Abstract
Background
Pharmacist-physician co-management of hypertension has been shown to improve office blood pressures (BP). We sought to describe the effect of such a model on 24-hour ambulatory BPs.
Methods
We performed a prospective, cluster-randomised controlled clinical trial in 179 patients with uncontrolled hypertension from five primary care clinics in Iowa City, Iowa. Patients were randomized by clinic to receive pharmacist-physician collaborative management of hypertension (intervention) or usual care (control) for a 9-month period. In the intervention group, pharmacists helped patients identify barriers to BP control, counselled on lifestyle and dietary modifications, and adjusted antihypertensive therapy in collaboration with the patient’s primary care provider. Patients were seen by pharmacists a minimum every 2 months. Ambulatory BP was obtained at baseline and study end.
Results
Baseline and end of study ambulatory BP profiles were evaluated for 175 patients. Ambulatory BPs were reduced to a greater extent in the intervention compared to control group (daytime ΔSBP [SD] 15.2[11.5] vs 5.5[13.5], p<0.001; nighttime ΔSBP [SD] 12.2[14.8] vs 3.4[13.3], p<0.001; 24-hour ΔSBP [SD] 14.1[11.3] vs 5.5[12.5], p<0.001). More patients in the intervention group had BP controlled at the end of the study (75% vs 50.7%, p<0.001) as defined by overall 24-hour ambulatory BP monitoring.
Conclusions
Pharmacist-physician collaborative management of hypertension achieved consistent and significantly greater reduction in 24-hour BP and a high rate of BP control.
Keywords: Ambulatory blood pressure monitoring, pharmacist/physician collaboration, blood pressure, hypertension management, cardiovascular risk, team-based care
INTRODUCTION
Hypertension affects 65 million persons in the United States and is associated with increased risks for stroke, myocardial infarction, and heart failure.1, 2 Blood pressure (BP) control among patients with hypertension remains below national targets. A large number of effective antihypertensive medications are available, suggesting problems are not due to lack of effective drugs, but also include such barriers as access to care and clinical inertia. Many strategies have been evaluated to improve BP control including algorithmic approaches to drug selection, case-management, and collaborative team-based approaches.3–6
Previously, in a large, prospective, cluster randomized controlled trial, we demonstrated that pharmacist-physician co-management improved office BP control and significantly decreased mean systolic BP in patients with uncontrolled BP at baseline.7 In our study, 89.1% of the patients in the intervention group achieved their goal BP within 9 months, compared to only 52.9% in the control group (p<0.001). Although the results of this and other studies have documented the efficacy of collaborative management to improve BP control,5–10 the impact of these strategies on ambulatory BP measurements has not been reported. Ambulatory BP measurements more accurately reflect the patient’s true BP, and even after adjustment for traditional cardiovascular risk factors such as office BP, they remain strongly predictive of the risk of end-organ damage.11–13 Therefore, the true effect size of any intervention to control BP is best established using 24-hour ambulatory BP monitoring. Our objective here is to report the results of 24-hour ambulatory BP monitoring obtained during a pharmacist-physician collaborative model of hypertension management.7
METHODS
Study Design
The Collaborative Management of Hypertension Study design, methods, and results have been reported previously in greater detail.7,14–15 Briefly, patients with uncontrolled hypertension from five Iowa City-area primary care clinics were recruited. Patients were eligible for the study if they were males or females aged 21–85 years, and receiving zero to three antihypertensive agents with no changes to their regimen within the past four weeks. To qualify, non-diabetic patients were required to have a clinic systolic BP value (average of the last two readings) between 145–179 mmHg or diastolic BP of 95–109 mmHg. Diabetic patients were required to have clinic systolic BP readings of 135–179 mmHg or diastolic BP readings of 85–109 mmHg. Patients with serious renal or hepatic disease were excluded, as well as those with recent myocardial infarction or stroke, unstable angina, or New York Heart Association class III or IV congestive heart failure.
