Abstract
N-acetylcysteine (NAC) has been widely used in cell culture-based studies for the role of reactive oxygen species generation in apoptosis induction by isothiocyanates (ITCs). Here we have demonstrated, using radiolabeled 14C-phenethyl ITC and 14C-sulforaphane, that NAC pretreatment significantly reduces ITC cellular uptake by conjugating with ITCs in the medium, suggesting that reduced uptake of ITCs, rather than the antioxidant activity of NAC itself, is responsible for the diminished downstream apoptotic effects. The study provides a cautionary note on the assay in studying mechanisms of apoptosis by ITCs and other electrophilic and thiol-reactive compounds.
Keywords: N-acetylcysteine, isothiocyanates, reactive oxygen species, thiol-reactive compounds
Introduction
N-acetylcysteine (NAC) is a precursor of the intracellular antioxidant glutathione (GSH) (1). Both NAC and GSH are capable of neutralizing reactive oxygen species (ROS) and conjugating with electrophiles. NAC has been shown to prevent damage to DNA and proteins caused by mutagens and carcinogens. These activities may contribute to its inhibitory effect against multistage carcinogenesis in rodents (2).
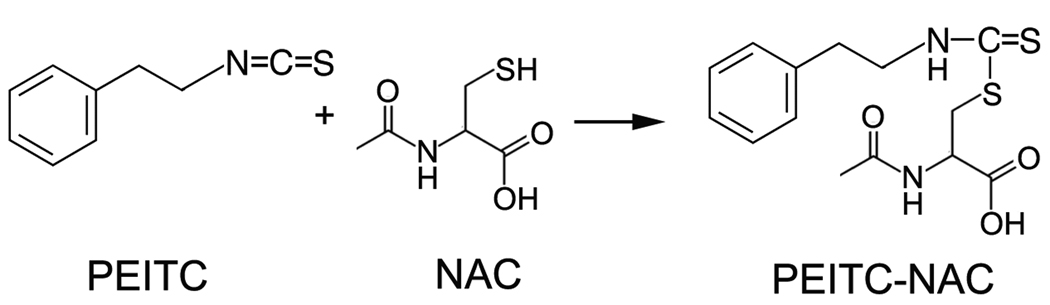
Cruciferous vegetable-derived isothiocyanates (ITCs) are versatile cancer chemopreventive compounds (3). Evidence obtained from both in vitro and in vivo studies supports ITCs anti-proliferative activity through cell cycle arrest and apoptosis induction in cancer cells (3). Although the detailed mechanisms underlying these events are still not fully understood, several cell culture-based studies have indicated that ROS generation, presumably induced by depleting cellular GSH via conjugation, might play an important role in the induction of apoptosis (4–7). A major piece of evidence supporting this claim is that pretreatment of cells with mM levels of NAC prevents GSH depletion, blocks ROS accumulation and oxidative damage in mitochondria, eliminates effects on a variety of signaling pathways, and eventually suppresses apoptosis and cell death induced by ITCs at the µM level (4–7). None of these reports indicate that pretreatment medium containing NAC was replaced before ITC treatment. Due to the fact that NAC is a ROS antagonist, these results have been interpreted to support the claim that ROS may underlie a mechanism by which ITCs induce apoptosis. However, it is also known that electrophilic ITCs can conjugate rapidly with NAC by forming ITC-NAC thiocarbamates (Figure 1) (8). We hypothesize that conjugate formation in the medium could affect the cellular uptake of ITCs, which may result in inhibition of downstream effects.
Figure 1.
Conjugate formation between PEITC and NAC.
