Abstract
Ascidians (tunicates; sea squirts) are sources of diverse, bioactive natural products, one of which is an approved drug and many of which are potent drug leads. It has been shown that symbiotic bacteria living with ascidians produce some of the bioactive compounds isolated from whole animals, and indirect evidence strongly implicates symbiotic bacteria in the synthesis of many others. However, for the majority the producing organism has not been identified. In cases where a symbiotic origin has been definitively assigned, the resulting data lead to improved paths to drug discovery and development from marine animals. This review traces evidence for symbiotic production where such evidence exists and describes the strengths and limitations of that evidence.
Introduction
Approximately 1,000 marine natural products have been reported from ascidians. Like sponges, ascidians are soft-bodied, benthic invertebrates that are likely to require chemical defenses for survival in high-predation environments.[1] This rationale has led to more than 35 years of investigation into ascidians as sources bioactive natural products,[2] resulting in the identification of many potent drug leads and even a clinically approved agent (Yondelis; Et-743).[3,4]
Although ascidians (Phylum Chordata) are not closely related to sponges (Phylum Porifera), they live a similar sedentary, filter-feeding lifestyle. As is the case with sponges, many metabolites first isolated from ascidians resemble or are identical to compounds from bacteria (Table 1). For this reason, as with sponges, it has been hypothesized that symbiotic bacteria are the true sources of many “ascidian” metabolites.[5] In addition, there are several ascidian compounds that are apparently found only in marine animals. The presence of these secondary metabolites across distantly related animal taxa is sometimes used as an argument for a potential symbiotic source.
Table.
| Ascidian Compound | Closest Bacterial Homolog | Compound Identity Level | Experimental Evidence |
|---|---|---|---|
| 1. Polyketides | |||
| A. Lipid-like | |||
| Sagittamides | Aflastatins, zwittermicin | Distant | None |
| B. Iejimalide group | |||
| Lobatamides | Oximidines I, II, III | Very similar | None |
| Patellazoles | Archazolid | Very similar | None |
| Palmerolide | Archazolid | Somewhat similar | trans-AT sequences identified by PCR |
| Haterumalides / biselides | Biselides | Very similar to identical | None |
| Iejimalides | Iejimalide A | Identical | None |
| Bistramides | Pederin / onnamides | Similar motifs | Compounds localized to Prochloron by cell separation |
| C. Odd-chain hydrocarbons | |||
| Lissoclinolide | Tetrenolin | Identical | None |
| Other C11 hydrocarbons | Nakienones | Very similar | Compounds localized to Prochloron by cell separation |
| D. Enediynes | |||
| Namenamicin / shishijimicins | Calicheamicin | Very similar | None |
| E. Rearranged polyketides | |||
| Enterocins | Enterocins | Identical | None |
| 2. Peptides | |||
| A. Nonribosomal | |||
| Didemnins | Statines and numerous nonribosomal depsipeptides | Similar motifs | None |
| Ecteinascidins | Saframycins and safracins | Very similar | Protein / gene isolation, 16S analysis |
| B. Uncertain | |||
| Diazonamide | Nonribosomal peptides, microcins, thiopeptides | Unusual, some common motifs | None |
| Vitilevuamide | Lantibiotics, microcins, nonribosomal peptides, thiopeptides | Unusual, some common motifs | None |
| B. Ribosomal | |||
| Westiellamide | Cycloxazoline | Identical | None |
| Other cyanobactins (patellamides, trunkamide, etc.) | Tenuecyclamides, prenylagaramides, others | Very similar | Whole genome sequencing and heterologous pathway expression |
| Virenamides | Aeruginosamide | Very similar | None |
| Cyclic (X-Pro)2 | Cyclic (X-Pro)2 | Identical | None |
| 3. Alkaloids | |||
| A. Indole alkaloids | |||
| Staurosporines | Staurosporines | Very similar to identical | None; for granulatimide, microscopy showed concentrated in animal cells |
| Pibocins | Brominated festuclavine (fungal derivative) | Almost identical to fungal metabolites | None |
| Fascaplysin | None | None | Also found in sponges |
| Pyridoacridines | None | None | Also found in sponges; localized to sponge cells |
| B. Pyrroles | |||
| Tambjamines | Tambjamines | Very similar to identical | Cultivated bacteria from ascidian |
| 4. Terpenes | |||
| Ritterazines | None | None | None |
| 5. Miscellaneous | |||
| Tubericidins | Tubericidins | Identical | None |
Recent advances add considerably to the evidence in favor of a bacterial origin for many ascidian compounds (Figure 1). A particularly well-studied interaction is the symbiosis between didemnid ascidians and Prochloron spp. photosynthetic cyanobacteria that inhabit their cellulose tunics.[6] Prochloron is sometimes required for the survival of the animals providing fixed carbon to the host and recycling nitrogen. Many other symbiotic interactions in ascidians exist but are not as well documented. Experimental evidence has shown that Prochloron produces some of the metabolites originally isolated from ascidians.[7,8••,9-10] In addition, other recent studies directly or indirectly implicate bacteria in the production of ascidian compounds.[11,12••]
Figure 1.