The study was a cluster-randomized design in which clinics were randomized to control or intervention groups. Subjects received either pharmacist-physician co- management (intervention) or usual care (control) according to their clinic randomization. This procedure was done to avoid contamination of the intervention at the physician level. Patients in both groups had scheduled, structured study visits with a research nurse at baseline, 2, 4, 6, 8, and 9 months. At each data collection visit the nurse measured the subjects’ BP three times with a mercury sphygmomanometer using standardized American Heart Association criteria. The second and third values were averaged and reported as the clinic BP as is often the standard procedure in other large clinical trials.16 The clinic BP values were provided to the primary care provider for patients in the usual care group, and follow-up interventions left to their discretion. For patients in the intervention group, clinical pharmacists reviewed patient data obtained by the research nurse and then interviewed the patient. During the interview, the pharmacists evaluated patient factors that might impede achieving goal BP and the patients’ current treatment strategies as compared to clinical guidelines. The pharmacists then discussed treatment recommendations with the patient’s physician. The physicians could choose to accept or reject the pharmacists’ recommendations at their discretion. Medication regimens in both the control and intervention groups could be adjusted at any time by the patient’s primary care provider outside of any study visits.
At the baseline and 9-month visits patients in both the intervention and control groups underwent an ambulatory BP monitoring session. All sessions were performed using the same type of BP monitor to insure uniformity (SpaceLabs 90217A, SpaceLabs Medical, Redmond, Washington). During the ambulatory BP monitoring session, BPs were measured every 20 minutes during the day (6:00 AM to 10:00 PM) and every 30 minutes at night (10:00 PM to 6:00 AM), according to accepted criteria.11 The physicians and pharmacists in the intervention clinics were blinded to the 24-hour BP results until the patient completed the trial. The results of the baseline ambulatory BP session, as well as the office BPs at the structured study visits were shared with physicians in the control clinics.
Data Analysis
The sample size calculation for this study was based on office BP as there have been no previously published studies examining the effects of collaborative management of hypertension using the outcome of 24 hour ambulatory BP measurements. Additionally, prior to this study, there were no clinical trials of this model that randomized by clinic. Thus, several fixed and random effects that affect power were unknown a priori, such as within- and between patient variability, between-physician variability, and between-clinic variability. Therefore, we used several techniques to estimate power and sample size; these techniques are described in further detail in a previous report.7 The estimated sample size to detect a 3.4 mmHg population standard deviation of the change in mean BP averaged across physicians in each clinic at the 80% level was 47 patients per group; however, since this was a longitudinal study with several fixed and random effects, so we inflated the sample size to 90 patients per group in consideration of this unknown variability.
There were eight (8%) subjects in the intervention group and 11 (15%) subjects of the control group with missing values for ambulatory BP measurements at the end of the study. To reduce bias in favor of the intervention group that could occur with the last observation carried forward method, we used the multiple imputation procedure described by Rubin for the missing values, with the assumption that the data are multivariate normally distributed and the missing data are missing at random.17
The primary outcome of this analysis was the comparison of the change in 24-hour mean systolic and diastolic ambulatory BP from baseline to 9 months between the intervention group and the control group. For analysis of the ambulatory BP monitoring data, daytime hours were defined in a clock-time-dependent manner with daytime hours from 6:00 AM to 10:00 PM, and nighttime hours from 10:00 PM to 6:00 AM. These were selected as modified from recommended guidelines.18,19
Values for the upper limit of normal ambulatory BPs were <130/80 mmHg for the overall 24-hour time period, <135/85 mmHg for the daytime period, and <120/70 mmHg for the nighttime period according to American Heart Association recommendations.20 The same goal ambulatory BP averages were used for all patients, including patients with diabetes as current ambulatory BP monitoring recommendations do not differentiate separate levels of BP control.
