Abstract
In the present study, we investigated the potential protective effects of three flavonoids (myricetin, myricitrin and rutin) derived from medicinal plants against methyl mercury (MeHg)-induced mitochondrial dysfunction in vitro. Incubation of mouse brain mitochondria with MeHg induced a significant decrease in mitochondrial function, which was correlated with decreased glutathione (GSH) levels and increased generation of reactive oxygen species (ROS) and lipid peroxidation. The co-incubation of mouse brain mitochondria with myricetin or myricitrin caused a concentration-dependent decrease of MeHg-induced mitochondrial dysfunction and oxidative stress. The flavonoid rutin was ineffective in counteracting MeHg toxicity. Among the three tested flavonoids, myricetin was the most efficient in protecting against MeHg-induced mitochondrial dysfunction. Moreover, myricetin completely blocked MeHg-induced ROS formation and lipid peroxidation and partially prevented MeHg-induced GSH depletion. The ability of myricetin to attenuate MeHg-induced mitochondrial dysfunction and oxidative stress appears to be related to its higher scavenging capability when compared to myricitrin and rutin. Overall, the results suggest that MeHg-induced mitotoxicity is associated with oxidative stress. The ability of myricetin to prevent MeHg-induced oxidative damage in brain mitochondria renders this flavonoid a promising molecule for further in vivo studies in the search for potential antidotes to counteract MeHg-induced neurotoxicity.
Keywords: Methylmercury, toxicity, mitochondria, flavonoids, myricetin
Introduction
Oxidative stress has been linked to several neuropathological conditions, such as neurodegenerative diseases and metal-induced neurotoxicity (Aschner et al 2007; Kamat et al 2008; Wang et al 2008). Of particular environmental concern, methylmercury (MeHg) is a ubiquitous environmental toxin that has been reported to cause neurological and developmental deficits both in animals and humans (Harada, 1995; Clarkson et al 2003). As a result of the biomethylation of mercury compounds released from anthropogenic sources in waterways, MeHg-containing fish represent a major source of human poisoning (Clarkson et al 2003; Myers et al 2007). Therefore, populations that rely heavily on fish for food may be exposed to neurotoxic levels of MeHg.
A number of synchronous mechanisms are likely to be associated with MeHg-induced neurotoxicity, including impairment of intracellular calcium homeostasis, alteration of glutamate homeostasis, oxidative stress and compromised cellular redox potential (Aschner et al 2000; Clarkson 2002; Manfroi et al 2004; Aschner et al 2007; Johansson et al 2007; Malagutti et al 2009). MeHg-induced oxidative stress is related to its direct chemical interaction with nonenzymatic antioxidants, such as glutathione (GSH), as well as changes in the activities of antioxidant enzymes (Ou et al 1999; Stringari et al 2006; Yin et al 2007; Franco et al 2009; Farina et al 2009). In this regard, it has been reported that MeHg exposure decreases the levels of GSH and increases the levels of peroxides and thiobarbituric acid reactive substances (Farina et al 2005; Shanker et al 2005; Kaur et al 2006; Franco et al 2006; Carvalho et al 2007; Stringari et al 2008).
Based on the pro-oxidative properties of MeHg, the co-adjuvant use of antioxidants (in association with the clinically available chelating therapies) might represent an efficient therapeutic strategy to counteract MeHg neurotoxicity (Carvalho et al 2007). Flavonoids, whose antioxidant properties have been well described (Rice-Evans et al 1996; Pietta 2000; Rice-Evans et al 2001), could represent important therapeutic choices. Consistent with this suggestion, a previous study from our group showed the beneficial in vivo effects of the hydroalcoholic extract of plants of the genus Polygala against MeHg-induced neurotoxicity in mice (Farina et al 2005). Moreover, an in vitro study showed that quercetin, a well known flavonoid with antioxidant properties, prevented the mitotoxic effects of MeHg by inhibiting MeHg-induced ROS formation (Franco et al 2007). In the same study, other compounds derived from Polygala, such as coumarins and xanthones, did not display protective effects, suggesting that flavonoids may represent promising neuroprotective agents to counteract MeHg-induced neurotoxicity.
Taking into account (i) the absence of effective strategies for treating MeHg poisoning (Tchounwou et al 2003), (ii) the neuroprotective effects of plant extracts against MeHg-induced neurotoxicity in in vivo animal models (Farina et al 2005; Lucena et al, 2007) and (iii) the promising role of flavonoids in counteracting MeHg-induced toxicity (Franco et al 2007), the present study was designed to investigate the potential protective effects of three flavonoids (myricetin, myricitrin and rutin) derived from Brazilian medicinal plants against MeHg-induced mouse brain mitochondrial dysfunction. To better characterize the molecular mechanisms associated with MeHg-induced toxicity and the potential protective effects inherent to these flavonoids, particular emphasis was directed at the number and position of hydroxyl/glycosyl groups in these flavonoids and how they correlate with the therapeutic efficacy.