A caveat: This field is in its infancy, and much more research is required to fully determine the origin of the diverse natural products isolated from ascidians. Although this review focuses on known or possible symbiotic sources, other possibilities include synthesis by the host animal, by eukaryotic microbes, or by multiple organisms, among others.
1. Polyketides
Sagittamide A was isolated from didemnid ascidians.[13] It was proposed that sagittamide A (1) could arise from polyketide extension with hydroxymalonyl CoA, such as is found in the bacterial zwittermicin (2) and aflastatins (Figure S1). If so, this could indicate a possible bacterial origin, but as the authors point out there are many other possibilities.
Compelling, but indirect, evidence exists that a series of ascidian polyketides exemplified by iejimalide (eg 3) may be produced by bacteria (Figures S2 and S3).[14] All of the compounds in this series except for bistramides [15] are strikingly similar in their probable biosynthesis, and they can be structurally aligned. The molecules have very similar putative starter units, followed by 0-2 amino acids. From there, a putative polyketide chain proceeds for 16-26 carbons, including a macrocycle of 13-24 atoms. Bistramides distantly resemble this family of ascidian polyketides, except that the amino acids are at the center of the compounds rather than at the starter side.
These compounds are similar to bacterial polyketides such as rhizoxins (eg 9) and archazolids (eg 10).[16,17•] Exact analogs of biselides and haterumalides (eg 11) have been isolated from soil and epiphytic bacteria, and haterumalides have also been isolated from sponges.[18•] Lobatamides are very similar to salicylhalamides from sponges as well as to oximidines I, II and III (12) from bacteria. Iejimalide A was isolated from a marine cyanobacterial mat.[19•] Bistramides more closely resemble sponge polyketides that have been traced to symbiotic proteobacteria, such as mycalamide A (13).[20••]
The source of the iejimalide relative, palmerolide A in Synoicum adareanum from Antarctica was studied using a metagenomic approach.[21•] Fragments of polyketide synthase genes from the trans-AT group were identified by PCR, and proteobacteria were identified using a 16S analysis. Further data are required to tie the reported sequences to palmerolide. Bistramides were identified in tropical ascidians, Lissoclinum bistratum, that harbor Prochloron cyanobacterial symbionts. Two localization studies have examined the origin of bistramides, both indicating that the bistramides were more concentrated in Prochloron than in the ascidian host.[22,23]
A number of C11 polyketides has been reported from several ascidians (Figure S4).[24] All members of the group contain odd-chain, highly oxidized hydrocarbons with a central methyl sidechain. The canonical represenative of this class is lissoclinolide (14), which is identical to the actinomycete compound tetrenolin. A series of related derivatives (eg 15-18) has been isolated from sponges, ascidians, and a cyanobacterial mat.[25-28] The ascidian Diplosoma virens was separated from symbiotic Prochloron cyanobacteria, and C11 compounds could be detected in the resulting bacterial acetone extracts, indicating a potential Prochloron origin.[27•]
Intriguingly, ascidian enediynes namenamicin (19) and the shishijimicins (20) contain core structures that are nearly identical to that of calicheamicin (eg 21), an actinomycete natural product that is part of the clinically used Mylotarg (Figure S5).[29-31] Cultivation attempts have not met with reported success.[32]
Enterocins (eg 22) have been isolated from ascidians and bacteria. (Figure S6).[33] In the original isolation of enterocin derivatives from Didemnum sp. ascidians, the authors unsuccessfully attempted to cultivate bacterial producers of enterocins, and they also noted the presence of V-shaped, Arthrobacter-like bacteria in fixed tissue samples.[33]
2. Peptides and primarily peptidic compounds
Most of the drug development activity with ascidian compounds has focused on peptides, including both peptides of probable nonribosomal origin and those of probable or demonstrated ribosomal origin.
Ecteinascidin 743 (Et-743; Yondelis; 23), which is an approved anticancer agent in Europe and in advanced trials in the US, is of possible nonribosomal origin. The ecteinascidins were isolated from the ascidian Ecteinasidia turbinata and E. thurstoni (Figure S7).[34] They are related to the safracins and saframycins from bacteria, as well as to renieramycins, jorumycins, and similar compounds from sponges. In bacteria, a nonribosomal peptide synthetase (NRPS) encoding either 3 or 4 modules is solely responsible for synthesis of a core tetrapeptide, and for reductive cyclization of the tetrapeptide to form 3 new heterocycles. The bacterial tetrapeptide is composed of alanine or derivative thereof, glycine, and two units of highly modified tyrosine.[35] Et-743 is apparently composed of cysteine (in place of alanine), glycolate, and two subunits of modified tyrosine. There is a twist: an additional dopamine derivative is added to the N-terminal side of the peptide in Et-743.