The mean BP reductions were compared between the control group and the intervention group using two-sample t-test, and the BP controlled proportion was compared between the control group and the intervention group using the Chi-square test. All analyses were performed using statistical package SPSS for Windows version 17.0 (SPSS, Chicago, Illinois, USA) and SAS (Version 9.2. SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 179 patients were enrolled in the study. Nineteen patients withdrew from the study; 10 from the control group and 9 from the intervention group. Of the total 179 patients, 24-hour ambulatory BP profiles were obtained for 175 patients at baseline (100 in intervention group and 75 in control group). At the end of study ambulatory BP monitoring data was collected for 156 patients (92 in the intervention and 64 in the control group). Patient demographic data for the 175 patients included in these analyses are summarized in Table 1. The mean (SD) age for the intervention and control groups was 59.6 (13.7) and 61.9 (11.3), respectively. No significant differences were noted between the groups, with the exception of a higher percentage of patients with a history of coronary artery bypass grafting in the control group (7.7 vs 1.0%; p=0.044).
TABLE 1.
Demographic data of study participants.
| Intervention (n=101) No. of Patients |
Control (n=78) No. of Patients |
||
|---|---|---|---|
| Gender | Male | 42 (41.6%) | 36 (46.2%) |
| Female | 59 (58.4%) | 42 (53.8%) | |
| Race | Caucasian | 89 (88.1%) | 74 (94.9%) |
| Non-Caucasian | 12 (11.9%) | 4 (5.1%) | |
| Insurance Status | Ind. or Group Plan | 89 (88.1%) | 65 (83.3%) |
| Medicare/Medicaid | 12 (15.4%) | 7 (6.9%) | |
| Self-pay or other | 1 (1.3%) | 5 (5.0%) | |
| Education beyond high school | 64 (63.4%) | 42 (53.9%) | |
| Mean age (SD) | 59.6 (13.7) | 61.9 (11.3) | |
| Mean Body Mass Index (SD) | 32.3 (7.7) | 31.8 (14.7) | |
| Co-morbid Conditions | |||
| Diabetes | 25 (24.8%) | 19 (24.4%) | |
| Stroke or TIA | 9 (8.9%) | 2 (2.6%)a | |
| Myocardial Infarction | 4 (4.0%) | 5 (6.4%) | |
| Peripheral arterial disease | 3 (3.0%) | 2 (2.6%) | |
| Angina | 2 (2.0%) | 0 (0.0%) | |
| Heart failure | 2 (2.0%) | 0 (0.0%) | |
| Coronary artery bypass | 1 (1.0%) | 6 (7.7%)b | |
| Nephropathy | 1 (1.0%) | 0 (0.0%) | |
| Mean (SD) Systolic BP | 153.1 (10.0) | 150.3 (9.0) | |
| Mean(SD) Diastolic BP | 84.9 (12.0) | 85.4 (11.0) | |
Unless otherwise indicated, groups were not significantly different.
p=0.117,
p=0.044,
Fisher’s exact test.
By the end of the 9-months of study, the mean (SD) number of antihypertensive medications increased from 1.5 (1.0) to 2.4 (0.9) in the intervention group and 1.4 (1.0) to 1.9 (1.0) in the control group (P<0.01 for comparison between groups).7 Mean office systolic BP decreased by 28.9 mmHg in the intervention group compared to 17.3 mmHg in the control group (p<0.001 for between group difference).7 Table 2 summarizes the mean BP reduction at the end of the study for daytime, nighttime, and overall 24-hour ambulatory BP measurements for the intervention and control groups. While both the intervention group and control group began the study with similar BP readings, there was a significantly greater mean systolic BP reduction in the intervention group for daytime, nighttime, and overall 24-hour period. The overall 24-hour mean (SD) systolic BP for the intervention group was reduced from 135.5 (11.3) mmHg to 121.4 (9.7) mmHg compared to a reduction from 136.0 (13.3) mmHg to 130.5 (11.4) mmHg in the control group (p<0.001 for intervention vs control).
TABLE 2.