Materials and Methods
Materials
Adult Swiss Albino male mice (2 months old) were bred in the animal facilities of the Universidade Federal de Santa Catarina. The animals were maintained according to the Animal Care Guidelines from the National Institutes of Health of the United States of America, and all experiments were approved by our ethic committee for animal use (Comissão de Ética no Uso de Animais - UFSC - 00084 and P00373/CEUA; 23080.030005/2009-66/UFSC). Animals were maintained at 23°C on a 12 h light/dark cycle with free access to water and food (Nuvital, PR, Brazil). Methylmercury (II) chloride, mercuric chloride, 5,5′-dithiobis-(2-nitrobenzoic acid), and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St Louis, MO). All other chemicals were of analytical grade. Rutin was isolated from the ethanolic crude extract of Polygala paniculata, as previously described (Farina et al 2005). Myricitrin was isolated by chromatography fractionation from the crude ethanolic extract of Eugenia uniflora leaves. Its aglycone myricetin was obtained through chemical hydrolysis of myricitrin with 10% HCl in EtOH/H2O (1:1) and purified by column chromatography. The isolated flavonoids were identified by 1H and 13C NMR spectral analyses and showed higher than 98% purity. The flavonoids were diluted in ethanol prior to use at a final ethanol concentration of 0.1%.
Preparation of mouse brain mitochondrial-enriched fractions
Mouse brain mitochondrial-enriched fractions were prepared as previously described (Franco et al 2007). A total of thirty animals were used in the study. Briefly, adult (8–10 weeks) male Swiss mice were sacrificed by decapitation. The whole brains (except cerebellum) were removed and homogenized on ice in 10 volumes of isolation medium (10 mM HEPES buffer pH 7.0 containing 220 mM mannitol, 68 mM sucrose, 10 mM KCl and 0.1% serum albumin) and the homogenate was centrifuged at 4°C for 10 min at 1000 x g. The supernatant was then centrifuged at 17,500 x g for 10 min at 4°C, resulting in a myelin-rich supernatant and a pellet (P2) consisting of synaptosomes and free (extra-synaptosomal) mitochondria. The supernatant was discarded, and the pellet was suspended in a medium similar to the isolation medium without albumin. The samples were kept on ice until the experiments were performed, usually within 10–15 min.
Incubations and biochemical determinations
P2 (2 mg of protein) was incubated with MeHg (100 μM) diluted in incubation buffer, and/or flavonoids (0.03, 0.1 and 0.3 mM) in a incubation medium containing 10 mM HEPES buffer (pH 7.0), 220 mM mannitol, 68 mM sucrose and 10 mM KCl (total incubation volume = 300 μL). Incubations were carried out at 25°C for 15 minutes (for measuring ROS formation), 30 minutes (for measuring GSH levels and MTT reduction) or 60 minutes (for measuring lipid peroxidation end products). The specific incubation periods prior to ROS and GSH levels and lipid peroxidation end product measurements were based on temporal events related to MeHg-induced oxidative damage (Franco et al 2007), commencing with ROS generation, followed by GSH oxidation and ultimately lipid peroxidation-end product formation. Parallel experiments with the presence of catalase (200 units) were also carried out in order to elucidate molecular mechanisms of toxicity and protection. Mitochondrial function was assessed by the conversion of the metabolic dye methylthiazolyldiphenyl-tetrazolium bromide (MTT) to formazan (Denizot et al 1986). GSH content was measured as nonprotein thiols according to a method previously described (Ellman 1959). The lipid peroxidation end products were determined by the thiobarbituric acid reactive substances (TBARS) assay originally described by Ohkawa et al (1979). ROS formation was determined according to the method described by Ali et al (1992), using the fluorescent dye 2′7′-dichlorofluorescein diacetate (DCFDA).
Assessment of protein content
Protein concentration was determined according to Bradford (1976), using a bovine serum albumin as a standard.
Statistical analysis
Statistical differences among groups were analyzed by one-way ANOVA analysis of variance followed, when appropriate, by the Duncan's multiple range test. Data are presented as mean ± standard error (SE) and differences were considered statistically significant when P<0.05.
