Abstract
The goal of this study was to determine whether the endocannabinoid system is altered by chronic antidepressant treatment. The effects of three week administration of the monoamine oxidase inhibitor, tranylcypromine (10 mg/kg) and the selective serotonin reuptake inhibitor, fluoxetine (5 mg/kg) on cannabinoid CB1 receptor densities and endocannabinoid contents were determined in limbic brain regions of the rat. Tranylcypromine significantly reduced tissue content of the endocannabinoid N-arachidonylethanolamine (anandamide) in the prefrontal cortex, hippocampus and hypothalamus and increased 2-arachidonoylglycerol content in the prefrontal cortex. Tranylcypromine treatment significantly increased CB1 receptor binding density in the prefrontal cortex and hippocampus, but not in the hypothalamus. Treatment with fluoxetine increased CB1 receptor density in the prefrontal cortex, but had no effect on endocannabinoid contents in any brain region examined. These data suggest that monoaminergic neurotransmission can regulate the endocannabinoid system and further indicates a role of the endocannabinoid system in affective illness and its treatment.
Keywords: cannabinoid, depression, antidepressant, SSRI, MAOI
Introduction
The endocannabinoid system is a neuromodulatory system in the brain composed of a G-protein coupled receptor (CB1) and at least two arachidonate-derived endogenous ligands, N-arachidonylethanolamine (anandamide or AEA; Devane et al., 1992) and 2-arachidonoylglycerol (2-AG; Sugiura et al., 1995). Several lines of evidence suggest that the endocannabinoid system is a possible target for the treatment of neuropsychiatric disease, particularly depression (Hill and Gorzalka, 2005b; Mangieri and Piomelli, 2007; Witkin et al., 2005). For example, the cannabinoid CB1 receptor is located in brain regions involved in stress regulation and emotion, such as the prefrontal cortex, hypothalamus and hippocampus (Herkenham et al., 1991). Furthermore, the endocannabinoid system regulates neurovegetative, motivational and emotional processes, all of which are disturbed in depression (reviewed in Hill and Gorzalka, 2005b).
In line with a role of the endocannabinoid system in depressive illnes, many of the neural systems implicated in the etiology of depression exert regulation over the endocannabinoid system. Specifically, a large body of evidence implicates corticosteroids in the pathophysiology of depression (Holsboer, 2000; Parker et al., 2003), and it has been shown that the endocannabinoid system is down regulated in the hippocampus by chronic stress or glucocorticoid administration (Hill et al., 2005, 2008a,b). Similarly, there is evidence that monoaminergic neurotransmission may be altered in depression (Ressler and Nemeroff, 2000); however, the extent to which endocannabinoid activity is modulated by monoamines is not well understood. Serotonin is believed to play an important role in permitting CB1 receptor signaling (Devlin and Christopolous, 2002), and dopamine receptor agonists and antagonists have been shown to regulate endocannabinoid content (Patel et al., 2003; Tzavara et al., 2006), but few other studies have examined the extent to which monoaminergic signaling modulates the endocannabinoid system.
The current study determined the effects of two conventional antidepressants, which potentiate monoaminergic neurotransmission via different mechanisms, on endocannabinoid signaling as an approach to clarify the both the role of endocannabinoid signaling in the mechanism of action of anti-depressants and its regulation by monoamine systems. A recent report from our group demonstrated that the tricyclic antidepressant, desipramine (which largely functions as a noradrenergic uptake inhibitor), increases cannabinoid CB1 receptor density in the hypothalamus and hippocampus (Hill et al., 2006); however, the extent to which these findings can be generalized to other classes of antidepressants is unknown. In the present study, we have examined the effect of a three week treatment of rats with drugs from two other major classes of antidepressants, monoamine oxidase inhibitors (MAOI) and selective serotonin reuptake inhibitors (SSRI), on biochemical parameters of the endocannabinoid system in brain regions implicated in depression.