In E. turbinata, molecular methods identified the γ-proteobacterium, Candidatus Endoecteinascidia frumentensis, as a potentially vertically transmitted, intracellular bacterium associated with E. turbinata in the Meditteranean.[36••] Later, E. turbinata collected in 3 different locations in the Caribbean was analyzed for the presence of persistent bacteria, which might be associated with compound production. Only E. frumentensis was found in all 3 animals, validating this strain as a persistent symbiont.[37] However, no peer-reviewed data have been presented linking the bacteria to compounds as of this writing (but see [12••]).
The didemnin family of ascidian natural products has been described from several ascidians (Figure S8).[38] Included in this family is aplidine, which was given orphan drug status in Europe for cancer. The didemnins are cyclic depsipeptides of possible nonribosomal origin. In addition, there are 1-2 polyketide-like units, forming isostatine and the statine-like Hip group. Most of the variability in the didemnin family occurs at the side chain, which contains diverse amino acids and acyl moieties. The only exception to this is in the tamandarins, which have a slightly different composition. So far, mixed polyketide-peptide biosynthesis has only been described in bacteria and lower eukaryotes, indicating that the compounds could be derived from symbiotic bacteria. Statines and isostatines are thought to be largely associated with bacteria.[39]
Two peptide families may be either ribosomal or nonribosomal, exemplified by diazonamides (eg 29), from Diazonia sp., and vitilevuamide (30), from P. lithostrotum and Didemnum cuculliferum (Figure S9).[40,41] Both molecules have features that have been previously found only in nonribosomal or only in ribosomal peptides isolated from bacteria.[42,43]
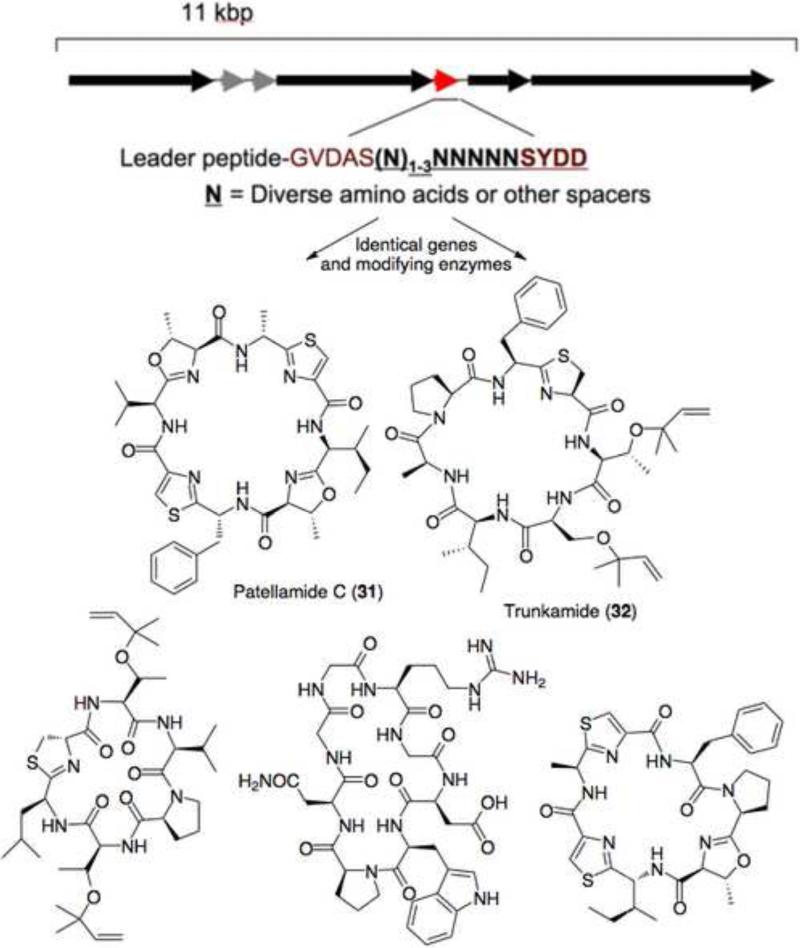
The biggest single class of complex natural products from ascidians is the ribosomal peptide group, with more than 60 representatives (eg, 31-33). The cyanobactin family includes N-C circular peptides that are further modified by heterocyclization or prenylation at cysteine, serine, and threonine, and several other modifications (Figure S10).[7,44,45] Cyanobactins have been isolated from both free-living cyanobacteria and from ascidians. Virenamides (eg 34) are biosynthetically similar to cyanobactins.[46] Westiellamide / cycloxazoline has been isolated both from cyanobacteria and from ascidians, while virenamide-like molecules (eg 35) have also been isolated from cyanobacteria.[47]
Genetic methods were used to definitively show that uncultivated symbiotic cyanobacteria, Prochloron spp., synthesize cyanobactins in ascidians. Genes were identified from coral reef animals by metagenome sequencing or by random library generation and functionally expressed in Escherichia coli.[9,10] Cyanobactin biosynthetic genes were localized to the Prochloron genome in whole-genome sequencing studies, showing that the compounds originate in Prochloron.[9] No such genes were found in the animal host or in other bacteria in the assembly. Representatives of all compound classes have been identified in ascidian symbionts using genetic methods, accounting for about 6% of known ascidian natural products.[7,8••] Taken together, the evidence demonstrates that symbiotic cyanobacteria synthesize this group of ascidian natural products.