BP Results and mean BP reduction from baseline to end of study (N=175; Control, 75; Intervention, 100)
| Daytime | Nighttime | Overall 24-hr | Office BP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | ||
| Control | Baseline | 140.0 ± 13.1 | 79.2 ± 10.7 | 128.4 ± 17.8 | 71.5 ± 11.0 | 136.0 ± 13.3 | 76.6 ± 9.9 | 150.3 ± 9.0 | 85.4 ± 11.0 |
| End of study | 134.6 ± 11.6 | 76.6 ± 9.8 | 122.7 ± 15.2 | 68.1 ± 10.0 | 130.5 ± 11.4 | 73.7 ± 9.0 | 133.0 ± 14.2 | 78.5 ± 10.9 | |
| Mean BP Reduction | 5.5 ± 13.5 | 2.7 ± 6.7 | 5.7 ± 14.3 | 3.5 ± 8.8 | 5.5 ± 12.5 | 2.8 ± 6.5 | N/A | N/A | |
| Intervention | Baseline | 140.7 ± 11.1 | 79.7 ± 10.7 | 125.5 ± 15.7 | 68.8 ± 10.3 | 135.5 ± 11.3 | 76.0 ± 9.8 | 153.1 ± 10.0 | 84.9 ± 12.0 |
| End of Study | 125.5 ± 10.1 | 72.3 ± 9.8 | 113.4 ± 13.9 | 63.2 ± 9.3 | 121.4 ± 9.7 | 69.2 ± 8.7 | 124.2 ± 9.7 | 74.7 ± 9.6 | |
| Mean BP Reduction | 15.2 ± 11.5 | 7.4 ± 5.9 | 12.2 ± 14.8 | 5.6 ± 9.1 | 14.1 ± 11.3 | 6.8 ± 5.9 | N/A | N/A | |
| P* | <0.001 | <0.001 | 0.004 | 0.122 | <0.001 | <0.001 | <0.001 | <0.001 | |
p-value for comparing mean BP reduction between the control group and the intervention group.
SBP = systolic blood pressure
DBP = diastolic blood pressure
N/A = not available
In addition to the multiple imputation method, we performed a sensitivity analysis on complete data only (summarized in Table 3). Under both methods of analysis, the between-group differences were similar and remained statistically significant (mean systolic BP reduction changed from 5.5±12.5 to 3.4±11.6 for the control group and 14.1±11.3 to 14.4±11.0 for the intervention group; p<0.001 for between-group comparison).
TABLE 3.
Ambulatory BP Results and mean BP reduction from baseline to end of study (completers only; N=156; Control, 64; Intervention, 92)
| Daytime | Nighttime | Overall 24-hour | |||||
|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | SBP | DBP | ||
| Control | Baseline | 138.8 ± 12.7 | 78.5 ± 9.3 | 126.3 ± 17.6 | 70.3 ± 9.9 | 134.7 ± 13.0 | 75.7 ± 8.6 |
| End of study | 135.6 ± 11.7 | 76.7 ± 8.8 | 122.5 ± 15.7 | 67.5 ± 9.3 | 131.3 ± 11.8 | 73.7 ± 8.0 | |
| Mean BP Reduction | 3.1 ± 12.5 | 1.7 ± 6.6 | 3.8 ± 13.9 | 2.8 ± 8.6 | 3.4 ± 11.6 | 2.0 ± 6.4 | |
| Intervention | Baseline | 141.0 ± 11.2 | 79.8 ± 10.9 | 125.0 ± 15.9 | 68.6 ± 10.6 | 135.5 ± 11.3 | 76.0 ± 10.0 |
| End of Study | 125.4 ± 10.2 | 72.3 ± 9.8 | 112.9 ± 14.3 | 63.1 ± 9.1 | 121.2 ± 9.9 | 69.1 ± 8.6 | |
| Mean BP Reduction | 15.5 ± 11.3 | 7.4 ± 5.7 | 12.1 ± 14.6 | 5.5 ± 9.0 | 14.4 ± 11.0 | 6.9 ± 5.7 | |
| P* | <0.001 | <0.001 | 0.001 | 0.062 | <0.001 | <0.001 | |
p-value for comparing mean BP reduction between the control group and the intervention group.