Methods
Subjects and Treatment
Male Sprague-Dawley rats (70 days of age, 250–300 g) housed in groups of three in triple wire mesh cages were used in this study. Colony rooms were maintained at 21 °C, and on a reverse 12 h light/dark cycle, with lights on at 0700 h. All rats were given ad libitum access to Purina Rat Chow and tap water. Subjects were randomly assigned to one of three conditions for chronic antidepressant administration: a) vehicle (0.9% saline); b) tranylcypromine (10 mg/kg; Sigma, Canada); c) fluoxetine (5 mg/kg; Pfizer). All subjects were administered a daily intraperitoneal injection of their respective drug or vehicle for 21 days. Twenty h following the last injection, all animals were rapidly decapitated and their brains were removed and the prefrontal cortex, hippocampus and hypothalamus were sectioned out on dry ice and flash frozen in liquid nitrogen as previously described (Hill et al., 2006). Tissues were stored at−80 °C until analysis. All animal protocols were approved by the Animal Ethics Committee of the University of British Columbia and were consistent with the standards of the Canadian Council for Animal Care.
CB1 Receptor Binding Assay
Dissected brain sections were homogenized in 10 volumes of 0.32 M sucrose containing 3 mM HEPES (pH 7.5; N-2-Hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and 1 mM EDTA (ethylenediaminetetraacetic acid). The homogenates were centrifuged at 1,000 × g for 10 min, and the supernatant was centrifuged again at 18,000 × g for 20 min after which the remaining supernatant was rapidly decanted. The remaining pellet, which is the membrane fraction, was resuspended in 1–2 ml TME buffer (50 mM Tris HCl, pH 7.4; 1 mM EDTA and 3 mM MgCl2) containing 1 mM sodium orthovanadate. Protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA).
CB1 receptor binding assays were performed using a Multiscreen Filtration System with Durapore 1.2-μM filters (Millipore, Bedford, MA) as described previously (Hillard et al., 1995). Incubations (total volume = 0.2 mL) were carried out using TME buffer containing 1 mg/mL bovine serum albumin (TME/BSA). Membranes (10 μg protein per incubate) were added to the wells containing 0.25, 0.5, 1.0, or 2.5 nM 3H-CP 55,940, a cannabinoid CB1 receptor agonist. Ten μM Δ9-THC was used to determine non-specific binding.
Endocannabinoid Quantification
Brain tissue samples were subjected to a lipid extraction process exactly as described previously (Patel et al., 2003). The content of both AEA and 2-AG within lipid extracts were determined using isotope-dilution liquid chromatography/mass spectrometry as described previously (Patel et al., 2005).
Statistics
Comparison of the effects of chronic antidepressant treatment on parameters of CB1 receptor binding (n = 3–4 animals/group) and endocannabinoid content (n = 6–8 animals/group) were analyzed using a one way analysis of variance. Following ANOVA, a Dunnett’s test was employed to compare each group to vehicle treated animals. Based on our previous study demonstrating that chronic tricyclic antidepressant treatment increased CB1 receptor density in the hippocampus and hypothalamus (Hill et al., 2006), our a priori hypothesis was that MAOI and SSRI antidepressants would do the same; thus, Dunnett’s tests were one tailed in the analyses of Bmax values. There were no a priori hypotheses made for endocannabinoid content in any brain region. Significance was established against an alpha level of .05.
