotometer at 340 nm. Glutathione reductase (GR) activity was determined by the method described by Carlberg and Mannervik (1985). The rate of GSSG reduction was indirectly determined through monitoring the NADPH disappearance at 340 nm.
Glutathione levels and lipid peroxidation
Glutathione was measured as nonprotein thiols based on Ellman (1959) with minor modifications. Briefly, samples (200 µg of protein) were precipitated in cooled trichloroacetic acid 10% and centrifuged at 15,000×g for 2 min, and the supernatant was incubated with DTNB in a 0.5 M phosphate buffer, pH 8.0. Absorbances were measured at 412 nm. Lipid peroxidation was measured as thiobarbituric acid reactive substances (TBARS) based on Ohkawa et al. (1979). Briefly, samples (200 µg of protein) were incubated in a reaction media containing 0.28% 2-thiobarbituric acid (TBA), 1.2% SDS, and 0.45 M/0.12 M acetic acid/HCl buffer (pH 3.4). After incubation at 95 °C for 60 min, TBARS were measured at 532 nm and compared to a standard curve of malondialdehyde (MDA). Although some lines of evidence suggests the absence of quantitative relationship among sample MDA content and lipid peroxidation (Janero, 1990), the MDA/TBA reactivity has been shown to be an acurrate indicator for measuring lipoperoxidative damage in murine brain tissues (Carvalho et al., 2007; da Silva et al., 2008).
Determination of protein
The protein content was quantified by the method of Bradford (1976), using bovine serum albumin as a standard.
Statistical analysis
Differences between groups (MeHg or quercetin effects) were evaluated by one-way analysis of variance, followed by Duncan's multiple range tests when appropriate. Two-way analysis of variance was also performed to evaluate quercetin vs. MeHg interactions.
Results
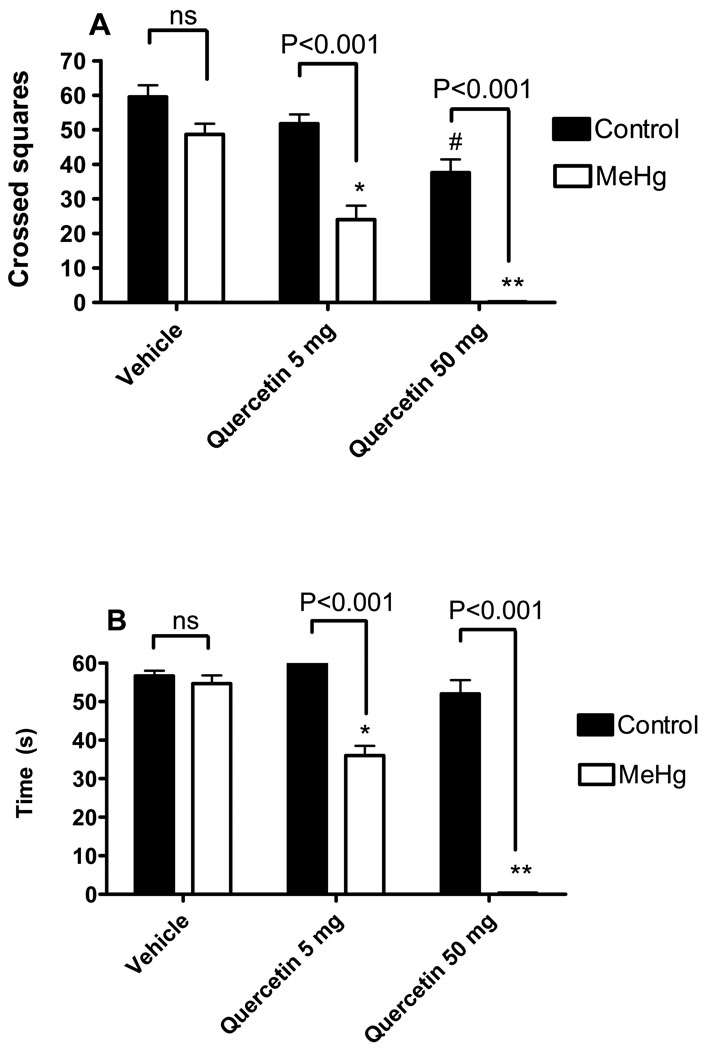
Liquid and solid consumptions and body weight did not differ between groups during the treatment period (data not shown). MeHg alone did not decrease the locomotor activity in the open field test in the absence of quercetin (Figure 2A). However, a significant interaction between MeHg and quercetin was observed [F2,24 = 6.683; P = 0.0049], which indicates a synergistic negative effect of both compounds when co-administrated. Even though neither MeHg nor quercetin affected motor performance of animals in the rotaroad task, the co-exposure to both compounds displayed a significant motor deficit and such phenomenon was dependent on the flavonoid dosage (Figure 2B). In fact, two-way ANOVA showed a significant [F2,24 = 53.77; P<0.0001] interaction between MeHg and quercetin toward the motor performance of animals, which reinforces the idea of a synergistic negative effect of both compounds when co-administrated.