The cyanobactins story illustrates the biotechnological application of symbiosis studies (Figure 2). The rare but preclinical lead cyanobactin trunkamide was produced in E. coli culture.[7] Many other cyanobactins and cyclic peptides, both natural and unnatural, have been produced in vivo in E. coli or using recombinant pure enzymes for in vitro synthesis.[8,48,49••] The cyanobactin biosynthetic pathway is the most substrate-flexible natural product assembly line so far characterized, and studies of symbiosis were the key to rapidly understanding this flexibility. Briefly, identical enzymes tolerate extremely diverse substrates, catalyzing N-C circularization, heterocyclization, and prenylation through simple genetic engineering of short 18-24 nt DNA cassettes.
Figure 2.
Other simple cyclic peptides, cyclo[Leu-Pro]2, cyclo[Phe-Pro]2, and cyclo[Val-Pro]2, have been isolated from ascidians, bacteria, and other organisms.[50] Dragmacidin piperazine derivatives have been isolated both from sponges and from Didemnum candidum ascidians.[51]
3. Alkaloids
Tambjamines and staurosporines are the only ascidian alkaloids for which indirect experimental evidence indicates a possible bacterial source (Figure S11). Tambjamines (eg 36) are pyrrole alkaloids that have been isolated from bryozoans and ascidians as well as their nudibranch predators, where they are likely used as defense against predation by fish.[1,52] In addition, tambjamines have been isolated from actinomycete bacteria,[53] and they are closely related to the prodigiosin-type alkaloids that are common in diverse bacterial strains.[54] A tambjamine derivative (YP1; 37) was isolated from the marine bacterium, Pseudoalteromonas tunicata, which in turn was isolated from the surface of an ascidian, but one that does not contain tambjamines.[11]
Staurosporine (38) and related metabolites such as granulatimides (eg 39) are isolated from diverse taxonomic groups (Figure S11).[55,56] These compounds are extremely common in bacteria, and they have been isolated from a number of ascidians, especially Eudistoma toalensis and its predatory flatworm, where they serve as potential feeding deterrents.[57] Although the animal compounds are of presumed bacterial origin, as of this writing there is no direct experimental evidence supporting this view. A potential contrary example is found in the granulatimides, which were identified in ascidian cells of Didemnum sp.[58] Cellular localization does not necessarily indicate the metabolic origin of metabolites, but these data could be interpreted as possibly indicating an animal source for the compounds.
The pyridoacridines (eg 40) are found in ascidians and sponges, with many identical compounds found in both taxa, but related compounds have not been found outside of these groups or in bacteria (Figure S12).[59,60] There is some indirect evidence based upon sulfur content that ascidian pyridoacridines may be localized in ascidian cells, and bacterial symbionts of these ascidians have also been described.[61] Sponge-derived pyridoacridines were localized to sponge cells, which was interpreted cautiously to indicate a potential eukaryotic origin for the compounds.[62]
The ascidian pibocins (eg 41) are closely related to fungal festuclavines (eg 42) (Figure S12).[63] Fascaplysin (43) has been isolated from ascidians and sponges.[64]
4. Terpenoids
The only ascidian isoprenoids with data supporting possible symbiotic origin are the ritterazines (eg 44), dimeric steroidal alkaloids isolated from Ritterella tokioka (Figure S13).[65] Because they bear a striking resemblance to the tubeworm-derived cephalostatins, it has been proposed that the compounds might be derived from a microbial source. A challenge to this idea is that cholestane sterols are not known to be made by bacteria
5. Miscellaneous metabolites
There are many other ascidian metabolites that cannot be discussed here. One group includes tubercidin analogs, which have been isolated from Didemnum voeltzkowi.[66] Identical compounds have been isolated from algae, while similar compounds are known from diverse sources including actinomycetes.
Conclusion
Although as apparent above the data are often limited and of varying quality, we believe that current data support the hypothesis that most of the potently bioactive ascidian natural products are made by symbiotic bacteria. Further understanding of the general rules behind these symbioses will thus be greatly useful to biotechnology and drug development, as exemplified by early work on the cyanobactin biosynthetic pathway.