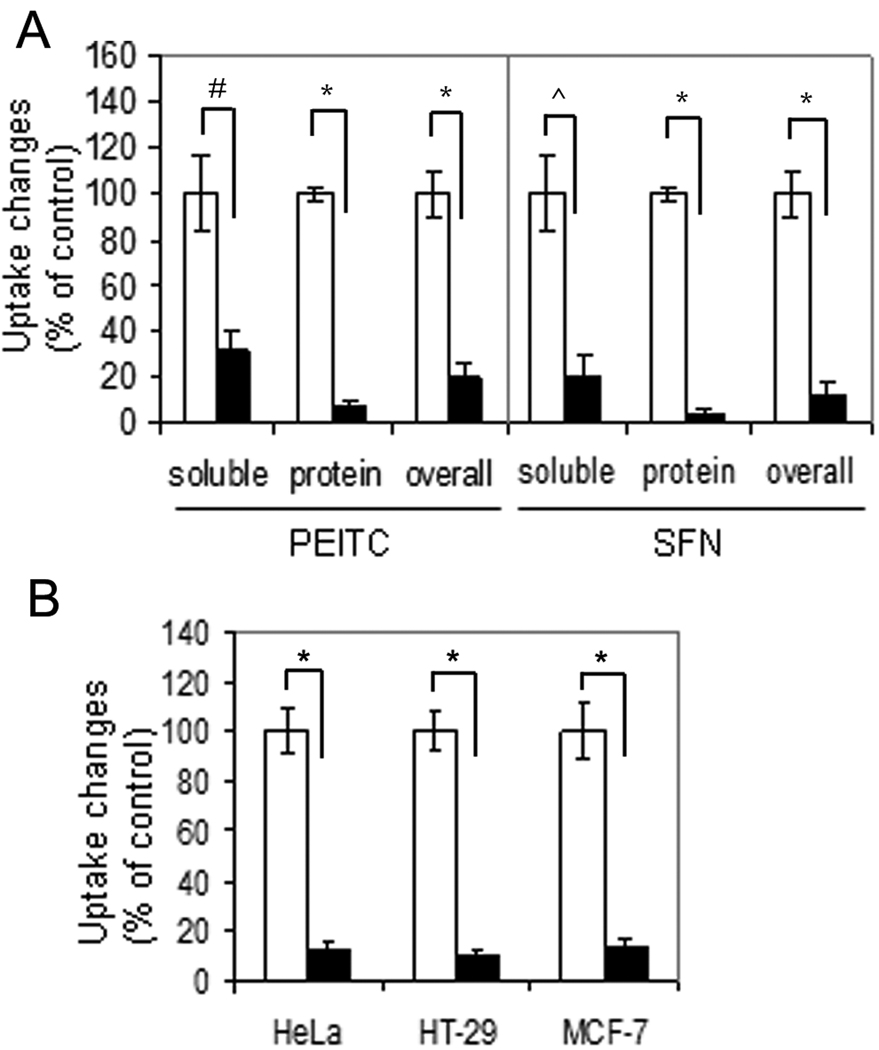
To confirm this hypothesis, we adapted the conditions reported by others (4) in which NAC was used as a ROS antagonist. T72Ras cells were treated with 5 µM 14C-PEITC (9) for 5 h with and without 3 mM NAC pretreatment for 1 h (molar ratio is NAC:PEITC=600:1) (see Supplementary material for detailed methods). After treatment, harvested cells were lysed and separated into two fractions, containing either small molecules or the precipitated proteins. The PEITC-associated radioactivity in these fractions was determined by scintillation counting. The results (Figure 2A left and Table S1) show that pretreatment with NAC reduced the cellular uptake of total PEITC radioactivity by 5-fold. The radioactivity in the supernatant containing small molecules (mainly GSH-PEITC conjugate) was reduced by 3-fold, and the radioactivity associated with protein conjugates by 14-fold. These results indicate that under these conditions NAC pretreatment can dramatically block cellular uptake of PEITC.
Figure 2.
NAC blocks uptake of ITCs in a variety of cells. (A) NAC significantly reduced radioactivity uptake of 14C-PEITC in both fractions and the whole cell lysate of T72Ras cells (left) and 14C-SFN in Hep3B cells (right). Open bar denotes cells without NAC pretreatment; solid bar denotes cells with NAC pretreatment. (B) NAC significantly reduced overall radioactivity uptake of 14C-PEITC in the whole cell lysate of HeLa, HT-29, and MCF-7 cells. #: p=0.0012; ^: p=0.001; *, p<0.001.
To investigate whether uptake of SFN, like PEITC, was also affected by NAC, we incubated Hep3B cells under the conditions reported (5), with 10 µM 14C- SFN (10) for 4 h with and without 5 mM NAC pretreatment (molar ratio is NAC:SFN=500:1). The results (Figure 2A right and Table S2) show that NAC pretreatment inhibited uptake of total radioactivity from SFN by 8-fold — with approximately 5-fold decrease in the small molecule fraction and more than 28-fold in the protein fraction, indicating that NAC pretreatment can greatly suppress the cellular uptake of SFN.
To study whether NAC blocks ITC uptake in other cell types, we expanded our study by including three more cancer cell lines, HeLa, HT-29, and MCF-7. Cells were treated with 5 mM NAC for 1 h followed by 5 µM 14C-PEITC for 5 h (molar ratio is NAC:PEITC=1000:1). The results (Figure 2B) show that the overall uptake of PEITC radioactivity, including both the soluble and protein fractions, was reduced by about 10-fold in the NAC–added medium, compared to the control, indicating that the ability of NAC to reduce cellular uptake of PEITC is not cell-type specific.