Results
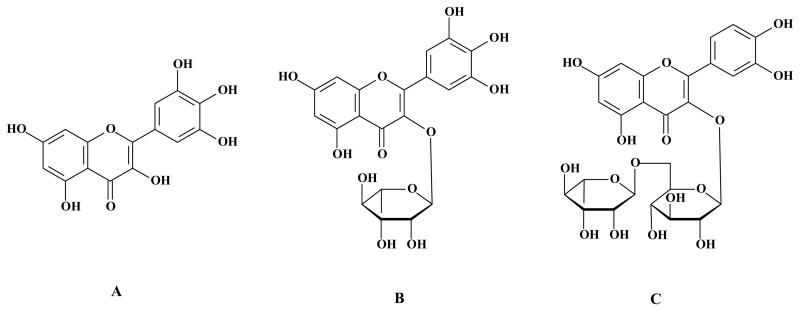
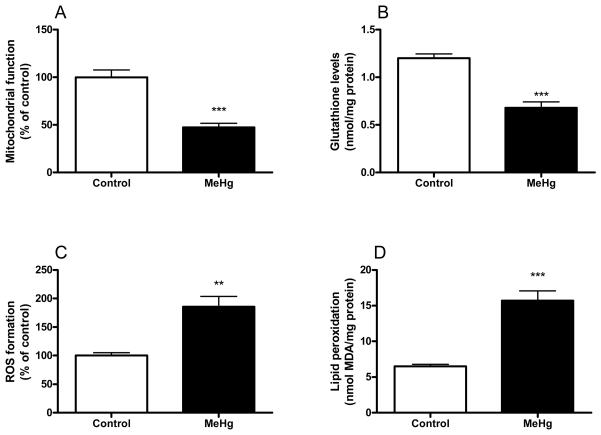
Figure 1 (A-C) illustrates the chemical structures of the flavonoids myricetin (3,3′,4′,5,5′,7-hexahydroxyflavone), myricitrin (myricetin-3-O-rhamnoside) and rutin (quercetin-3-O-rutinoside). The incubation of mouse brain mitochondria with 100 μM MeHg induced a significant decrease in the mitochondrial function (Figure 2A). This event was paralleled by a significant decrease in GSH levels (Figure 2B). MeHg also increased ROS generation (Figure 2C) and TBARS levels (Figure 2D). The simultaneous incubation of mitochondria with catalase (200 U) completely inhibited MeHg-induced mitochondrial dysfunction and lipid peroxidation, indicating that removal of hydrogen peroxide confers protective effects against the deleterious effects of MeHg in mouse brain mitochondria (data not shown).
Figure 1.
Chemical structures of the flavonoids (A) myricetin, (B) myricitrin and (C) rutin.
Figure 2.
Effects of MeHg on mitochondrial function and oxidative stress markers. Mitochondrial enriched fractions were isolated from mouse brains and incubated in the absence (white bars) or in the presence (black bars) of 100 μM MeHg. Subsequently, (A) mitochondrial function, (B) GSH levels, (C) ROS formation, and (D) lipid peroxidation were determined. ROS, GSH and lipid peroxidation were measured at 15, 30 and 60 minutes after incubations with MeHg, respectively. Data are expressed as nmol/mg protein (A and C) or percent of controls (B and D) (mean ± SE; n = 6).
** (p<0.01), *** (p<0.001) when compared to the control group by one-way ANOVA, followed by Duncan's multiple range test.
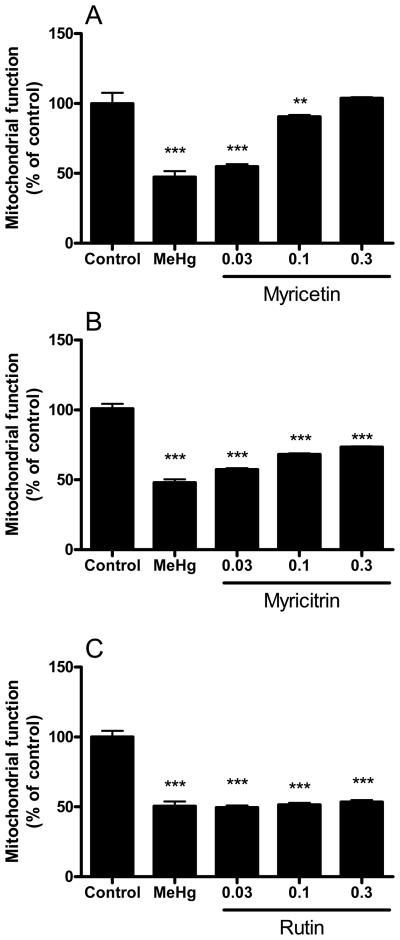
Myricetin restored mitochondrial function in a concentration-dependent manner (Figure 3A). The flavonoid myricitrin was somewhat effective in reversing the effects of MeHg on mitochondrial function after MeHg incubation (Figure 3B); however, this effect was incomplete and even at the highest concentrations of the flavonoid (0.1 and 0.3 mM), remaining statistically significant (P<0.001) from the controls. Rutin was ineffective in attenuating the MeHg-induced loss of mitochondrial function (Figure 3C).
Figure 3.
Effects of flavonoids against MeHg-induced mitochondrial dysfunction. Mitochondrial enriched fractions were isolated from mouse brains and incubated with MeHg (100 μM) and (A) myricetin, (B) myricitrin and (C) rutin (0.03, 0.1 and 0.3 mM). After incubations (30 min at 25 °C), mitochondrial activity was determined by the MTT reduction assay. Data are expressed as percent of controls (mean ± SE; n = 6).
** (p<0.01), *** (p<0.001) when compared to the control group by one-way ANOVA, followed by Duncan's multiple range test.
