Abstract
Insulin-like growth factor binding protein (IGFBP)-3 exerts either proapoptotic or growth stimulatory effects depending upon the cellular context. IGFBP-3 is overexpressed frequently in esophageal cancer. Yet, the role of IGFBP-3 in esophageal tumor biology remains elusive. To delineate the functional consequences of IGFBP-3 overexpression, we stably transduced Ha-RasV12-transformed human esophageal cells with either wild-type or mutant IGFBP-3, the latter incapable of binding Insulin-like growth factor (IGFs) as a result of substitution of amino-terminal Ile56, Leu80, and Leu81 residues with Glycine residues. Wild-type, but not mutant, IGFBP-3 prevented IGF-I from activating the IGF-1 receptor and AKT, and suppressed anchorage-independent cell growth. When xenografted in nude mice, in vivo bioluminescence imaging demonstrated that wild-type, but not mutant IGFBP-3, abrogated tumor formation by the Ras-transformed cells with concurrent induction of apoptosis, implying a prosurvival effect of IGF in cancer cell adaptation to the microenvironment. Moreover, there was more aggressive tumor growth by mutant IGFBP-3 overexpressing cells than control cell tumors, without detectable caspase-3 cleavage in tumor tissues, indicating an IGF-independent growth stimulatory effect of mutant IGFBP-3. In aggregate, these data suggest that IGFBP-3 contributes to esophageal tumor development and progression through IGF-dependent and independent mechanisms.
Keywords: IGFBP-3, IGF, Ras, esophageal cancer, in vivo bioluminescence
INTRODUCTION
The insulin-like growth factor (IGF) system comprises a family of interacting ligands, receptors, and IGF binding proteins (IGFBPs). Amongst six IGFBPs, IGFBP-3 is the major carrier protein for IGF-I and IGF-II in circulation, binding over 90% of IGF detectable in the serum.1,2 The full-length form of IGFBP-3 has molecular masses of 43–45 kDa, depending upon posttranslational modifications such as glycosylation and phosphorylation.1,3 IGFBP-3 is secreted by many cell types, including fibroblasts, endothelial cells and epithelial cells.4–6
IGFBP-3 exerts antiproliferative or proapoptotic effects through IGF-dependent as well as IGF-independent mechanisms in vitro.1,7,8 The IGF-dependent functions of IGFBP-3 have been demonstrated by experiments using IGF-I analogues that can bind and activate IGF-IR, but cannot bind IGFBP-3. The IGF-independent mechanisms have been documented by experiments using IGF-IR knockout cells or mutant IGFBP-3I56G/L80G/L81G that cannot bind IGF.9–11 IGFBP-3 transgenic mice demonstrated impaired intrauterine and postnatal growth upon ubiquitous overexpression driven by the cytomegalovirus promoter,12 whereas proliferation of epidermal keratinocytes was reduced despite no gross morphological change in the skin.13 In a transgenic mouse model of prostate cancer, IGFBP-3 appeared to inhibit tumor growth.14 Several studies using tumor cell xenografts have demonstrated also in vivo growth inhibitory effects of IGFBP-3 in non-small lung cancer and prostate cancer cell lines.15–17 On the contrary, there are several in vitro observations indicating that IGFBP-3 may have growth stimulatory effects.1,7 However, it remains unclear if the growth stimulatory effects of IGFBP-3 are IGF-dependent or IGF-independent. In addition, it is not known if IGFBP-3 mediated growth-promoting effects occur in vivo. Epidemiological studies show a high level of serum IGFBP-3 as a protective factor against cancers including prostate and lung cancers, thus implying IGFBP-3 as an anti-cancer molecule, while several studies indicate that IGFBP-3 may be rather a risk factor for certain cancer types including those of the breast and the colon.18
Esophageal cancer is amongst deadliest cancers worldwide.19 Esophageal cancer comprises two major types, namely squamous cell carcinoma (ESCC) and adenocarcinoma (EADC). Both involves alterations in epidermal growth factor receptor (EGFR), cyclin D1, p16INK4a, and p53.20 EGFR is overexpressed frequently in invasive cancer as well as premalignant lesions such as squamous dysplasia and Barrett’s epithelium.21,22 Although IGF-IR overexpression is not common in cancer,23 IGF-I mRNA is increased in primary esophageal tumor tissues compared with adjacent normal mucosa while IGF-II mRNA is overexpressed in 80% of ESCC.24 However, the role of the IGF system in esophageal cancer remains elusive. We have found that IGFBP-3 is frequently overexpressed in esophageal cancer with concordant overexpression of EGFR.25 EGFR tyrosine kinase activity was implicated in regulating IGFBP-3 expression in vitro. However, epidermal growth factor (EGF) inhibited IGFBP-3 expression in a subset of esophageal cancer cell lines.25 Such EGF-mediated inhibition of IGFBP-3 appeared to occur through the Ras-MAPK signaling pathway, resulting in full activation of IGF-IR by IGF-I in immortalized human esophageal cells.26 Thus, EGF may positively regulate the IGF signaling pathway through inhibition of IGFBP-3,25,26 accounting for a synergistic effect of IGF-I and EGF upon esophageal epithelial cells.27,28 IGFBP-3 inhibition by RNA interference augments cell proliferation in TE11 esophageal cancer cells implying the growth inhibitory role of IGFBP-3.25 These observations, however, do not unravel the role of IGFBP-3 overexpression in esophageal cancer.