Figure 2.
Effects of MeHg and/or quercetin treatments on the motor profile of female mice. Locomotor activity (A) is presented as crossed squares in the open field arena. Motor performance (B) is presented as falling latency on the rotarod apparatus (seconds). *Statistically different when compared to animals treated with MeHg alone; **Statistically different when compared to animals treated with MeHg alone or animals treated with MeHg + quercetin 5 mg/Kg; #Statistically different when compared to control group (animals treated with vehicle and water as liquid source). Data were analyzed by ANOVA, followed by Duncan's multiple range tests, and are expressed as mean ± SEM. N = 6 animals per group. P values above bars indicate significant effects of MeHg. ns = not significant.
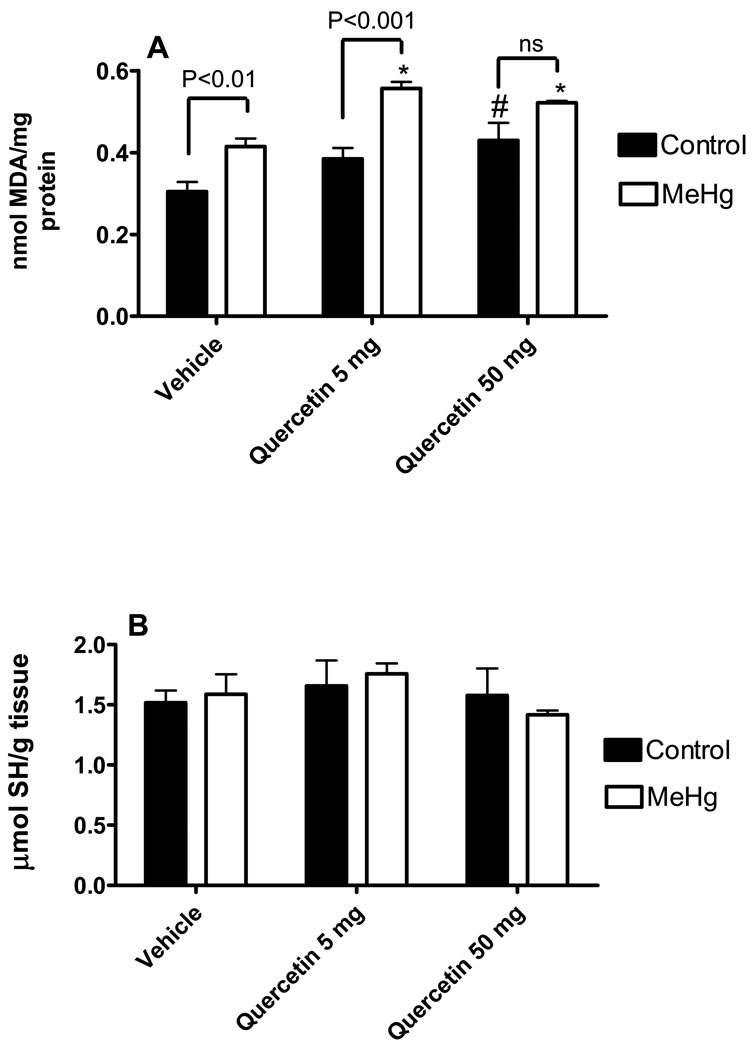
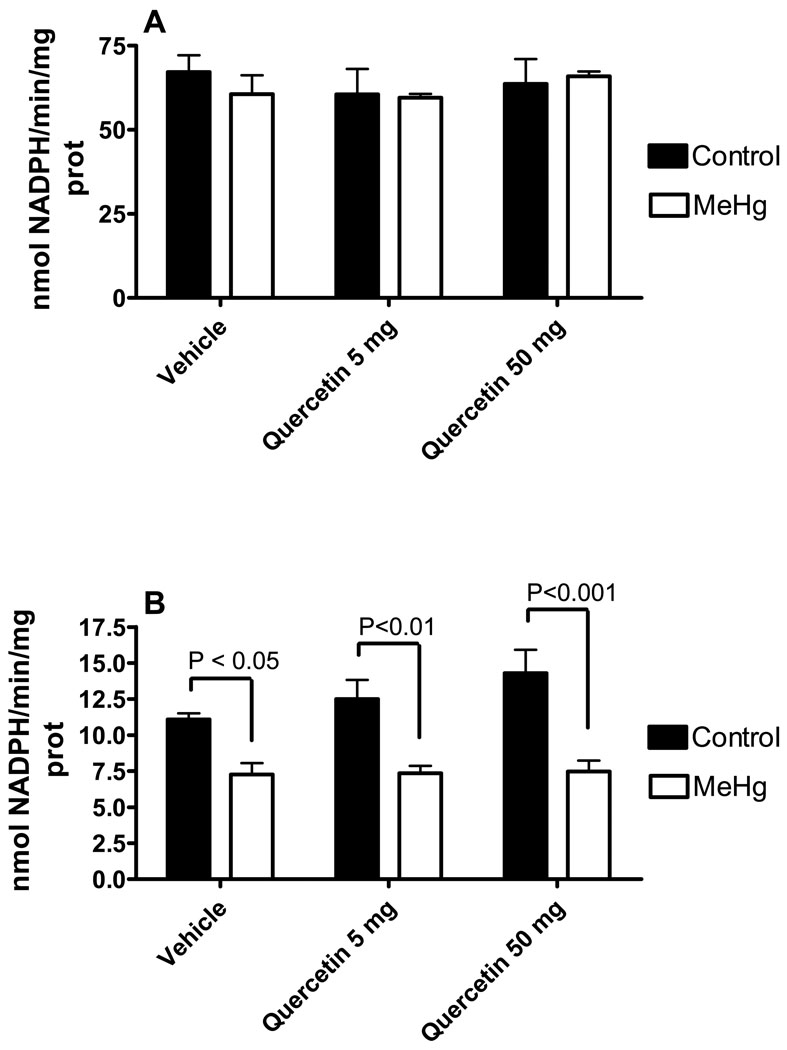
With respects to the biochemical parameters, cerebellar lipid peroxidation, glutathione levels, glutathione peroxidase (GPx) and glutathione reductase (GR) activities were evaluated. Either MeHg [F1,24 = 32.79; P<0.0001] or quercetin [F2,24 = 12.10; P = 0.0002] alone displayed a significant stimulatory effect toward the cerebellar levels of thiobarbituric acid reactive substances (TBARS), a marker of lipid peroxidation (Figure 3A). However, no significant interaction was observed [F2,24 = 1.239; P = 0.3076]. Cerebellar glutathione levels (Figure 3B) and cerebellar glutathione reductase activity (Figure 4A) were not changed by either MeHg or quercetin treatments. MeHg exposure decreased glutathione peroxidase activity [F1,27 = 48.92; P<0.0001], but quercetin co-exposure did not [F2,27 = 1.711; P = 0,1998] lead to an added effect (Figure 4B). No significant MeHg vs. quercetin interaction was observed [F2,27 = 1.329; P = 0.2814].