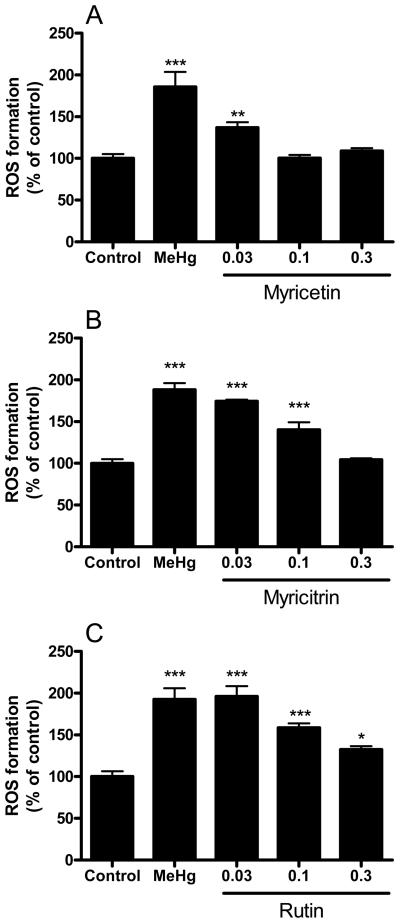
Figure 4 depicts the protective effects of the flavonoids against MeHg-induced ROS generation (DCFDA assay). Myricetin completely prevented MeHg-induced ROS generation at 0.1 and 0.3 mM (Figure 4A). Myricitrin prevented MeHg-induced ROS generation only at 0.3 mM (Figure 4B). The protective effects of rutin against MeHg-induced ROS generation were partial and observed only at 0.1 and 0.3 mM (Figure 4C).
Figure 4.
Effects of flavonoids against MeHg-induced mitochondrial ROS formation. Mitochondrial enriched fractions were isolated from mouse brains and incubated with MeHg (100 μM) and (A) myricetin, (B) myricitrin and (C) rutin (0.03, 0.1 and 0.3 mM). After incubations (15 min at 25 °C), mitochondrial ROS formation was determined by DCF fluorescence. Data are expressed as percent of controls (mean ± SE; n = 6).
* (p<0.05), ** (p<0.01), *** (p<0.001) when compared to the control group by one-way ANOVA, followed by Duncan's multiple range test.
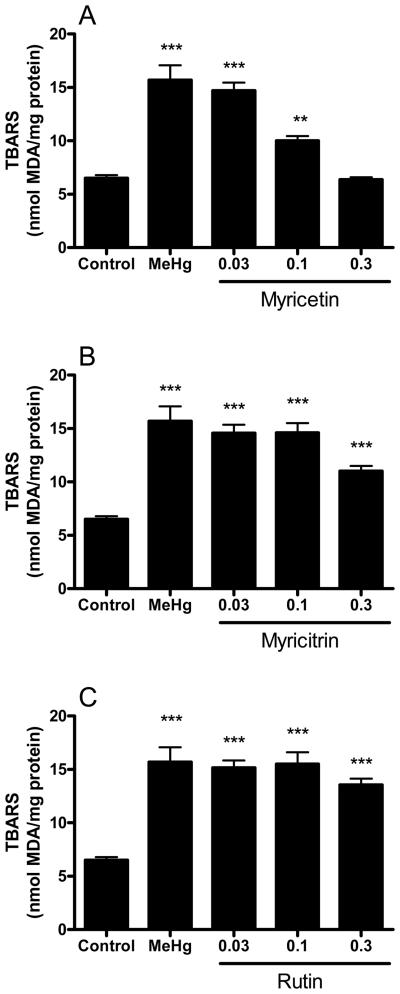
MeHg-induced lipid peroxidation in mouse brain mitochondria was completely prevented by 0.3 mM myricetin (Figure 5A). Myricitrin partially inhibited MeHg-induced lipid peroxidation at 0.3 mM (Figure 5B) and rutin did not show significant effects (Figure 5C).
Figure 5.
Effects of flavonoids against MeHg-induced mitochondrial TBARS formation. Mitochondrial enriched fractions were isolated from mouse brains and incubated with MeHg (100 μM) and (A) myricetin, (B) myricitrin and (C) rutin (0.03, 0.1 and 0.3 mM). After incubations (60 min at 25 °C), mitochondrial thiobarbituric acid reactive substances (TBARS) was determined. Data are expressed as nmol of MDA (malondialdehyde)/mg protein (mean ± SE; n = 6).
** (p<0.01), *** (p<0.001) when compared to the control group by one-way ANOVA, followed by Duncan's multiple range test.