Supplementary Material
Acknowledgment
Our work on ascidians is funded by NIH (GM0171425).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paul VJ, Lindquist N, Fenical W. Chemical defenses of the tropical ascidian Atapozoa sp. and its nudibranch predators Nembrotha spp. Mar Ecol Prog Ser. 1990;59:109–118. [Google Scholar]
- 2.Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2010;27:165–237. doi: 10.1039/b906091j. [DOI] [PubMed] [Google Scholar]
- 3.Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci. 31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat Rev Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 5.Ireland C, Scheuer PJ. Ulicyclamide and ulithiacyclamide, 2 new small peptides From a marine tunicate. J Am Chem Soc. 1980;102:5688–5691. [Google Scholar]
- 6.Gault PM, Marler HJ. Handbook on cyanobacteria : biochemistry, biotechnology and applications. Nova Science Publishers; New York: 2009. [Google Scholar]
- 7.Donia MS, Ravel J, Schmidt EW. A global assembly line for cyanobactins. Nat Chem Biol. 2008;4:341–343. doi: 10.1038/nchembio.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••8.Donia MS, Hathaway BJ, Sudek S, Haygood MG, Rosovitz MJ, Ravel J, Schmidt EW. Natural combinatorial peptide libraries in cyanobacterial symbionts of marine ascidians. Nat Chem Biol. 2006;2:729–735. doi: 10.1038/nchembio829. [The diverse cyanobactin pathways from ascidians are shown to be derived from symbiotic cyanobacteria] [DOI] [PubMed] [Google Scholar]
- 9.Schmidt EW, Nelson JT, Rasko DA, Sudek S, Eisen JA, Haygood MG, Ravel J. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc Natl Acad Sci U S A. 2005;102:7315–7320. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long PF, Dunlap WC, Battershill CN, Jaspars M. Shotgun cloning and heterologous expression of the patellamide gene cluster as a strategy to achieving sustained metabolite production. Chembiochem. 2005;6:1760–1765. doi: 10.1002/cbic.200500210. [DOI] [PubMed] [Google Scholar]
- 11.Burke C, Thomas T, Egan S, Kjelleberg S. The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Environ Microbiol. 2007;9:814–818. doi: 10.1111/j.1462-2920.2006.01177.x. [DOI] [PubMed] [Google Scholar]
- ••12.Rath CM, Yu F, Janto B, Inai M, Williams R, Ehrlich G, Hakansson K, Sherman DH. LC-FTICR-MS for targeted proteomics: characterization of tetrahydroquinoline natural product biosynthetic proteins of symbiotic bacteria from macroorganismal assemblies. American Society for Mass Spectrometry Annual Meeting.; Salt Lake City. 2010; [In this poster, the Sherman group describes identifying the Et-743 biosynthetic gene cluster in symbiotic bacteria] [Google Scholar]
- 13.Schuetz A, Junker J, Leonov A, Lange OF, Molinski TF, Griesinger C. Stereochemistry of sagittamide A from residual dipolar coupling enhanced NMR. J Am Chem Soc. 2007;129:15114–15115. doi: 10.1021/ja075876l. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi J, Cheng J, Ohta T, Nakamura H, Nozoe S, Hirata Y, Ohizumi Y, Sasaki T. Iejimalides A and B, novel 24-membered macrolides with potent antileukemic activity from the Okinawan tunicate Eudistoma cf. rigida. J Org Chem. 1988;53:6147–6150. [Google Scholar]
- 15.Biard J-F, Roussakis C, Kornprobst J-M, Gouiffes-Barbin D, Verbist J-F, Cotelle P, Foster MP, Ireland CM, Debitus C. Bistramides A, B, C, D, and K: a new class of bioactive cyclic polyethers from Lissoclinum bistratum. J Nat Prod. 1994;57:1336–1345. doi: 10.1021/np50112a002. [DOI] [PubMed] [Google Scholar]
- 16.Sasse F, Steinmetz H, Hofle G, Reichenbach H. Archazolids, new cytotoxic macrolactones from Archangium gephyra (Myxobacteria). Production, isolation, physico-chemical and biological properties. J Antibiot. 2003;56:520–525. doi: 10.7164/antibiotics.56.520. [DOI] [PubMed] [Google Scholar]