Results
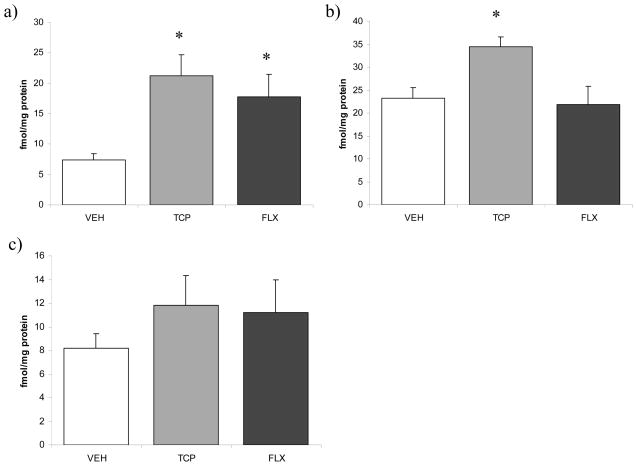
The effects of chronic antidepressant treatment on the endocannabinoid system were both region- and drug-specific. With regard to CB1 receptor binding, ANOVA indicated a significant effect of antidepressant treatment on the density (Bmax) of the CB1 receptor in the prefrontal cortex [F (2, 11) = 6.01, p < 0.03; Fig. 1]. Analysis with a Dunnett’s test demonstrated that both tranylcypromine (p < .01) and fluoxetine (p < .03) increased the Bmax for [3H]CP55940 binding to the CB1 receptor in the prefrontal cortex relative to vehicle treated animals. There was no effect of antidepressant treatment on the binding affinity (Kd) of [3H]CP55940 for the CB1 receptor [F (2, 11) = 1.54, p > 0.05; vehicle: 0.27 +/− 0.04 nM vs. tranylcypromine: 0.39 +/− 0.12 nM vs. fluoxetine: 0.78 +/−0.35 nM).
Figure 1.
The effects of 21 days of administration of varying antidepressants (VEH-vehicle; TCP-tranylcypromine [10 mg/kg]; FLX-fluoxetine [5 mg/kg]) on the maximal binding (Bmax) of the CB1 receptor (as determined through [3H]CP55940 binding to tissue homogenates) in the a) prefrontal cortex; b) hippocampus; c) hypothalamus. Values are denoted as means ± SEM. * Significantly different from control (p < .05).
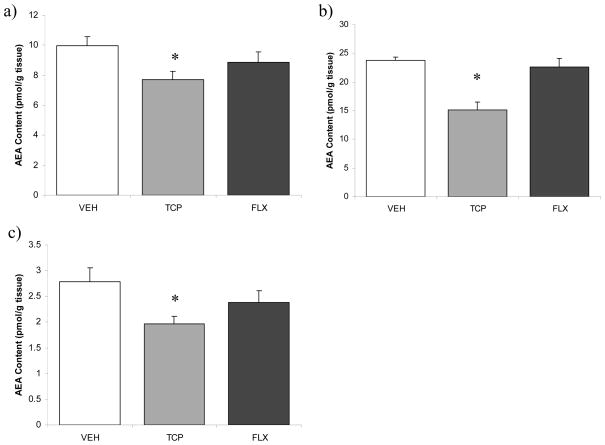
ANOVA revealed a nearly significant effect of antidepressant treatment on prefrontal cortical AEA content [F (2, 22) = 3.23, p = 0.06; Fig. 2]; a priori analysis, however, with a Dunnett’s t-test demonstrated that tranylcypromine treatment resulted in a significant reduction in AEA content (p < 0.04), whereas fluoxetine treatment had no effect (p > 0.05). Similarly, ANOVA revealed that there was no significant effect of antidepressant treatment on 2-AG content in the prefrontal cortex [F (2, 20) = 2.51, p > 0.05; Table 1], but a priori analysis with a Dunnett’s t-test demonstrated that tranylcypromine treatment significantly increased 2-AG content in the prefrontal cortex (p < 0.04).
Figure 2.
The effects of 21 days of administration of antidepressants (VEH-vehicle; TCP-tranylcypromine [10 mg/kg]; FLX-fluoxetine [5 mg/kg]) on anandamide content in the a) prefrontal cortex; b) hippocampus; c) hypothalamus. Values are denoted as means ± SEM. * Significantly different from control (p < .05).
Table 1.