Figure 3.
Effects of MeHg and/or quercetin treatments on the cerebellar levels of thiobarbituric acid reactive substances and glutathione of female mice. Thiobarbituric acid reactive substances (A) are presented as nmol of malondialdehyde (MDA)/mg protein. Glutathione levels (B) are presented as µmol/g tissue. *Statistically different when compared to animals treated with MeHg alone; **Statistically different when compared to animals treated with MeHg alone or animals treated with MeHg + quercetin 5 mg/Kg; #Statistically different when compared to control group (animals treated with vehicle and water as liquid source). Data were analyzed by ANOVA, followed by Duncan's multiple range tests, and are expressed as mean ± SEM. N = 6 animals per group. P values above bars indicate significant effects of MeHg. ns = not significant.
Figure 4.
Effects of MeHg and/or quercetin treatments on the cerebellar glutathione peroxidase and glutathione reductase activities of female mice. Glutathione reductase (A) and glutathione peroxidase (B) activities are presented as nmol NADPH oxidized/min/mg protein. *Statistically different when compared to animals treated with MeHg alone; **Statistically different when compared to animals treated w ith MeHg alone or animals treated with MeHg + quercetin 5 mg/Kg; #Statistically different when compared to control group (animals treated with vehicle and water as liquid source). Data were analyzed by ANOVA, followed by Duncan's multiple range tests, and are expressed as mean ± SEM. N = 6 animals per group. P values above bars indicate significant effects of MeHg. ns = not significant.
Discussion
A previous study from our group (Farina et al., 2005a) reported significant beneficial effects of the hydroalcoholic extract of the plant Polygala paniculata against MeHg-induced neurotoxicity in mice. In that study, the hydroalcoholic extract of Polygala paniculata prevented the behavioral and neurochemical changes induced by the oral exposure to MeHg, pointing to this plant as a potential therapeutic agent for the treatment of pathological conditions related to excitotoxicity and oxidative stress, including MeHg poisoning. In agreement, plants of the genus Polygala have been reported to display neuroprotective effects in several other neuropathological conditions related to excitotoxicity and oxidative stress (Lee et al., 2004; Park et al., 2006). A subsequent study from our group (Franco et al., 2007) indicated that the flavonoid quercetin, isolated from plants of the genus Polygala, also showed a highly beneficial effect in attenuating both organic (MeHg) and inorganic (HgCl2) mercury-induced mitochondrial toxicity and oxidative stress. Accordingly, we hypothesized that quercetin could effectively protect against MeHg-induced neurotoxicity in in vivo models. However, quercetin co-exposure (co-administrated with MeHg, at either 5 mg/Kg or 50 mg/Kg) failed to prevent MeHg-induced neurotoxicity. Conversely, quercetin synergistically potentiated the deleterious effects of MeHg on murine locomotor activity and motor performance. Moreover, quercetin when administered alone at the higher dose (50 mg/Kg) induced a small but significant increase in the cerebellar lipid peroxidation. This hormetic-like effect of this antioxidant flavonoid is likely related to the pro-oxidative properties of quercetin or its quinone metabolite(s) (Awad et al., 2002).
Although MeHg when administererd alone did not affect the locomotor activity in the open field and the motor performance in the rotarod apparatus, it displayed a significant main effect toward both behavioral parameters when administered with quercetin. Moreover, quercetin, which did not affected locomotor activity and slightly impaired motor performance per se, induced significant negative effects toward locomotor activity and motor performance when co-administered with MeHg. This phenomenon indicates that MeHg and quercetin synergistically interact to induce locomotor deficits and motor impairment in female mice. This hypothesis is supported by the significant interactions observed between MeHg and quercetin toward animal locomotor activity and motor performance.