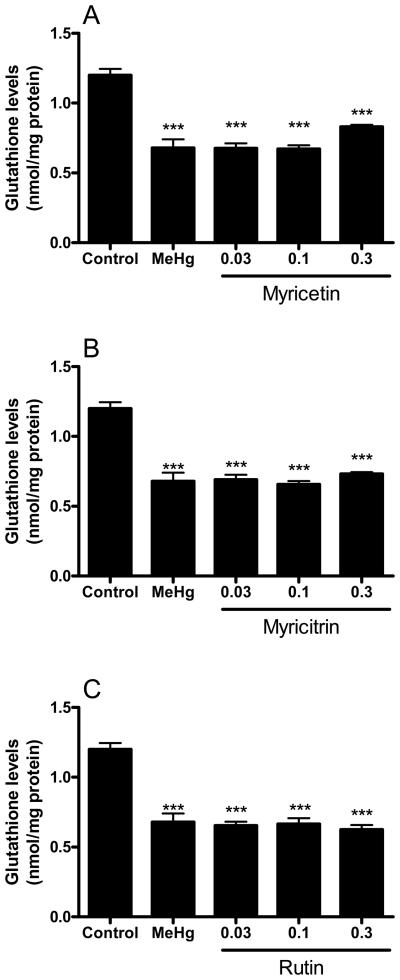
MeHg-induced GSH depletion was partially prevented by 0.3 mM myricetin (Figure 6A). The glycoside flavonoids myricitrin and rutin were unable to protect against MeHg-induced depletion of mitochondrial GSH (Figure 6B and C). The individual effects of the tested flavonoids (in the absence of MeHg) on mitochondrial function, ROS formation, lipid peroxidation and GSH levels were performed in parallel experiments and were not different when compared to the control conditions (data not shown).
Figure 6.
Effects of flavonoids against MeHg-induced mitochondrial GSH depletion. Mitochondrial enriched fractions were isolated from mouse brains and incubated with MeHg (100 μM) and (A) myricetin, (B) myricitrin and (C) rutin (0.03, 0.1 and 0.3 mM). After incubations (30 min at 25 °C), mitochondrial GSH levels were determined as non-protein thiols (NPSH). Data are expressed as nmol/mg protein (mean ± SE; n = 6).
*** (p<0.001) when compared to the control group by one-way ANOVA, followed by Duncan's multiple range test.
The ability of myricetin to counteract MeHg-induced mitochondrial dysfunction and oxidative stress was higher when compared to myricitrin and rutin. This was confirmed by analysing EC50 values (Table 1), which indicated that the protective potencies of the flavonoids against MeHg-induced mitotoxicity were myricetin > myricitrin > rutin.
Table 1.
EC50 values of the flavonoids in preventing MeHg (100 μM) induced mitochondrial dysfunction (MTT), ROS formation and lipid peroxidation (LPO).
| EC50 (μM) |
|||
|---|---|---|---|
| Flavonoid | MTT | ROS | LPO |
| Myricetin | 56.2 | 27.8 | 81.0 |
| Myricitrin | 270.7 | 94.6 | 297.6 |
| Rutin | --- | 217.5 | 451.4 |
Discussion
Previous studies (Franco et al 2007; Wagner et al 2010) have shown the protective effects of the flavonoid quercetin from MeHg-induced toxicity and oxidative stress in both mitochondria-enriched fractions and brain slices. In the present study, we observed that three additional flavonoids (derived from Polygala paniculata and Eugenia uniflora) protected against MeHg-induced mitochondrial dysfunction and oxidative stress. The results clearly showed that myricetin, which had high antioxidant capability, also displayed higher protective effect against MeHg-induced mitotoxicity when compared to myricitrin and rutin.
The available antidotal strategies to treat mercury poisonings are largely based on chelating therapies. The use of sulfhydryl (SH)-enriched chelators is based on the high affinity of MeHg to –SH groups, thus assisting in eliminating mercury from tissues predominantly via renal excretion. However, these drugs are of limited use, because of their adverse side effects (Tchounwou et al 2003). Moreover, chelating agents are generally used after exposures to inorganic mercury, such as mercury vapour and mercury salts. It has been proposed that chelating therapies are ineffective in poisonings with organic forms of mercury, such as MeHg and ethyl mercury (Clarkson et al 2003), although this issue remains controversial (Pingree et al 2001; Koh et al 2002; Carvalho et al 2007). Nevertheless, there is general agreement that metal chelators are unable to completely eliminate mercurials' body burden and by inference, toxicity. Accordingly, the use of compounds with antioxidant properties and no apparent side effects could represent an efficient co-adjuvant strategy to counteract MeHg-induced toxicity. In this context, flavonoids seem to be highly attractive candidates due to their well known antioxidant effects (Rice-Evans et al 1996; Pietta 2000; Rice-Evans 2001), as well as their potential metal-chelating properties (Renugadevi and Prabu, 2009). Such class of compounds has displayed neuroprotective effects in several experimental models of neurodegeneration (Lee et al 2008; Williams et al 2004; Lucena et al 2007; Shimmyo et al 2008). Of particular significance, we have previously demonstrated that flavonoid-containing plant extracts (obtained from the Brazilian plants Polygala paniculata or Cypura paludosa) effectively protected against MeHg-induced neurotoxicity in mice (Farina et al 2005; Lucena et al 2007). In the present study, the three studied flavonoids (myricetin, myricitrin and rutin) displayed differential effects in preventing MeHg-induced oxidative damage in mouse brain mitochondria. In fact, myricetin was the only flavonoid that completely inhibited MeHg-induced ROS formation and lipid peroxidation. The antioxidant ability of myricetin was correlated to its protective effects against MeHg-induced mitochondrial dysfunction, evaluated by its capability to reduce MTT (see Figure 2). In fact, significant negative correlations were detected for mitochondrial function vs. TBARS levels (Pearson coefficient = −0.9320; P<0.0001) and for mitochondrial function vs. ROS levels (Pearson coefficient = −0.8193; P<0.0001), indicating that the scavenger properties of myricetin were responsible, at least in part, for its protective effects against MeHg-induced mitochondrial dysfunction. Although myricitrin and rutin were also effective in protecting against MeHg-induced mitotoxicity and TBARS generation, they failed in most instances to fully reverse MeHg's effects, or when effective, the protection was reached only at higher concentrations than those required for myricetin.