The effects of chronic antidepressant treatment on brain regional 2-arachidonylglycerol content.
| Control | 2-AG (nmol/g tissue) | ||
|---|---|---|---|
| Tranylcypromine | Fluoxetine | ||
| Prefrontal Cortex | 4.58 +/− 0.43 | 5.69 +/− 0.23* | 5.16 +/− 0.36 |
| Hippocampus | 8.27 +/− 0.22 | 7.76 +/− 0.55 | 9.13 +/− 0.75 |
| Hypothalamus | 7.81 +/− 0.52 | 7.15 +/− 0.73 | 8.06 +/− 0.89 |
Twenty one days of tranylcypromine (10 mg/kg) or fluoxetine (5 mg/kg) treatment did not result in any changes in the content of 2-arachidonylglycerol (2-AG), except a significant increase in 2-AG content in the prefrontal cortex following tranylcypromine treatment. Data are presented as mean values +/− SEM.
Significantly different from control (p < .05).
In the hippocampus, antidepressant treatment had a significant effect on Bmax [3H]CP55940 binding to the CB1 receptor [F (2, 10) = 5.12, p < 0.05; Fig. 1], with a Dunnett’s test demonstrating that tranylcypromine treatment increased the Bmax of the CB1 receptor relative to control (p < 0.04), whereas fluoxetine had no effect (p > 0.05). There was no effect of antidepressant treatment on the Kd for [3H]CP55940 binding to the CB1 receptor in the hippocampus [F (2, 10) = 1.00, p > 0.05; vehicle: 1.14 +/− 0.15 nM vs. tranylcypromine: 1.81 +/− 0.18 nM vs. fluoxetine: 1.35 +/− 0.49 nM). I
In the hippocampus, ANOVA revealed that antidepressant treatment significantly affected AEA content [F (2, 21) = 16.95, p < 0.001; Fig. 2]. Analysis with a Dunnett’s test revealed that tranylcypromine produced a highly significant reduction in hippocampal AEA content (p < 0.001) while fluoxetine had no effect (p > 0.05). There was no effect of antidepressant treatment on 2-AG content in the hippocampus [F (2, 20) = 1.63, p > 0.05; Table 1].
In the hypothalamus, there was no significant effect of antidepressant treatment on the Bmax [F (2, 10) = 0.81, p > 0.05; Fig. 1] or the Kd [F (2, 10) = 0.32, p > 0.05; vehicle: 2.21 +/− 0.21 nM vs. tranylcypromine: 2.43 +/− 0.98 nM vs. fluoxetine: 3.08 +/− 0.92 nM) for [3H]CP55940 binding to the CB1 receptor.
Anandamide content in the hypothalamus was significantly reduced by antidepressant treatment [F (2, 22) = 3.56, p < 0.05; Fig. 2], with Dunnett’s test demonstrating that tranylcypromine treatment significantly reduced AEA content in the hypothalamus (p < 0.03), whereas fluoxetine had no effect (p > 0.05). There was no effect of antidepressant treatment on 2-AG content in the hypothalamus [F (2, 22) = 0.41, p > 0.05; Table 1].
Discussion
The current study demonstrates that chronic treatment with tranylcypromine and fluoxetine can elicit region-specific alterations in the endocannabinoid system that appear to be specific to the monoaminergic system targeted by each agent The data presented herein, together with our previous study (Hill et al., 2006) indicate that endocannabinoid signaling is regulated by chronic modulation of the monoaminergic systems by three different anti-depressant drug, but that there is no change in signaling that is common to all three classes.
