Abstract
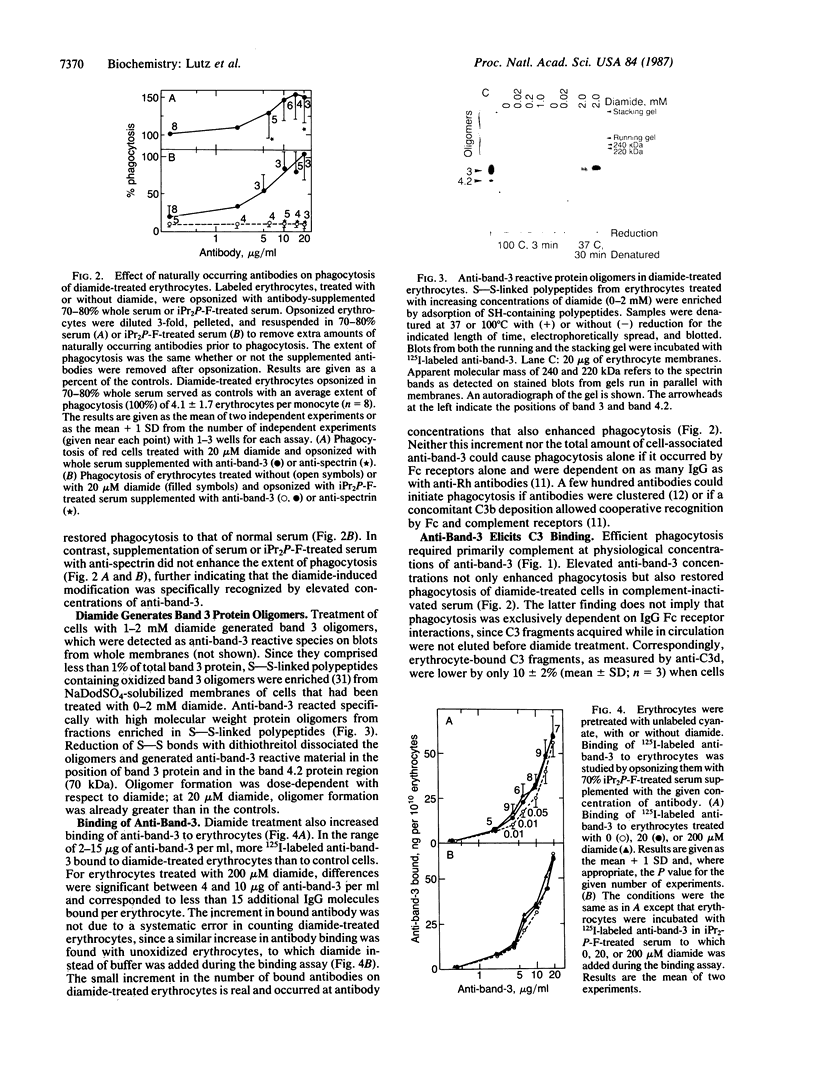
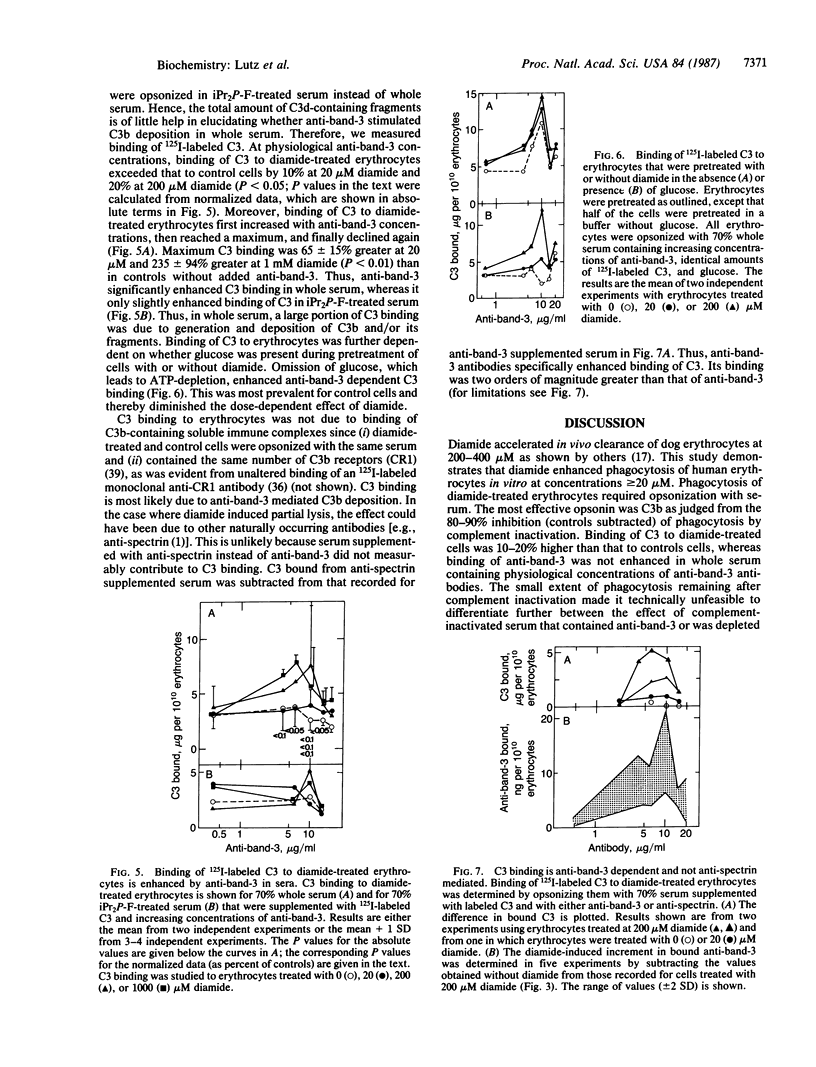
Treatment of erythrocytes with the thiol-specific oxidant azodicarboxylic acid bis(dimethylamide) (diamide) enhances their phagocytosis by adherent monocytes. Phagocytosis of diamide-treated erythrocytes required that the cells were opsonized with whole serum, since complement inactivation abolished phagocytosis. Opsonization with whole serum containing 20-100 times the physiological concentration of naturally occurring anti-band-3 antibodies enhanced phagocytosis of diamide-treated erythrocytes. High inputs of anti-band-3 also restored phagocytosis of erythrocytes that had been incubated with complement-inactivated serum. Elevated concentrations of anti-spectrin antibodies were ineffective in whole and complement-inactivated serum. Specific recognition of diamide-treated erythrocytes by anti-band-3 antibodies may be due to generation of anti-band-3 reactive protein oligomers on intact diamide-treated erythrocytes. Generation of such oligomers was dose-dependent with respect to diamide. Bound anti-band-3 alone was not sufficient to mediate phagocytosis. It resulted in deposition of complement component C3b on the cells through activation of the alternative complement pathway in amounts exceeding that of bound antibodies by two orders of magnitude. Thus, anti-band-3 and complement together mediate phagocytosis of oxidatively stressed erythrocytes, which stimulate senescent erythrocytes with respect to bound antibody and complement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Bussolino F., Turrini F., Arese P. Measurement of phagocytosis utilizing [14C]cyanate-labelled human red cells and monocytes. Br J Haematol. 1987 Jun;66(2):271–274. doi: 10.1111/j.1365-2141.1987.tb01311.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Coetzer T., Zail S. Membrane protein complexes in GSH-depleted red cells. Blood. 1980 Aug;56(2):159–167. [PubMed] [Google Scholar]
- Cook J., Fischer E., Boucheix C., Mirsrahi M., Jouvin M. H., Weiss L., Jack R. M., Kazatchkine M. D. Mouse monoclonal antibodies to the human C3b receptor. Mol Immunol. 1985 May;22(5):531–539. doi: 10.1016/0161-5890(85)90176-2. [DOI] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Chaiken I. M., McCormick W. M. Expression of multivalency in the affinity chromatography of antibodies. Appendix: Derivation and evaluation of equations for independent bivalent interacting systems in quantitative affinity chromatography. Biochemistry. 1979 Mar 6;18(5):790–795. doi: 10.1021/bi00572a008. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Properdin factor D: characterization of its active site and isolation of the precursor form. J Exp Med. 1974 Feb 1;139(2):355–366. doi: 10.1084/jem.139.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Fidalgo B. V., Katayama Y., Najjar V. A. The physiological role of the lymphoid system. V. The binding of autologous (erythrophilic) gamma globulin to human red blood cells. Biochemistry. 1967 Nov;6(11):3378–3385. doi: 10.1021/bi00863a007. [DOI] [PubMed] [Google Scholar]