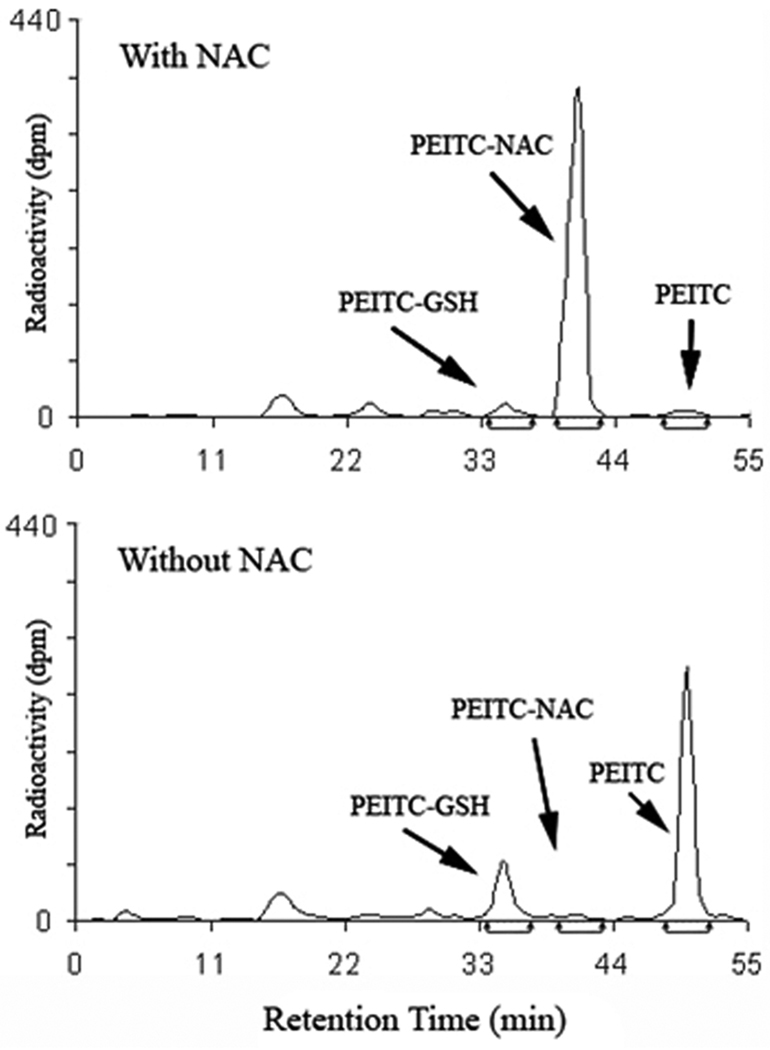
To confirm that NAC reduces PEITC cellular uptake through conjugation in the medium, we analyzed the radioactivity in the culture medium of T72Ras cell treatment by HPLC using a radioflow detector. The results (Figure 3) show that in contrast to PEITC in the medium without NAC, PEITC-NAC is the dominant species in the medium with NAC (>90% of the initial input PEITC), indicating that, as expected, NAC substantially form conjugate with PEITC. Therefore, conjugation with NAC in the medium is likely responsible for the reduced PEITC uptake. It has been reported that PEITC-GSH, once formed inside cells, can be rapidly excreted into the culture medium (11, 12). Our results also show that less PEITC-GSH was detected in the medium with NAC than without NAC, supporting the notion that NAC in the medium suppresses PEITC uptake.
Figure 3.
HPLC-radioflow analysis of PEITC and its metabolites in the culture medium of T72Ras cells treated with 14C-PEITC for 5 h with and without pretreatment with NAC. The identities of the metabolites were determined by comparing the retention times with those of the synthetic UV standards.
ITCs react with deprotonated thiols in both small molecules (such as GSH and NAC) and proteins. The reaction happens very rapidly (8). It is also reversible. Therefore, the NAC- and GSH- conjugates of ITCs, like free ITCs, can also induce apoptosis and cell cycle arrest (13), presumably via deconjugation to free ITCs. However, the large excess of NAC used in previous studies (4–7) would certainly keep ITCs in the conjugated forms in the medium and result in a significant decrease in ITC cellular uptake and ultimately ITC-induced downstream effects. The hypothesis that NAC is reducing ITC uptake is also supported by the inhibition of ITC-induced biological effects being neither target- nor pathway- specific (4–7). Simultaneous suppression of various intermediate signals and endpoint apoptosis may lead to false conclusions on these events, namely that they are ROS-dependent and are related.