One functional response to chronic treatment with some classes of antidepressants is a reduction in stress-induced hypothalamic/pituitary/adrenal (HPA) axis activation (Hill et al., 2006; Duncan et al., 1996; Connor et al., 2000). We have shown previously that 21 day treatment with desipramine produced a significant increase in hypothalamic CB1 receptor density and that this change was involved in the effects of desipramine to dampen HPA axis reactivity (Hill et al., 2006). However, we report herein that hypothalamic CB1 receptor density is not affected by 21 day treatment with either tranylcypromine or fluoxetine, so an increase in hypothalamic CB1 receptor density is not a common mechanism among anti-depressants. Interestingly, however, the tricyclics are known to be the most efficacious among the three classes we examined, for reducing HPA axis activation (Duncan et al., 1996; Connor et al., 2000) and the differential effect of desipramine compared to fluoxetine and tranylcyramine could underlie this characteristic. On the other hand, 21 day treatment with tranylcypromine significantly decreased AEA content in the hypothalamus. Deficits in endocannabinoid signaling, presumably within the hypothalamus, are associated with hyperresponsiveness (Cota et al., 2007; Patel et al., 2004) and/or reduced feedback regulation of the HPA axis (Cota et al., 2007). Interestingly, both of these phenomenon have been documented following chronic treatment with an MAOI antidepressant (Duncan et al., 1996; Kier et al., 2005). Taken together, our data suggest that differential effects on endocannabinoid signaling within the hypothalamus could contribute to the differential effects of pharmacologically distinct antidepressants on the HPA axis.
Both tranylcypromine and fluoxetine increased CB1 receptor density in the prefrontal cortex. This increase in CB1 receptor density in the prefrontal cortex is in agreement with a report that chronic fluoxetine treatment enhances signal transduction elicited by activation of the CB1 receptor in the prefrontal cortex (Pazos et al., 2005). Interestingly, it has recently been demonstrated that activation of CB1 receptors in the ventromedial PFC increases the firing activity of serotonergic neurons in the dorsal raphe (Bambico et al., 2007). Upon initial exposure to antidepressants, dorsal raphe neurons exhibit reductions in firing activity; following repeated antidepressant treatment, firing activity increases back to baseline levels, a phenomenon which is believed to correlate to the clinical onset of action of these drugs (Czachura and Rasmussen, 2000; Besson et al., 2000; Mongeau et al., 1997). It is believed that the increase in firing rate of raphe neurons following chronic fluoxetine is due to a disinhibition of inhibitory raphe 5-HT1A receptors (Mongeau et al., 1997; Le Poul et al., 1995); however, given the current data it is also possible that antidepressants which modulate serotonergic neurotransmission may increase prefrontal cortical CB1 receptor activity, in an attempt to increase and normalize raphe neuron firing. Recent reports, however, have demonstrated that prefrontal cortical CB1 receptors are increased in depressed, suicide victims (Hungund et al., 2004) and in rodents following chronic stress (Bortolato et al., 2007; Hill et al., 2008b). There are two possibilities to account for this apparent discrepancy with the current report. First, an increase in CB1 receptor binding in the PFC in depression and following chronic stress, could be a compensatory response launched to increase the activational drive on the raphe nulei and enhance serotonergic neurotransmission. Alternately, it is possible that CB1 receptors in distinct neuronal populations are differentially altered in depressed states or following antidepressant treatment. Determination of the neuronal localization of changes in CB1 receptor density in animal models of depression and antidepressant regimens is clearly needed and will aid significantly in the interpretation of these and other data.
It is interesting that 21 day treatment with tranylcypromine, but not fluoxetine, was found to evoke a ubiquitous reduction in AEA content in every limbic region examined in this study. An important difference between the mechanism of action of tranylcypromine compared to fluoxetine and desipramine (Hill et al., 2006) is that tranylcypromine inhibits monoamine oxidase activity, as opposed to uptake of specific monoaminergic neurotransmitters, and thus in addition to increasing serotonergic and noradrenergic activity, this agent exhibits the ability to augment dopamine transmission. Therefore, the differential effect of tranylcypromine to modulate AEA content could be via enhancement of dopaminergic signaling. This idea is in line with previous work demonstrating that both a dopamine reuptake inhibitor and a dopamine D1 receptor agonist reduced AEA content in the limbic forebrain (Patel et al., 2003), as well as the finding that mice lacking the dopamine transporter exhibit a selective reduction in AEA in the striatum (Tzavara et al., 2006).