- •17.Lackner G, Moebius N, Scherlach K, Partida-Martinez LP, Winkler R, Schmitt I, Hertweck C. Global distribution and evolution of a toxinogenic Burkholderia-Rhizopus symbiosis. Appl Environ Microbiol. 2009;75:2982–2986. doi: 10.1128/AEM.01765-08. [A prescient look at symbiosis globally, albeit between bacteria and fungi. The genes for these molecules are probably similar to those for some ascidian polyketides] [DOI] [PMC free article] [PubMed] [Google Scholar]
- •18.Kigoshi H, Hayakawa I. Marine cytotoxic macrolides haterumalides and biselides, and related natural products. Chem Rec. 2007;7:254–264. doi: 10.1002/tcr.20119. [An excellent and underappreciated review of compounds that are found in bacteria, sponges, and ascidians] [DOI] [PubMed] [Google Scholar]
- •19.Simmons TL, Coates RC, Clark BR, Engene N, Gonzalez D, Esquenazi E, Dorrestein PC, Gerwick WH. Biosynthetic origin of natural products isolated from marine microorganism-invertebrate assemblages. Proc Natl Acad Sci U S A. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [Describes the identification of iejimalide from a cyanobacterial mat, and also discusses trends in understanding symbiotic and other origins of natural products] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••20.Nguyen T, Ishida K, Jenke-Kodama H, Dittmann E, Gurgui C, Hochmuth T, Taudien S, Platzer M, Hertweck C, Piel J. Exploiting the mosaic structure of trans-acyltransferase polyketide synthases for natural product discovery and pathway dissection. Nat Biotechnol. 2008;26:225–233. doi: 10.1038/nbt1379. [The first application of an innovative phylogenetic method to pathway discovery, which has been especially influential in the study of metabolites from symbiotic bacteria] [DOI] [PubMed] [Google Scholar]
- •21.Riesenfeld CS, Murray AE, Baker BJ. Characterization of the microbial community and polyketide biosynthetic potential in the palmerolide-producing tunicate Synoicum adareanum. J Nat Prod. 2008 doi: 10.1021/np800287n. [The first comparative analysis of 16S and polyketide gene sequences in an ascidian] [DOI] [PubMed] [Google Scholar]
- 22.Biard JF, Grivois C, Verbist JF, Debitus C, Carre JB. Origin of bistramide A identified in Lissoclinum bistratum (Urochordata): possible involvement of symbiotic Prochlorophyta. J Mar Biol Assoc UK. 1990;70:741–746. [Google Scholar]
- 23.Degnan BM, Hawkins CJ, Lavin MF, McCaffrey EJ, Parry DL, Watters DJ. Novel cytotoxic compounds from the ascidian Lissoclinum bistratum. J Med Chem. 1989;32:1354–1359. doi: 10.1021/jm00126a035. [DOI] [PubMed] [Google Scholar]
- 24.Richardson AD, Ireland CM. A profile of the in vitro antitumor activity of lissoclinolide. Toxicol Appl Pharmacol. 2004;195:55–61. doi: 10.1016/j.taap.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Nagle DG, Gerwick WH. Nakienones A-C and nakitriol, new cytotoxic cyclic C11 metabolites from an Okinawan cyanobacterial (Synechocystis sp.) overgrowth of coral. Tetrahedron Lett. 1995;36:849–852. [Google Scholar]
- 26.Ogi T, Taira J, Margiastuti P, Ueda K. Cytotoxic metabolites from the Okinawan ascidian Diplosoma virens. Molecules. 2008;13:595–602. doi: 10.3390/molecules13030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •27.Ogi T, Margiastuti P, Teruya T, Taira J, Suenaga K, Ueda K. Isolation of C11 cyclopentenones from two didemnid species, Lissoclinum sp. and Diplosomasp. Mar Drugs. 2009;7:816–832. doi: 10.3390/md7040816. [Identical molecules, or nearly identical molecules, were found in cyanobacteria and ascidians. Family relationships are coherently described] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wratten SJ, Faulkner DJ. Antimicrobial metabolites from the marine sponge Ulosa sp. Tetrahedron Lett. 1978:961–964. [Google Scholar]