As an alternative to the ROS theory, we have proposed that binding to cellular protein targets may underlie ITC-induced apoptosis (12). Recently, we found that benzyl ITC (BITC) and PEITC bind specifically to certain cysteine residues in tubulin inducing tubulin conformation changes, microtubule disruption, tubulin precipitation, and degradation (14–16). ITC binding to tubulin correlates well with the effects of cell cycle arrest and apoptosis by ITCs. Also, these tubulin-related events are independent of ROS generation (15, 16).
The results of this study indicate that NAC should be used with caution in experiments designed to ascertain the role of ROS in apoptosis induced by ITCs and other electrophilic and thiol-reactive compounds. One of the most convincing strategies to gain insights of ROS-mediated mechanisms is through regulating levels of catalase and SOD by either transient or stable transfection.
Supplementary Material
List of Abbreviations
- BITC
benzyl isothiocyanate
- GSH
glutathione
- ITCs
isothiocyanates
- NAC
N-acetylcysteine
- PEITC
phenethyl isothiocyanates
- SFN
sulforaphane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgan LR, Holdiness MR, Gillen LE. N-acetylcysteine: its bioavailability interaction with ifosfamide metabolites. Semin. Oncol. 1983;10:56–61. [PubMed] [Google Scholar]
- 2.Kelloff GJ, Boone CW, Crowell JA, Steele VE, Lubet R, Doody LA. Surrogate endpoint biomarkers for phase II cancer chemoprevention trials. J. Cell Biochem. Suppl. 1994;19:1–9. [PubMed] [Google Scholar]
- 3.WHO. Lyon, France: IARC; IARC Handbook on Cancer Prevention, Vol. 9: Cruciferous Vegetables, Isothiocyanates and indoles. 2004
- 4.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Kim EH, Eom YW, Kim WH, Kwon TK, Lee SJ, Choi KS. Sulforaphane Sensitizes Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)–Resistant Hepatoma Cells to TRAIL-Induced Apoptosis through Reactive Oxygen Species–Mediated Up-regulation of DR5. Cancer Res. 2006;66:1740–1750. doi: 10.1158/0008-5472.CAN-05-1568. [DOI] [PubMed] [Google Scholar]
- 6.Shankar S, Ganapathy S, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin. Cancer Res. 2008;14:6855–6866. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]
- 7.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J. Biol. Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 8.Podhradsky D, Drobnica L, Kristian P. Reaction of Cysteine, its derivatives, glutathione, coenzyme A, and dihydrolipoic acid with isothiocyanates. Experientia. 1979;35:154–155. doi: 10.1007/BF01920581. [DOI] [PubMed] [Google Scholar]
- 9.Conaway CC, Jiao D, Kohri T, Liebes L, Chung FL. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in F344 rats. Drug Metab. Dispos. 1999;27:13–20. [PubMed] [Google Scholar]
- 10.D'Souza CA, Amin S, Desai D. A facile and efficient synthesis of 14C-labelled sulforaphane. J. Labelled Comp. and Radiopharma. 2003;46:851–859. [Google Scholar]
- 11.Zhang YS, Talalay P. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Res. 1998;58:4632–4639. [PubMed] [Google Scholar]
- 12.Mi L, Wang X, Govind S, Hood BL, Veenstra T, Conrads TP, Saha DT, Goldman R, Chung FL. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67:6409–6416. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- 13.Yang YM, Jhanwar-Uniyal M, Schwartz J, Conaway CC, Halicka HD, Traganos F, Chung FL. N-acetylcysteine conjugate of phenethyl isothiocyanate enhances apoptosis in growth-stimulated human lung cells. Cancer Res. 2005;65:8538–8547. doi: 10.1158/0008-5472.CAN-05-0236. [DOI] [PubMed] [Google Scholar]
- 14.Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, Conrads TP, Veenstra TD, Chung FL. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J. Biol. Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mi L, Gan N, Cheema A, Dakshanamurthy S, Wang X, Yang DC, Chung FL. Cancer preventive isothiocyanates induce selective degradation of cellular {alpha}- and {beta}-tubulins by proteasomes. J. Biol. Chem. 2009;284:17039–17051. doi: 10.1074/jbc.M901789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mi L, Gan N, Chung FL. Aggresome-like structure induced by isothiocyanates is novel proteasome-dependent degradation machinery. Biochem. Biophys. Res. Commun. 2009;388:456–462. doi: 10.1016/j.bbrc.2009.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.