- Freedman J. Membrane-bound immunoglobulins and complement components on young and old red cells. Transfusion. 1984 Nov-Dec;24(6):477–481. doi: 10.1046/j.1537-2995.1984.24685066804.x. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Gaither T. A., Hammer C. H., Frank M. M. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med. 1984 Dec 1;160(6):1640–1655. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Clark M. R., Shohet S. B. Excessive binding of natural anti-alpha-galactosyl immunoglobin G to sickle erythrocytes may contribute to extravascular cell destruction. J Clin Invest. 1986 Jan;77(1):27–33. doi: 10.1172/JCI112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano J. H., DeFuria F. G., Cerami A., Reece R. P. The use of 14C-cyanate as a method for determining erythrocyte survival. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):326–328. doi: 10.3181/00379727-144-37583. [DOI] [PubMed] [Google Scholar]
- Green G. A., Rehn M. M., Kalra V. K. Cell-bound autologous immunoglobulin in erythrocyte subpopulations from patients with sickle cell disease. Blood. 1985 May;65(5):1127–1133. [PubMed] [Google Scholar]
- Grob P. J., Frommel D., Isliker H. C., Masouredis S. P. Interaction of IgG and its fragments with red cells. Immunology. 1967 Nov;13(5):489–499. [PMC free article] [PubMed] [Google Scholar]
- Haest C. W., Kamp D., Plasa G., Deuticke B. Intra- and intermolecular cross-linking of membrane proteins in intact erythrocytes and ghosts by SH-oxidizing agents. Biochim Biophys Acta. 1977 Sep 5;469(2):226–230. doi: 10.1016/0005-2736(77)90186-9. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hebbel R. P., Miller W. J. Phagocytosis of sickle erythrocytes: immunologic and oxidative determinants of hemolytic anemia. Blood. 1984 Sep;64(3):733–741. [PubMed] [Google Scholar]
- Jain S. K., Hochstein P. Polymerization of membrane components in aging red blood cells. Biochem Biophys Res Commun. 1980 Jan 15;92(1):247–254. doi: 10.1016/0006-291x(80)91545-4. [DOI] [PubMed] [Google Scholar]
- Johnson G. J., Allen D. W., Flynn T. P., Finkel B., White J. G. Decreased survival in vivo of diamide-incubated dog erythrocytes. A model of oxidant-induced hemolysis. J Clin Invest. 1980 Nov;66(5):955–961. doi: 10.1172/JCI109964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Shapiro S. S., Bendich A., Bassel P. S. Oxidation as a possible mechanism of cellular aging: vitamin E deficiency causes premature aging and IgG binding to erythrocytes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2463–2467. doi: 10.1073/pnas.83.8.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Localization of senescent cell antigen on band 3. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Khansari N., Fudenberg H. H. Phagocytosis of senescent erythrocytes by autologous monocytes: requirement of membrane-specific autologous IgG for immune elimination of aging red blood cells. Cell Immunol. 1983 May;78(1):114–121. doi: 10.1016/0008-8749(83)90264-2. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- Low P. S., Waugh S. M., Zinke K., Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985 Feb 1;227(4686):531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- Lutz H. U. Elimination alter Erythrozyten aus der Zirkulation: Freilegung eines zellater-spezifischen Antigens auf alternden Erythrozyten. Schweiz Med Wochenschr. 1981 Oct 10;111(41):1507–1517. [PubMed] [Google Scholar]