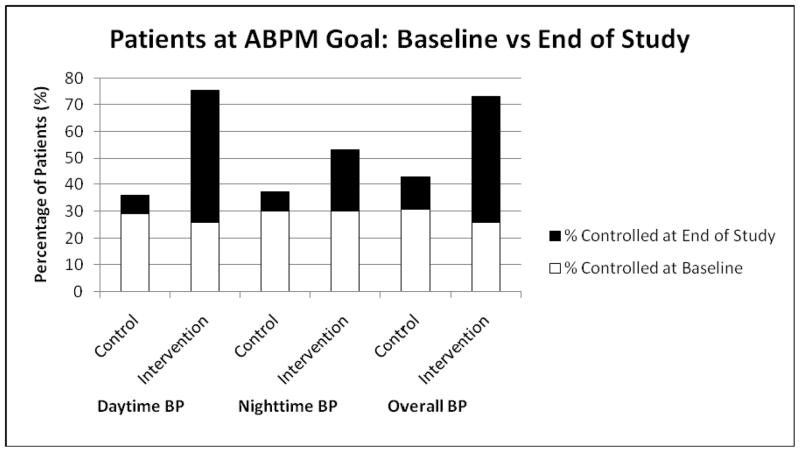
Figure 1 illustrates the percentage of patients in both groups with BP control at baseline and at the end of the study as defined by ambulatory BP monitoring criteria. Again, while both the control and intervention groups had a similar percentage of patients at goal at baseline, the intervention group achieved higher control rates by the end of the study across all time periods (daytime, nighttime, and overall 24-hour) of the 24-hour session.
FIGURE 1.
ABPM = ambulatory blood pressure monitoring
Control defined as follows according to American Heart Association recommendations.20
Daytime: <135/85 mmHg
Nighttime: <120/70 mmHg
Overall: <130/80 mmHg
Data regarding pharmacist recommendations have been previously reported.14 Briefly, changes in drug therapy were recommended 267 times for the 101 intervention patients, where most recommendations for a change in treatment involved adding a new antihypertensive medication (46.4%) or up-titrating the dose of an existing medication (33.3%). Of the pharmacist recommendations to add a new antihypertensive medication, 36.3% were for the addition of a thiazide-type diuretic. Physicians accepted and implemented 95.9% of the 267 pharmacist recommendations to modify drug therapy.
DISCUSSION
Pharmacist-physician co-management is an effective method for improving BP control in hypertensive patients, as traditionally defined by office BP readings.5,9,10 Our study is the first team-based care approach to evaluate the impact of the intervention on ambulatory BP measurements, and demonstrates that pharmacist intervention results in greater decreases in the mean BP and an increase in the number of patients meeting their BP goal (75% vs 50.7%) compared to usual care. The lower BPs achieved in the pharmacist intervention group were primarily attributable to the increased number of medications per patient (1.5±1.0 to 2.4±0.9) compared to the control group (1.4±1.0 to 1.9±1.0), suggesting such a strategy can reduce clinical inertia. As typical physician visits are short and may not provide adequate time to address multiple issues, the collaborative management with pharmacists allowed specific time to focus on improving medication regimens to meet BP goals. The rate of acceptance of the pharmacist recommendations (95.9%) indicates a relatively high level of satisfaction with the pharmacist-physician co-management model on the part of physicians in the clinics randomized to the intervention.
Our present study showed that daytime, nighttime, and overall 24-hour BPs were consistently lower in the pharmacist intervention group. These findings indicate that pharmacist-physician co-management can effectively sustain BP control throughout the entire 24-hour period. This is an important finding, as traditional office BP measurements would likely be taken during a time of peak antihypertensive activity during daytime hours, thus potentially overestimating the effect of the intervention. Our findings are also noteworthy considering the control group was subjected to structured research nurse visits and the BP data were regularly shared with the patient’s primary care provider. Patients in the control group were informed of their BP results and their goal BP in a structured, consistent manner, and were provided with written information on BP management. In this respect, the ‘control’ group represented a more “enhanced usual care” than what is traditionally found in team-based BP intervention studies. In this group, the BP decreased from baseline to the end of the study and BP control rates also increased; however, the intervention group saw greater BP reduction in both areas of measurement that remained statistically significantly lower.