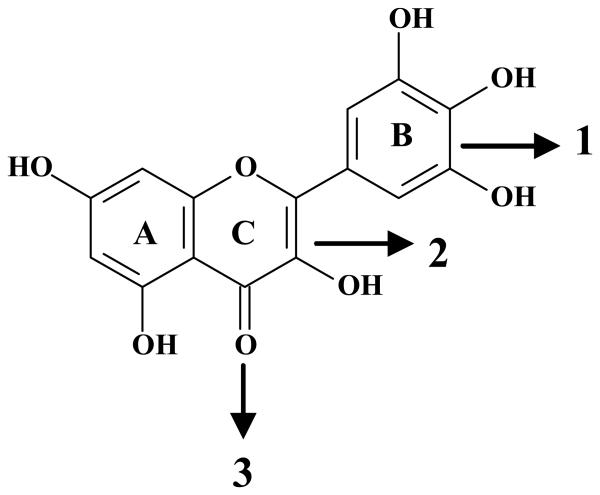
As already mentioned, the protective effects of myricetin were related to its antioxidant properties (Figures 3, 4, and 5). The antioxidant capacity of a given flavonoid depends upon its chemical structure. There are several molecular characteristics that confer the ability of a given flavonoid to promptly donate electrons and reduce reactive species. Basically, polyphenolic flavonoids possess a diphenylpropane (C6C3C6) skeleton (Rice-Evans et al 1996). The presence of hydroxyl groups linked to phenolic rings correlates with their capability to donate electrons (Harborne 1986). The positions and, more importantly, the amounts of hydroxyl groups present in the polyphenolic skeleton increase their ability to neutralize reactive species (Rice-Evans et al 1996). Based on these observations, one could posit that the increased antioxidant efficacy of myricetin in the present study is based on its higher number of hydroxyl functional groups, which can scavenge ROS generated during MeHg exposure. From a molecular point of view, the presence of three hydroxyl groups in the B-ring of myricetin is noteworthy. Lack of saturation at the C-ring is another structural property that confers antioxidant ability to flavonoids (see Figure 7). The removal of this functional group from flavonoids has been reported to impair their antioxidant potency (Shahidi et al 1992). Similarly, the blockade of the hydroxyl group in the C-ring through glycosylation has also been reported to decrease the antioxidant ability of this class of compounds (Shahidi et al 1992). This evidence is in agreement with the reduced protective effects of myricitrin and rutin against MeHg toxicity when compared to myricetin (see Figure 7). This idea is reinforced by the fact that the Trolox equivalent antioxidant activity (TEAC) of myricetin is higher when compared to rutin and miricitrin (Rice-Evans et al 1996; Hopia et al 1999). Although the protective effectiveness of myricetin was clearly higher when compared to myricitrin and rutin, the in vivo protective properties of such flavonoids against MeHg-induced neurotoxicity have yet to be addressed. In this regard, it is important to take into consideration that glycosylated myricetin derivatives can undergo hydrolysis in the gastro-intestinal tract, releasing the polyphenolic skeleton and increasing antioxidant capabilities (Harborne 1986).
Figure 7. The chemical structure of the flavonoid myricetin and its functional groups.
The most important chemical properties of flavonoid compounds, which support their antioxidant activity, are the presence of a catechol or dihydroxylated B-ring (1), the presence of unsaturation in the C-ring (2) and a 4-oxo function in the C-ring (3) (Rice-Evans et al 1996).
From a molecular point of view, it is also important to note that the toxicity elicited by MeHg is related, at least in part, to the increased generation and/or decreased detoxification of ROS (Carvalho et al 2007; Franco et al 2007; Lucena et al 2007; Stringari et al 2008; Farina et al 2009). Of particular importance, hydrogen peroxide has been proposed as a key molecule involved with the pro-oxidative damage induced by MeHg. Allen and collaborators showed that catalase, an enzyme that catalyzes the conversion of hydrogen peroxide to water, was able to prevent the decrease of glutamate transport induced by MeHg in cultured astrocytes (Allen et al 2001). In agreement, our group has previously shown that hydrogen peroxide was able to decrease the function of mouse brain mitochondria induced by MeHg under in vitro conditions and that this event was blunted by the addition of catalase in the reaction medium (Franco et al 2007). These observations corroborate the fact that compounds that stimulate (Farina et al 2009) or mimic (Farina et al 2003; de Freitas et al 2009) glutathione peroxidase activity are protective against MeHg-induced toxicity both in vitro and in vivo. Corroborating the fact that hydrogen peroxide is able to generate hydroxyl radical via Fenton's reaction, lipid peroxidation has been proposed as an important event related to the cytotoxicity elicited by MeHg (Manfroi et al 2004; Franco et al 2006; Wagner et al 2010). With a particular relevance to the present study, it is noteworthy that the flavonoid myricetin, which possesses the highest ability to scavenge ROS due to the aforementioned structural properties (hydroxyl functional groups and unsaturations in the C-ring), also possessed the highest protective effects against MeHg-induced mitochondrial dysfunction.