There is some evidence that cerebellar cells are selectively targeted by mercury compounds in vivo (Sanfeliu et al., 2003) and that MeHg neurotoxicity affects the motor system (Grandjean et al., 1997). Indeed, the relationship between MeHg-induced motor deficit and MeHg-induced cerebellar damage is a well-described process (Sakamoto et al., 1993). In this regard, we have reported motor deficits in animals exposed to MeHg during adulthood (Dietrich et al., 2005, Farina et al., 2005b) as well as in the suckling period (Franco et al., 2006). Here, we observed that MeHg exposure affects the motor coordination of mice in the rotarod task and the locomotor activity in the open field. Based on literature data, these results suggest that the harmful effect of MeHg on the motor coordination is related, at least in part, to its deleterious pro-oxidative effects on mouse cerebellum. The observed increased levels in cerebellar lipid peroxidation reinforce such idea.
Mechanistically, it is well known that MeHg-induced neurotoxicity is related to oxidative stress, which is closely associated with glutamate and calcium dyshomeostasis (Soares et al., 2003; Aschner et al., 2007). Notably, the glutathione antioxidant system has been reported to represent a molecular target for the deleterious effects of MeHg in the central nervous system (Stringari et al., 2008). In the present study, cerebellar GSH levels and glutathione reductase activity were not changed in response to MeHg exposure. It is notable that cerebellar GSH levels were measured as non-protein thiols based on the protocol developed by Ellman (1959), which does not discriminate between reduced glutathione and other non-protein thiols. Because GSH accounts for approximately 90% of the total non-protein thiols in cells (Cooper, 1998; Franco and Cidlowski, 2006), one could suppose that the absence of significant effects of treatments toward non-protein thiols levels also represents non-significant effects toward cerebellar GSH levels. On the other hand, cerebellar GPx activity was significantly lower in MeHg-exposed mice when compared to control animals. Since GPx catalyzes the reduction of hydrogen peroxide, phospholipid-hydroperoxide, and other organic hydroperoxides at the expense of GSH (Flohe, 1971), the increased cerebellar levels of TBARS (marker of lipid peroxidation) in MeHg-exposed animals was an expected observation. On the other hand, quercetin, which synergically decreased locomotor activity and motor performance when administered with MeHg, did not affect cerebellar GPx activity, but showed a significant main effect toward cerebellar lipid peroxidation. Taking into account that both MeHg and quercetin induced positive main effects toward cerebellar lipid peroxidation and that MeHg vs. quercetin interaction was not significant, it is reasonable to suggest that both compounds increased cerebellar lipid peroxidation in an additive, but not synergic manner. The potential molecular mechanism related to MeHg-induced lipid peroxidation should be related, at least in part, to the decreased cerebellar GPx activity. Regarding to the potential mechanisms related to quercetin-induced cerebellar lipid peroxidation and motor/locomotor impairments, our findings do not point to the GSH antioxidant system as a potential molecular target involved with such phenomena. However, pro-oxidative effects of quercetin or its quinone metabolite(s) (Awad et al., 2002), might be responsible for the observed events.
In conclusion, even though Polygala paniculata (Farina et al., 2005a) and quercetin (Franco et al., 2007) possess beneficial effects against MeHg induced toxicity under in vivo and in vitro conditions, respectively, quercetin does not possess such beneficial effects under our in vivo conditions. Conversely, quercetin synergystically increased such toxicity when co-administrated with MeHg and this phenomenon appears to be related to the pro-oxidative effects of the flavonoid and/or its metabolite(s).