It is well established that traditional office BP measurements do not provide the most accurate assessment of the true BP. Banegas, et al. found that office BP underestimated BP control 33.4% of the time and overestimated BP control 5.4% of the time.21 This has been shown specifically in patients on hydrochlorothiazide,22 and may be due in part to the fact that BP measurement often occurs during the peak effect of many antihypertensive medications during the day. As 36.3% of the pharmacist recommendations in our study to add therapy were for the addition of a thiazide-type diuretic, it is noteworthy that the intervention group maintained significant lower BP throughout the full 24-hour period. Gorostidi, et al. found significant discrepancies between office BP measurement and ambulatory BP measurements in high-risk patients, and that 60% of patients demonstrated a non-dipper pattern.23 Ambulatory BP monitoring is able to provide a more accurate picture of overall CV risk since it includes nocturnal BPs, which are known to correlate strongly with CV risk.12,13,24 Patients receiving pharmacist-physician co-management in our study experienced significantly reduced BPs, including during the important nighttime period (mean systolic BP reduction 12.2 vs 5.7).
Our study should be interpreted within the context of several limitations. First, our analysis defined daytime and nighttime periods in a fixed-clock manner rather than patient-specific wake/sleep periods. Patient-specific measurement of awake/sleep cycles can been performed using actigraphy; however, this method is not standard among all BP studies and there is little evidence to suggest that definitions of daytime and nighttime based on fixed-clock intervals or actual time spent in bed significantly impacts the determination of cardiovascular risk.25 Secondly, the differential attrition rate with eight (8%) subjects in the intervention group and 11 (15%) subjects of the control group with missing values at the end-of-study, would potentially bias the results in favor of the intervention. To control for this potential bias, we performed the multiple imputation procedure to replace missing values, and also performed an additional analysis only on completed data. In both analyses, the results remained significant. While we do not know the reason for withdrawal in these patients, we suspect the lower rate of attrition in the intervention group may be partially due to the relationship formed between patients and clinical pharmacists and the incentive of receiving enhanced care.
A final limitation is the unknown generalizability of our intervention. The patient population examined was largely Caucasian hypertensive subjects with relatively few co-morbidities. We do not know the scalability of the model as we have not yet conducted a cost-effectiveness analysis or examined the efficiency of the model. A larger study of this model conducted in 27 clinics serving large minority populations across the United States is underway and will answer many of these questions.26
CONCLUSIONS
Despite smaller changes in ambulatory BP readings than office BPs, pharmacist- physician co-management achieved significantly greater reduction in BP and a high rate of BP control compared to patients receiving usual care. Given the strong correlation between ambulatory BP and cardiovascular risks, our study findings suggest that pharmacist-physician co-management of hypertension can reduce these risks in uncontrolled hypertensive patients through sustained 24-hour BP lowering.
Acknowledgments
Funding: This work was funded in part by the National Heart, Lung, and Blood Institute (1 R01 HL069801-01A1).
Footnotes
clinicaltrials.gov identifier: NCT00201045
Dr. Ernst reports having received consultant fees from Takeda Pharmaceuticals. All other authors report no conflicts of interest.