While the effects of tranylcypromine on AEA were regionally non-specific, tranycypromine elicited a significant increase in 2-AG content in the PFC exclusively. Some data in the literature suggest that the reciprocal changes in AEA and 2-AG are mechanistically related. For example, genetic overexpression of FAAH in the prefrontal cortex, which results in a substantial reduction in AEA content, causes a significant increase in 2-AG levels in the PFC (Rubino et al., 2008). Maccarrone and colleagues (2008) have recently shown that AEA inhibits 2-AG synthesis and physiological actions in the striatum. These studies suggest that tranylcypromine downregulates AEA content in the PFC, which in turn results in an increase in 2-AG activity. However, this relationship requires further exploration, including an explanation for why this is only observed in the PFC.
In the hippocampus, 21 day treatment with tranylcypromine but not fluoxetine produced a significant increase in the density of CB1 receptors and a significant reduction in tissue AEA content. It is possible that the increase in CB1 receptor binding sites produced by tranylcypromine is a compensatory response to reduced levels of its ligand, AEA; however, CB1 receptor regulation is not always coupled to tissue content of ligand and does not behave like similar G-protein coupled receptors, which exhibit agonist dependent regulation of expression (Hill et al., 2005; Kola et al., 2005; Romero et al., 1995; Maccarrone et al., 2008). Further, if the primary signaling ligand for the CB1 receptor is 2-AG and not AEA (Makara et al., 2005; Hashimotodani et al., 2007), than the reduction in AEA content may be irrelevant with regards to mechanisms of CB1 receptor regulation. Given these bidirectional effects of tranylcypromine on the endocannabinoid system in the hippocampus, the functional relevance of these changes is currently unclear.
In conclusion, these data demonstrate that the endocannabinoid system is differentially regulated by two classes of antidepressants in a region specific fashion throughout the limbic system. The mechanistic differences between the antidepressants used suggests that dopaminergic transmission could be integral for regulating limbic AEA content, whereas 5-HT may be particularly relevant for CB1 receptor regulation in the PFC. To date, preclinical research has produced a contradictory body of evidence regarding the potential role of the endocannabinoid system in affective diseases such as depression, with studies indicating that either facilitation (Hill and Gorzalka, 2005a,b; Hill et al., 2007; Gobbi et al., 2005; Bortolato et al., 2007; Mangieri and Piomelli, 2007) or inhibition (Witkin et al., 2005; Griebel et al., 2005) of endocannabinoid signaling could represent a novel target for the development of antidepressants. The current data add to the growing body of literature by demonstrating that the endocannabinoid system is modified by antidepressant treatment. However, there is no single change that is common across all classes of antidepressants. Ultimately, these data highlight the importance of understanding the regional and neuronal localization of CB1 receptors as well as the different physiological functions of AEA and 2-AG before these data can be reconciled to a broader theory.
Acknowledgments
This research was supported by operating grants from the Canadian Institute of Health Research (CIHR) and the Natural Sciences and Engineering Council of Canada (NSERC) to BBG and by National Institute of Health (NIH) grant R21DA022439 to Cecilia J. Hillard, Ph.D.; partial funding was provided by Research for a Healthier Tomorrow, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin (CJH); by a NSERC postgraduate scholarship and a Michael Smith Foundation for Health Research (MSFHR) Trainee Award to MNH.