- 29.McDonald LA, Capson TL, Krishnamurthy G, Ding W-D, Ellestad GA, Bernan VS, Maiese WM, Lassota P, Discafani C. Namenamicin, a new enediyne antitumor antibiotic from the marine ascidian Polysyncraton lithostrotum. J Am Chem Soc. 1996;118:10898–10899. et a. [Google Scholar]
- 30.Oku N, Matsunaga S, Fusetani N. Shishijimicins A-C, novel enediyne antitumor antibiotics from the ascidian Didemnum proliferum. J Am Chem Soc. 2003;125:2044–2045. doi: 10.1021/ja0296780. [DOI] [PubMed] [Google Scholar]
- 31.Zein N, Sinha AM, McGahren WJ, Ellestad GA. Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science. 1988;240:1198–1201. doi: 10.1126/science.3240341. [DOI] [PubMed] [Google Scholar]
- 32.He H, Ding WD, Bernan VS, Richardson AD, Ireland CM, Greenstein M, Ellestad GA, Carter GT. Lomaiviticins A and B, potent antitumor antibiotics from Micromonospora lomaivitiensis. J Am Chem Soc. 2001;123:5362–5363. doi: 10.1021/ja010129o. [DOI] [PubMed] [Google Scholar]
- 33.Kang H, Jensen PR, Fenical W. Isolation of microbial antibiotics from a marine ascidian of the genus Didemnum. J Org Chem. 1996;61:1543–1546. [Google Scholar]
- 34.Sakai R, Rinehart KL, Guan Y, Wang AH. Additional antitumor ecteinascidins from a Caribbean tunicate: crystal structures and activities in vivo. Proc Natl Acad Sci U S A. 1992;89:11456–11460. doi: 10.1073/pnas.89.23.11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••35.Koketsu K, Watanabe K, Suda H, Oguri H, Oikawa H. Reconstruction of the saframycin core scaffold defines dual Pictet-Spengler mechanisms. Nat Chem Biol. 2010;6:408–410. doi: 10.1038/nchembio.365. [The first mechanistic look at a very strange new mechanism to isoquinolines. The uniqueness of this mechanism will allow the identification of Et-743 genes from ascidians to be much more readily established] [DOI] [PubMed] [Google Scholar]
- 36.Moss C, Green DH, Perez B, Velasco A, Henriquez R, McKenzie JD. Intracellular bacteria associated with the ascidian Ecteinascidia turbinata: phylogenetic and in situ hybridisation analysis. Mar Biol. 2003;143:99–110. [Google Scholar]
- 37.Perez-Matos AE, Rosado W, Govind NS. Bacterial diversity associated with the Caribbean tunicate Ecteinascidia turbinata. Antonie Van Leeuwenhoek. 2007;92:155–164. doi: 10.1007/s10482-007-9143-9. [DOI] [PubMed] [Google Scholar]
- 38.Rinehart KL, Jr., Gloer JB, Hughes RG, Jr., Renis HE, McGovren JP, Swynenberg EB, Stringfellow DA, Kuentzel SL, Li LH. Didemnins: antiviral and antitumor depsipeptides from a caribbean tunicate. Science. 1981;212:933–935. doi: 10.1126/science.7233187. [DOI] [PubMed] [Google Scholar]
- 39.Preciado A, Williams PG. A simple microscale method for determining the relative stereochemistry of statine units. J Org Chem. 2008;73:9228–9234. doi: 10.1021/jo8012429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicolaou KC, Chen DYK, Huang X, Ling T, Bella M, Snyder SA. Chemistry and biology of diazonamide A: First total synthesis and confirmation of the true structure. J Am Chem Soc. 2004;126:12888–12896. doi: 10.1021/ja040092i. [DOI] [PubMed] [Google Scholar]
- 41.Edler MC, Fernandez AM, Lassota P, Ireland CM, Barrows LR. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the vinca alkaloids, and dolastatin 10. Biochem Pharmacol. 2002;63:707–715. doi: 10.1016/s0006-2952(01)00898-x. [DOI] [PubMed] [Google Scholar]
- 42.McIntosh JA, Donia MS, Schmidt EW. Ribosomal peptide natural products: bridging the ribosomal and nonribosomal worlds. Nat Prod Rep. 2009;26:537–559. doi: 10.1039/b714132g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh CT, Acker MG, Bowers AA. Thiazolyl peptide antibiotic biosynthesis: a cascade of posttranslational modifications on ribosomal nascent proteins. J Biol Chem. 2010 doi: 10.1074/jbc.R110.135970. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt EW, Donia MS. Mander L, Liu H-W, editors. Cyanobactins, ubuquitous cyanobacterial metabolites. Comprehensive Natural Products II. 2010.