In conclusion, the novel results presented herein show the in vitro protective effects of the flavonoids myricetin, myricitrin and rutin against MeHg-induced mitochondrial dysfunction and oxidative stress. The molecular mechanisms associated with this protection appear to be related to the antioxidant properties of the flavonoids, which counteract MeHg-induced ROS formation. This idea is reinforced by the fact that myricetin, which possesses an increased antioxidant capability when compared to its glycoside derivatives, presented the highest protective efficacy in our experimental protocol. Although the results of this study cannot be translated into human or animal models, it is noteworthy that flavonoids have been reported to display neuroprotective effects under in vivo conditions (Das et al 2008; Hamaguchi et al 2009) and their metabolites have been found in the brain tissues of rodents after oral administration (Paulke et al 2006), suggesting their ability to cross the blood-brain barrier. Thus, the ability of myricetin to attenuate and reverse MeHg-induced oxidative damage in brain mitochondria under in vitro conditions warrants further in vivo studies in the search for potential antidotes to counteract MeHg-induced neurotoxicity.
Acknowledgements
This study was supported by grants from FAPESC (Jovens Pesquisadores – FAPESC/CNPq 04/2007) and CNPq (No. 479239/2007-0) to Marcelo Farina, and National Institutes of Health (EHS07731) to Michael Aschner. The authors are also thankful to the FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)” # 01.06.0842-00 and INCT for Excitotoxicity and Neuroprotection-MCT/CNPq. Jeferson Luis Franco and Thais Posser received CAPES fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001;902:92–100. doi: 10.1016/s0006-8993(01)02375-7. [DOI] [PubMed] [Google Scholar]
- Ali SF, LeBel CP, Bondy SC. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13:637–648. [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz. J. Med. Biol. Res. 2007;40:285–291. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Aschner M, Yao CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem. Int. 2000;37:199–206. doi: 10.1016/s0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carvalho MC, Franco JL, Ghizoni H, Kobus K, Nazari EM, Rocha JB, Nogueira CW, Dafre AL, Muller YM, Farina M. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on methylmercury-induced locomotor deficits and cerebellar toxicity in mice. Toxicology. 2007;239:195–203. doi: 10.1016/j.tox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. The three modern faces of mercury. Environ. Health Perspect. 2002;110(Suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury--current exposures and clinical manifestations. N. Engl. J. Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Das S, Mandal AK, Ghosh A, Panda S, Das N, Sarkar S. Nanoparticulated quercetin in combating age related cerebral oxidative injury. Curr. Aging Sci. 2008;1:169–174. doi: 10.2174/1874609810801030169. [DOI] [PubMed] [Google Scholar]
- de Freitas AS, Funck VR, Rotta M. dos S., Bohrer D, Mörschbächer V, Puntel RL, Nogueira CW, Farina M, Aschner M, Rocha JB. Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Res. Bull. 2009;79:77–84. doi: 10.1016/j.brainresbull.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Meth. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Farina M, Campos F, Vendrell I, Berenguer J, Barzi M, Pons S, Sunol C. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol. Sci. 2009;112:416–426. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- Farina M, Franco JL, Ribas CM, Meotti FC, Missau FC, Pizzolatti MG, Dafre AL, Santos AR. Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J. Pharm. Pharmacol. 2005;57:1503–1508. doi: 10.1211/jpp.57.11.0017. [DOI] [PubMed] [Google Scholar]
- Farina M, Frizzo ME, Soares FA, Schwalm FD, Dietrich MO, Zeni G, Rocha JB, Souza DO. Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicol. Lett. 2003;144:351–357. doi: 10.1016/s0378-4274(03)00242-x. [DOI] [PubMed] [Google Scholar]
- Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, Leal RB, Santos AR, Dafre AL, Pizzolatti MG, Farina M. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem. Res. Toxicol. 2007;20:1919–1926. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy AC, Marques MR, Dafre AL, Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic. Biol. Med. 2009;47:449–457. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Franco JL, Teixeira A, Meotti FC, Ribas CM, Stringari J, Garcia Pomblum SC, Moro AM, Bohrer D, Bairros AV, Dafre AL, Santos AR, Farina M. Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ. Res. 2006;102:22–28. doi: 10.1016/j.envres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Ono K, Murase A, Yamada M. Phenolic compounds prevent Alzheimer's pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009;175:2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Nature, distribution and function of plant flavonoids. Prog. Clin. Biol. Res. 1986;213:15–24. [PubMed] [Google Scholar]