References
- 1.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in the United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Feldman RD, Zou GY, Vandervoort MK, Wong CJ, Nelson SAE, Feagan BG. A simplified approach to the treatment of uncomplicated hypertension. Hypertension. 2009;53(4):646–53. doi: 10.1161/HYPERTENSIONAHA.108.123455. [DOI] [PubMed] [Google Scholar]
- 4.Jones DW, Peterson ED. Improving hypertension control rates: technology, people or systems? JAMA. 2008;299(24):2896–8. doi: 10.1001/jama.299.24.2896. [DOI] [PubMed] [Google Scholar]
- 5.Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299(24):2857–67. doi: 10.1001/jama.299.24.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169(21):1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich) 2008;10(4):260–71. doi: 10.1111/j.1751-7176.2008.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahey T, Schroeder K, Ebrahim S. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2006;(2):CD005182. doi: 10.1002/14651858.CD005182.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Bogden PE, Abbott RD, Williamson P, Onopa JK, Koontz LM. Comparing standard care with a physician and pharmacist team approach for uncontrolled hypertension. J Gen Intern Med. 1998;13(11):740–5. doi: 10.1046/j.1525-1497.1998.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169(19):1748–55. doi: 10.1001/archinternmed.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst ME, Bergus GR. Non-invasive, 24-hour ambulatory blood pressure monitoring: overview of technology and clinical applications. Pharmacotherapy. 2002;22(5):597–612. doi: 10.1592/phco.22.8.597.33212. [DOI] [PubMed] [Google Scholar]
- 12.Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial investigators. JAMA. 1999;282(6):539–46. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 13.Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348(24):2407–15. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 14.Von Muenster SJ, Carter BL, Weber CA, et al. Description of pharmacist interventions during physician-pharmacist co-management of hypertension. Pharm World Sci. 2008;30(1):128–35. doi: 10.1007/s11096-007-9155-6. [DOI] [PubMed] [Google Scholar]
- 15.Weber CA, Leloux MR, Carter BL, Farris KB, Xu Y. Reduction in adverse symptoms as blood pressure becomes controlled. Pharmacotherapy. 2008;28(9):1104–14. doi: 10.1592/phco.28.9.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright JT, Jr, Bakris G, Greene T, et al. African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–89. [Google Scholar]
- 18.Consensus document on non-invasive ambulatory blood pressure monitoring. The Scientific Committee. J Hypertens Suppl. 1990;8(6):S135–40. [PubMed] [Google Scholar]
- 19.Fagard RH, Staessen JA, Thijs L. Optimal definition of daytime and night-time blood pressure. Blood Press Monit. 1997;2(6):315–21. [PubMed] [Google Scholar]
- 20.Pickering TG, White WB American Society of Hypertension Writing Group. ASH Position Paper: Home and ambulatory blood pressure monitoring. When and how to use self (home) and ambulatory blood pressure monitoring. J Clin Hypertens (Greenwich) 2008;10(11):850–5. doi: 10.1111/j.1751-7176.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banegas JR, Segura J, Sobrino J, et al. Effectiveness of blood pressure monitoring outside the medical setting. Hypertension. 2007;49(1):62–8. doi: 10.1161/01.HYP.0000250557.63490.55. [DOI] [PubMed] [Google Scholar]
- 22.Finkielman JD, Schwartz GL, Chapman AB, Boerwinkle E, Turner ST. Lack of agreement between office and ambulatory blood pressure responses to hydrochlorothiazide. Am J Hypertens. 2005;18(3):398–402. doi: 10.1016/j.amjhyper.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Gorostidi M, Sobrino J, Segura J, et al. Ambulatory blood pressure monitoring in hypertensive patients with high cardiovascular risk: a cross-sectional analysis of a 20,000-patient database in Spain. J Hypertens. 2007;25(5):929–33. doi: 10.1097/HJH.0b013e32809874a2. [DOI] [PubMed] [Google Scholar]
- 24.Ernst ME. Nighttime blood pressure is the blood pressure. Pharmacotherapy. 2009;29(1):3–6. doi: 10.1592/phco.29.1.3. [DOI] [PubMed] [Google Scholar]
- 25.Verdecchia P, Angeli F, Sardone M, Borgioni C, Garofoli M, Reboldi G. Is the definition of daytime and nighttime blood pressure prognostically relevant? Blood Press Monit. 2008;13(3):153–5. doi: 10.1097/MBP.0b013e3282fd1709. [DOI] [PubMed] [Google Scholar]
- 26.Carter BL, Clarke WR, Ardery G, et al. A cluster-randomized effectiveness trial of a physician\pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2010 doi: 10.1161/CIRCOUTCOMES.109.908038. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]