References
- Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A, Haddjeri N, Blier P, de Montigny C. Effects of the co-administration of mirtazapine and paroxetine on serotonergic neurotransmission in the rat brain. Eur Neuropsychopharmacol. 2000;10:177–188. doi: 10.1016/s0924-977x(00)00069-9. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Kelliher P, Shen Y, Harkin A, Kelly JP, Leonard BE. Effect of subchronic antidepressant treatments on behavioral, neurochemical, and endocrine changes in the forced-swim test. Pharmacol Bioc Behav. 2000;65:591–597. doi: 10.1016/s0091-3057(99)00192-6. [DOI] [PubMed] [Google Scholar]
- Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grubler Y, Stalla J, Pasquali R, Lutz B, Staller GK, Pagotto U. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology. 2007;148:1571–1581. doi: 10.1210/en.2005-1649. [DOI] [PubMed] [Google Scholar]
- Czachura JF, Rasmussen K. Effects of acute and chronic administration of fluoxetine on the activity of serotonergic neurons in the dorsal raphe nucleus of the rat. Naunyn Schmiedebergs Arc Pharmacol. 2000;362:266–75. doi: 10.1007/s002100000290. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Devlin MG, Christopolous A. Modulation of cannabinoid agonist binding by 5-HT in the rat cerebellum. J Neurochem. 2002;80:1095–1102. doi: 10.1046/j.0022-3042.2002.00797.x. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Johnson KB, Breese GR. Functional classification of antidepressants based on antagonism of swim stress-induced fos-like immunoreactivity. J Pharmacol Exp Ther. 1996;277:1076–1089. [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008a;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, Gorzalka BB. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008b;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB(1) receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005a;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav Pharmacol. 2005b;16:333–352. doi: 10.1097/00008877-200509000-00006. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Sinopoli KJ, Viau V, Hillard CJ, Gorzalka BB. Involvement of the endocannabinoid system in the ability of long-term tricyclic treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2006;31:2591–2599. doi: 10.1038/sj.npp.1301092. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Campbell WB. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J Neurochem. 1995;64:677–683. doi: 10.1046/j.1471-4159.1995.64020677.x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Hill MN, Carrier EJ, Shi L, Cullinan WE, Gorzalka BB. Regulation of cannabinoid receptor expression by chronic, unpredictable stress in rats and mice. Soc Neurosci Abstr. 2006:746.19. [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, Mann JJ, Arango V. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- Kier A, Han J, Jacobson L. Chronic treatment with the monoamine oxidase inhibitor phenelzine increases hypothalamic-pituitary-adrenocortical activity in male C57BL/6 mice: relevance to atypical depression. Endocrinology. 2005;146:1338–1347. doi: 10.1210/en.2004-0650. [DOI] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mithcell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Acrh Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabo SL, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Mangieri RA, Piomelli D. Enhancement of endocannabinoid signaling and the pharmacotherapy of depression. Pharmacol Res. 2007;56:360–366. doi: 10.1016/j.phrs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeau R, Blier P, de Montigny C. The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res Rev. 1997;23:145–195. doi: 10.1016/s0165-0173(96)00017-3. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Pazos A, Valdizan E, Mato S. The antidepressant fluoxetine increases both the constitutive and agonist-dependent functionality of brain CB1 receptors. Soc Neurosci Abstr. 2005:447.7. [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia L, Fernandez-Ruiz JJ, Cebeira M, Ramos JA. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1995;51:731–737. doi: 10.1016/0091-3057(95)00023-p. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Comm. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Li DL, Moutsimilli L, Bisogno T, Di Marzo V, Phebus LA, Nomikos GG, Giros B. Endocannabinoids activate transient receptor potential vanilloid 1 receptors to reduce hyperdopaminergia-related hyperactivity: therapeutic implications. Biol Psychiatry. 2006;59:508–515. doi: 10.1016/j.biopsych.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Davis RJ, Li X, Nomikos GG. A therapeutic role for cannabinoid CB1 receptor antagonists in major depressive disorders. Trends Pharmacol Sci. 2005;26:609–617. doi: 10.1016/j.tips.2005.10.006. [DOI] [PubMed] [Google Scholar]