- Hopia A, Heinonen M. Antioxidant activity of flavonol aglycones and their glycosides in methyl linoleate. J. Am. Oil Chem. Soc. 1999;76:139–144. [Google Scholar]
- Johansson C, Castoldi AF, Onishchenko N, Manzo L, Vahter M, Ceccatelli S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox. Res. 2007;11:241–260. doi: 10.1007/BF03033570. [DOI] [PubMed] [Google Scholar]
- Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J. Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Aschner M, Syversen T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology. 2006;27:492–500. doi: 10.1016/j.neuro.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Koh AS, Simmons-Willis TA, Pritchard JB, Grassl SM, Ballatori N. Identification of a mechanism by which the methylmercury antidotes Nacetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: Transport by the renal organic anion transporter-1. Mol. Pharmacol. 2002;62:921–926. doi: 10.1124/mol.62.4.921. [DOI] [PubMed] [Google Scholar]
- Lee KH, Choi EM. Myricetin, a naturally occurring flavonoid, prevents 2-deoxy-D-ribose induced dysfunction and oxidative damage in osteoblastic MC3T3-E1 cells. Eur. J. Pharmacol. 2008;591:1–6. doi: 10.1016/j.ejphar.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Lucena GMRDS, Franco JL, Ribas CM, Azevedo MS, Meotti FC, Gadotti VM, Dafre AL, Santos ARS, Farina M. Cipura paludosa extract prevents methyl mercury-induced neurotoxicity in mice. Basic & Clin. Pharmacol. & Toxicol. 2007;101:127–131. doi: 10.1111/j.1742-7843.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Malagutti KS, da Silva AP, Braga HC, Mitozo PA, Santos ARS, Dafre AL, de Bem AF, Faarina M. 17β-estradiol decreases methylmercury-induced neurotoxicity in male mice. Environ Toxicol Pharmacol. 2009;27:293–297. doi: 10.1016/j.etap.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, Rocha JBT, Frizzo MES, Souza DO, Farina M. Maternal milk as methylmercury source for suckling mice: Neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol. Sci. 2004;81:172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Strain JJ. Nutrient and methyl mercury exposure from consuming fish. J. Nut. 2007;137:2805–2808. doi: 10.1093/jn/137.12.2805. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for Lipid Peroxides in Animal-Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ou YC, White CC, Krejsa CM, Ponce RA, Kavanagh TJ, Faustman EM. The role of intracellular glutathione in methylmercury-induced toxicity in embryonic neuronal cells. Neurotoxicology. 1999;20:793–804. [PubMed] [Google Scholar]
- Paulke A, Schubert-Zsilavecz M, Wurglics M. Determination of St. John's wort flavonoid-metabolites in rat brain through high performance liquid chromatography coupled with fluorescence detection. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci. 2006;832:109–113. doi: 10.1016/j.jchromb.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Pietta PG. Flavonoids as antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Pingree SD, Simmonds PL, Woods JS. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol. Sci. 2001;61:224–233. doi: 10.1093/toxsci/61.2.224. [DOI] [PubMed] [Google Scholar]
- Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009;256:128–134. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. Flavonoid antioxidants. Curr. Med. Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Shanker G, Syversen T, Aschner JL, Aschner M. Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Brain Res. Mol. Brain Res. 2005;137:11–22. doi: 10.1016/j.molbrainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Multifunction of myricetin on A beta: neuroprotection via a conformational change of A beta and reduction of A beta via the interference of secretases. J. Neurosci. Res. 2008;86:368–377. doi: 10.1002/jnr.21476. [DOI] [PubMed] [Google Scholar]
- Stringari J, Meotti FC, Souza DO, Santos AR, Farina M. Postnatal methylmercury exposure induces hyperlocomotor activity and cerebellar oxidative stress in mice: dependence on the neurodevelopmental period. Neurochem. Res. 2006;31:563–569. doi: 10.1007/s11064-006-9051-9. [DOI] [PubMed] [Google Scholar]
- Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Tox.. Appl. Pharmacol. 2008;227:147–154. doi: 10.1016/j.taap.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003;18:149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- Wagner C, Vargas AP, Roos DH, Morel AF, Farina M, Nogueira CW, Aschner M, Rocha JB. Comparative study of quercetin and its two glycoside derivatives quercitrin and rutin against methylmercury (MeHg)-induced ROS production in rat brain slices. Arch. Toxicol. 2010;84:89–97. doi: 10.1007/s00204-009-0482-3. [DOI] [PubMed] [Google Scholar]
- Wang G, Fowler BA. Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Tox. Appl. Pharmacol. 2008;233:92–99. doi: 10.1016/j.taap.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signaling molecules? Free Radic. Biol. Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, Sidoryk M, Albrecht J, Aschner M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]