Acknowledgements
This study was supported by grants from FAPESC (Jovens Pesquisadores - FAPESC/CNPq 04/2007) and CNPq (479239/2007-0) to Marcelo Farina, as well as by grants from the National Institute of Environmental Health Sciences (US Public Health Service Grant ES007331) to Michael Aschner. The authors are also tankfull to the FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)” # 01.06.0842-00. Martins RP received a CNPq/PIBIC (117644/2006-4) fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aposhian HV, Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z, Hurlbut KM, Junco-Munoz P, Dart RC, Aposhian MM. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology. 1995;97:23–38. doi: 10.1016/0300-483x(95)02965-b. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz. J. Med. Biol. Res. 2007;40:285–291. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Awad HM, Boersma MG, Boeren S, van der Woude H, van Zanden J, van Bladeren PJ, Vervoort J, Rietjens IM. Identification of o-quinone/quinone methide metabolites of quercetin in a cellular in vitro system. FEBS Lett. 2002;520(1–3):30–34. doi: 10.1016/s0014-5793(02)02754-0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. Glutathione reductase. Meth. Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Carvalho MC, Franco JL, Ghizoni H, Kobus K, Nazari EM, Rocha JB, Nogueira CW, Dafre AL, Müller YM, Farina M. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on methylmercury-induced locomotor deficits and cerebellar toxicity in mice. Toxicology. 2007;239:195–203. doi: 10.1016/j.tox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury-current exposures and clinical manifestations. N. Engl. J. Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Conklin DR, Tan KH, Aschner M. Dimethyl sulfoxide, but not acidosis-induced metallothionein mRNA expression in neonatal rat primary astrocyte cultures is inhibited by the bioflavonoid, quercetin. Brain Res. 1998;794:304–308. doi: 10.1016/s0006-8993(98)00241-8. [DOI] [PubMed] [Google Scholar]
- Cooper AJL. Role of astrocytes in maintaining cerebral glutathione homeostasis and in protecting the brain against xenobiotics and oxidative stress. In: Shaw CA, editor. The Role of Glutathione in the Nervous System. Washington: Taylor and Francis; 1998. pp. 91–115. [Google Scholar]
- da Silva AP, Farina M, Franco JL, Dafre AL, Kassa J, Kuca K. Temporal effects of newly developed oximes (K027, K048) on malathion-induced acetylcholinesterase inhibition and lipid peroxidation in mouse prefrontal cortex. Neurotoxicology. 2008;29:184–189. doi: 10.1016/j.neuro.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Mantese CE, Anjos GD, Souza DO, Farina M. Motor impairment induced by oral exposure to methylmercury in adult mice. Environ. Toxicol. Pharmacol. 2005;19:169–175. doi: 10.1016/j.etap.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Duham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Farina M, Dahm KC, Schwalm FD, Brusque AM, Frizzo ME, Zeni G, Souza DO, Rocha JB. Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicol. Sci. 2003;73:135–140. doi: 10.1093/toxsci/kfg058. [DOI] [PubMed] [Google Scholar]
- Farina M, Franco JL, Ribas CM, Meotti FC, Pizzolatti MG, Dafre AL, Santos ARS. Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J. Pharma. Pharmacol. 2005a;57:1503–1508. doi: 10.1211/jpp.57.11.0017. [DOI] [PubMed] [Google Scholar]
- Farina M, Cereser V, Portela LV, et al. Methylmercury increases S100B content in rat cerebrospinal fluid. Environ Toxicol Pharmacol. 2005b;19:249–253. doi: 10.1016/j.etap.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J. Biol. Chem. 2006;281:29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- Franco JL, Teixeira A, Meotti FC, et al. Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ. Res. 2006;102:22–28. doi: 10.1016/j.envres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Franco JL, Braga HC, Stringari J, Missau F, Posser T, Mendes B, Leal RB, Santos ARS, Dafre AL, Pizzolatti MG, Farina M. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem. Res. Toxicol. 2007;20:1919–1926. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorense N, Dahl R, Jorgense PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;49:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Gupta R, Flora SJ. Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats. J. Appl. Toxicol. 2006;26:213–222. doi: 10.1002/jat.1131. [DOI] [PubMed] [Google Scholar]
- Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1999;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Ban JY, Koh SB, Seong NS, Song KS, Bae KW, Seong YH. Polygalae radix extract protects cultured rat granule cells against damage induced by NMDA. Am. J. Chin. Med. 2004;32:599–610. doi: 10.1142/S0192415X04002235. [DOI] [PubMed] [Google Scholar]
- Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ. Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain Res. 2006;171(2):251–260. doi: 10.1016/j.bbr.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, Rocha JB, Frizzo ME, Souza DO, Farina M. Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol. Sci. 2004;81:172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- Meotti FC, Fachinetto R, Maffi LC, Missau FC, Pizzolatti MG, Rocha JB, Santos AR. Antinociceptive action of myricitrin: involvement of the K+ and Ca2+ channels. Eur J Pharmacol. 2007;567:198–205. doi: 10.1016/j.ejphar.2007.03.039. [DOI] [PubMed] [Google Scholar]
- Mishra D, Flora SJ. Quercetin administration during chelation therapy protects arsenic-induced oxidative stress in mice. Biol Trace Elem Res. 2008;122:137–147. doi: 10.1007/s12011-007-8064-9. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim JS, Jang DS, Lee SM. Effect of Polygala tenuifolia root extract on cerebral ischemia and reperfusion. Am. J. Chin. Med. 2006;34:115–123. doi: 10.1142/S0192415X06003680. [DOI] [PubMed] [Google Scholar]
- Pingree SD, Simmonds PL, Woods JS. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol Sci. 2001;61(2):224–233. doi: 10.1093/toxsci/61.2.224. [DOI] [PubMed] [Google Scholar]
- De Rosa E, Sullivan EV. Enhanced release from proactive interference in nonamnesic alcoholic individuals: implications for impaired associative binding. Neuropsychology. 2003;17(3):469–481. doi: 10.1037/0894-4105.17.3.469. [DOI] [PubMed] [Google Scholar]
- Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Exp Brain Res. 1997;117(3):428–436. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Nakano A, Kajiwara Y, Naruse I, Fujisaki T. Effects of methyl mercury in postnatal developing rats. Environ. Res. 1993;61:43–50. doi: 10.1006/enrs.1993.1048. [DOI] [PubMed] [Google Scholar]
- Sanfeliu C, Sebastia J, Cristofol R, Rodriguez-Farre E. Neurotoxicity of organomercurial compounds. Neurotox. Res. 2003;5:283–305. doi: 10.1007/BF03033386. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Atchison WD. Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicol. Appl. Pharmacol. 2000;167:1–11. doi: 10.1006/taap.2000.8967. [DOI] [PubMed] [Google Scholar]
- Stringari J, Meotti FC, Souza DO, Santos AR, Farina M. Postnatal methylmercury exposure induces hyperlocomotor activity and cerebellar oxidative stress in mice: dependence on the neurodevelopmental period. Neurochem. Res. 2006;4:563–569. doi: 10.1007/s11064-006-9051-9. [DOI] [PubMed] [Google Scholar]
- Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol. Appl. Pharmacol. 2008;227(1):147–154. doi: 10.1016/j.taap.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003;18:149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- Wagner C, Fachinetto R, Dalla Corte CL, Brito VB, Severo D, de Oliveira Costa Dias G, Morel AF, Nogueira CW, Rocha JB. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 2006;1107:192–198. doi: 10.1016/j.brainres.2006.05.084. [DOI] [PubMed] [Google Scholar]
- Wendel A. Glutathione peroxidase. Meth. Enzymol. 1981;77:325–333. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li G, Han C, Sun L, Zhao R, Cui S. Protective effects of Hippophae rhamnoides L. juice on lead-induced neurotoxicity in mice. Biol. Pharm. Bull. 2005;28:490–494. doi: 10.1248/bpb.28.490. [DOI] [PubMed] [Google Scholar]