- Lutz H. U., Fehr J. Total sialic acid content of glycophorins during senescence of human red blood cells. J Biol Chem. 1979 Nov 25;254(22):11177–11180. [PubMed] [Google Scholar]
- Lutz H. U., Flepp R., Stringaro-Wipf G. Naturally occurring autoantibodies to exoplasmic and cryptic regions of band 3 protein, the major integral membrane protein of human red blood cells. J Immunol. 1984 Nov;133(5):2610–2618. [PubMed] [Google Scholar]
- Lutz H. U., Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol. 1982 Apr;128(4):1695–1699. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Müller H., Lutz H. U. Binding of autologous IgG to human red blood cells before and after ATP-depletion. Selective exposure of binding sites (autoantigens) on spectrin-free vesicles. Biochim Biophys Acta. 1983 Apr 6;729(2):249–257. doi: 10.1016/0005-2736(83)90491-1. [DOI] [PubMed] [Google Scholar]
- Nelson B., Ruddy S. Enhancing role of IgG in lysis of rabbit erythrocytes by the alternative pathway of human complement. J Immunol. 1979 May;122(5):1994–1999. [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Rank B. H., Carlsson J., Hebbel R. P. Abnormal redox status of membrane-protein thiols in sickle erythrocytes. J Clin Invest. 1985 May;75(5):1531–1537. doi: 10.1172/JCI111857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A. Interaction of divalent antibody with cell surface antigens. Biochemistry. 1979 Jan 23;18(2):264–269. doi: 10.1021/bi00569a004. [DOI] [PubMed] [Google Scholar]
- Salama A., Mueller-Eckhardt C. Binding of fluid phase C3b to nonsensitized bystander human red cells. A model for in vivo effects of complement activation on blood cells. Transfusion. 1985 Nov-Dec;25(6):528–534. doi: 10.1046/j.1537-2995.1985.25686071424.x. [DOI] [PubMed] [Google Scholar]
- Schlüter K., Drenckhahn D. Co-clustering of denatured hemoglobin with band 3: its role in binding of autoantibodies against band 3 to abnormal and aged erythrocytes. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6137–6141. doi: 10.1073/pnas.83.16.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. R., Griffin F. M., Jr Phagocytosis requires repeated triggering of macrophage phagocytic receptors during particle ingestion. Nature. 1981 Jan 29;289(5796):409–411. doi: 10.1038/289409a0. [DOI] [PubMed] [Google Scholar]
- Szymanski I. O., Odgren P. R., Valeri C. R. Relationship between the third component of human complement (C3) bound to stored preserved erythrocytes and their viability in vivo. Vox Sang. 1985;49(1):34–41. doi: 10.1111/j.1423-0410.1985.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam P., Petz L. D., Spath P. Detection of IgG sensitization of red cells with 125I staphylococcal protein A. Am J Hematol. 1982 Jun;12(4):337–346. doi: 10.1002/ajh.2830120405. [DOI] [PubMed] [Google Scholar]