- 45.Sivonen K, Leikoski N, Fewer DP, Jokela J. Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria. Appl Microbiol Biotechnol. 86:1213–1225. doi: 10.1007/s00253-010-2482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carroll AR, Feng Y, Bowden BF, Coll JC. Studies of Australian ascidians. 5. Virenamides A-C, new cytotoxic linear peptides from the colonial didemnid ascidian Diplosoma virens. J Org Chem. 1996;61:4059–4061. doi: 10.1021/jo951379o. [DOI] [PubMed] [Google Scholar]
- 47.Lawton LA, Morris LA, Jaspars M. A bioactive modified peptide, aeruginosamide, isolated from the cyanobacterium Microcystis aeruginosa. J Org Chem. 1999;64:5329–5332. doi: 10.1021/jo990247i. [DOI] [PubMed] [Google Scholar]
- ••48.McIntosh JA, Donia MS, Schmidt EW. Insights into heterocyclization from two highly similar enzymes. J Am Chem Soc. 2010;132:4089–4091. doi: 10.1021/ja9107116. [The first example of using symbiosis to inform an enzymological experiment. The enzymes in question are identical in their catalytic domains, differing in their substrate-binding domains, yet they synthesize different products using identical substrates] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J, McIntosh J, Hathaway BJ, Schmidt EW. Using marine natural products to discover a protease that catalyzes peptide macrocyclization of diverse substrates. J Am Chem Soc. 2009;131:2122–2124. doi: 10.1021/ja8092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rungprom W, Siwu ERO, Lambert LK, Dechsakulwatana C, Barden MC, Kokpol U, Blanchfield JT, Kita M, Garson MJ. Cyclic tetrapeptides from marine bacteria associated with the seaweed Diginea sp. and the sponge Halisarca ectofibrosa. Tetrahedron. 2008;64:3147–3152. [Google Scholar]
- 51.Capon RJ, Rooney F, Murray LM, Collins E, Sim ATR, Rostas JAP, Butler MS, Carroll AR. Dragmacidins: new protein phosphatase inhibitors from a Southern Australian deep-water marine sponge, Spongosorites sp. J Nat Prod. 1998;61:660–662. doi: 10.1021/np970483t. [DOI] [PubMed] [Google Scholar]
- 52.Lindquist N, Fenical W. New tambjamine class alkaloids from the marine ascidian Atapozoa sp. and its nudibranch predators. Origin of the tambjamines in Atapozoa. Experientia. 1991;47:504–506. [Google Scholar]
- 53.Nakajima S, Kojiri K, Suda H. A new antitumor substance, BE-18591, produced by a streptomycete. II. Structure determination. J Antibiot. 1993;46:1894–1896. doi: 10.7164/antibiotics.46.1894. [DOI] [PubMed] [Google Scholar]
- 54.Williamson NR, Fineran PC, Leeper FJ, Salmond GP. The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol. 2006;4:887–899. doi: 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]
- 55.Horton PA, Longley RE, McConnell OJ, Ballas LM. Staurosporine aglycone (K252-c) and arcyriaflavin A from the marine ascidian, Eudistoma sp. Experientia. 1994;50:843–845. doi: 10.1007/BF01956468. [DOI] [PubMed] [Google Scholar]
- 56.Berlinck RGS, Britton R, Piers E, Lim L, Roberge M, Moreira dRR, Andersen RJ. Granulatimide and isogranulatimide, aromatic alkaloids with G2 checkpoint inhibition activity isolated from the Brazilian ascidian Didemnum granulatum: Structure elucidation and synthesis. J Org Chem. 1998;63:9850–9856. [Google Scholar]
- 57.Schupp P, Eder C, Proksch P, Wray VV, Schneider B, Herderich M, Paul VV. Staurosporine derivatives from the ascidian Eudistoma toealensis and its predatory flatworm Pseudoceros sp. J Nat Prod. 1999;62:959–962. doi: 10.1021/np980527d. [DOI] [PubMed] [Google Scholar]
- 58.Seleghim MHR, Lira SP, Campana PT, Berlinck RGS, Custodio MR. Localization of granulatimide alkaloids in the tissues of the ascidian Didemnum granulatum. Mar Biol. 2007;150:967–975. [Google Scholar]
- 59.Marshall KM, Barrows LR. Biological activities of pyridoacridines. Nat Prod Rep. 2004;21:731–751. doi: 10.1039/b401662a. [DOI] [PubMed] [Google Scholar]
- 60.Molinski TF. Marine pyridoacridine alkaloids: structure, synthesis, and biological chemistry. Chem Rev. 1993;93:1825–1838. [Google Scholar]
- 61.Turon X, López-Legentil S, Banaigs B. Cell types, microsymbionts, and pyridoacridine distribution in the tunic of three color morphs of the genus Cystodytes (Ascidiacea, Polycitoridae). Invert Biol. 2005;124:355–369. [Google Scholar]
- 62.Salomon CE, Deerinck T, Ellisman MH, Faulkner DJ. The cellular localization of dercitamide in the Palauan sponge Oceanapia sagittaria. Mar Biol. 2001;139:313–319. [Google Scholar]
- 63.Makarieva TN, Ilyin SG, Stonik VA, Lyssenko KA, Denisenko VA. Pibocin, the first ergoline marine alkaloid from the Far-Eastern ascidian Eudistoma sp. Tetrahedron Lett. 1999;40:1591–1594. [Google Scholar]
- 64.Foderaro TA, Barrows LR, Lassota P, Ireland CM. Bengacarboline, a new beta-carboline from a marine ascidian Didemnum sp. J Org Chem. 1997;62:6064–6065. [Google Scholar]
- 65.Moser BR. Review of cytotoxic cephalostatins and ritterazines: Isolation and synthesis. J Nat Prod. 2008;71:487–491. doi: 10.1021/np070536z. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell SS, Pomerantz SC, Concepcion GP, Ireland CM. Tubercidin analogs from the ascidian Didemnum voeltzkowi. J Nat Prod. 1996;59:1000–1001. doi: 10.1021/np960457f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.