We have recently established an immortalized cell line T-Te29 by stably transducing EPC2 primary human esophageal epithelial cells30,31 with retrovirus expressing hTERT and simian virus 40 large tumor (SV40T) antigen. While T-Te cells are non-tumorigenic, additional transduction with oncogenes such as Ha-RasV12, AKT, and EGFR resulted in transformation, allowing tumor formation upon xenograft transplantation in athymic nude mice.29 Importantly, T-Te cell-derived tumors present unique gene expression signatures similar to primary esophageal cancers. Moreover, T-Te cells express firefly luciferase, enabling in vivo bioluminescence imaging of the tumor, thus providing an ideal model of esophageal cancer to investigate the effect of any genetic or pharmacological factors upon tumor biology in vivo. In this study, we used Ha-RasV12 transformed T-Te cells to delineate the functional consequences of IGFBP-3 overexpression and found that IGFBP-3 may exert IGF-dependent and independent effects upon esophageal tumor formation and growth in a xenograft transplantation model.
MATERIALS AND METHODS
Cell Lines
T-Te and Ha-RasV12-transformed T-Te (T-Te-Ras) cells were established and characterized as described previously.29 Cells were grown at 37°C under 5% CO2 in a 1:1 mixture of Dulbecco’s Modified Eagle Medium (DMEM)(Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS)(Hyclone, Logan, UT) and Keratinocyte-SFM (KSFM) containing 5 µg/ml insulin, 1 ng/ml EGF, 40 µg/ml bovine pituitary extract (BPE), 6.7 ng/ml of triiodithyronine, 74 ng/ml of hydrocortisone (Invitrogen). The medium was also supplemented with 100 units/ml penicillin, 100 µg/ml streptomycin (Invitrogen). Cells were counted using Coulter™ Z1 Counter (Beckman Coulter, Fullerton, CA). Cell viability was determined by trypan blue exclusion. For growth factor deprivation, cells were rinsed twice with Dulbecco’s PBS (DPBS) without calcium chloride and magnesium chloride and exposed to a 1:1 mixture of DMEM without serum and keratinocyte basal medium (KBM; Bio Whittaker; Walkersville, MD), which is devoid of the growth factors and hormones contained in KSFM.
Retrovirus-Mediated Gene Transduction
A 0.9 kb cDNA fragment each of wild-type and mutant IGFBP-3I56G/L80G/L81G was isolated from pBabe-puro-hIGFBP-3 and pBabe-puro-hIGFBP-3-GGG,26 respectively, and subcloned into the pBabe-bla retrovirus expression vector carrying a blasticidin S resistance gene as a selection marker, resulting in generation of pBabe-bla-hIGFBP-3 and pBabe-bla-IGFBP-3-GGG. Retrovirus production and infection were carried out as described previously. 26,30 Following retrovirus infection, cells were treated with 300 µg/ml of blasticidin S (Invitrogen) for seven days.
Soft Agar Colony Formation Assays
Cells suspended in 0.67% agarose containing DMEM-KBM (1:1) medium supplemented with 5% FCS, 30 µg/ml BPE, and 0.5 ng/ml EGF were overlaid on top of a 1% agarose containing the medium (2.5×104 cells per well), and allowed to grow and form colonies in the soft agar for a week at 37°C under 5% CO2. Then, each well was treated with or without 20 ng/ml of recombinant human IGF-I for an additional one week. Colonies were stained with 0.02% Accustatin® Giemsa stain in a buffered methanol solution, pH6.9 (Sigma, St. Louis, MO), observed under the Eclipse TS100 inverted microscope (Nikon, Melville, NY) and the images were obtained using the MetaVue™ Imaging System (Molecular Devices, Downingtown, PA) to measure the size and number of the colonies.
Western Blot Analysis
Western blot analysis was carried out as described previously.26 In brief, 20 µg of cell lysates were denatured and fractionated on a NuPAGE Bis-Tris 4–12% gel using the NuPAGE System (Invitrogen) and electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore). The membrane was incubated with primary antibody [anti-human IGFBP-3 (DSL-R00536, Diagnostic Systems Laboratories; Webster, TX), anti-β-actin (Sigma), anti-phospho-IGF-IR (Tyr1135/1136) antibody (19H7, no. 3024, Cell Signaling Technology), anti-IGF-IRβ (C-20, sc-713, Santa Cruz Biotechnology; Santa Cruz, CA), anti-phospho-Akt (Ser473) antibody (no. 9271, Cell Signaling Technology), anti-Akt antibody (no. 9272, Cell Signaling Technology), anti-caspase-3 antibody (no. 9662, Cell Signaling Technology)]. The signal was detected by horseradish peroxidase-conjugated secondary antibody [donkey anti-goat IgG (sc-2020, Santa Cruz Biotechnology), donkey anti-rabbit IgG (Amersham Biosciences), or sheep anti-mouse IgG (Amersham Biosciences)], visualized by an enhanced chemiluminescence solution (ECL Plus; Amersham Biosciences Pharmacia Biotech), and exposed to X-Omat LS film (Eastman Kodak; New York, NY).
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was performed using the Human IGFBP-3 DuoSet ELISA Development System (R&D Systems; Minneapolis, MN) as described previously.25,26
Xenograft Transplantation Experiments
For xenograft transplantation experiments 3 × 106 cells of the T-Te-Ras cell derivatives were suspended in 50% Matrigel (BD Biosciences Co., Franklin, NJ) and implanted subcutaneously in quadruplicate fashion into the dorsal skin of athymic nu/nu mice (4–6 weeks old)(Charles River Breeding Laboratories). Tumor growth was monitored by bioluminescence in vivo imaging. Tumor volumes were also measured. Mice were sacrificed to analyze the tumors morphologically and biochemically. One half of a tumor was homogenized to extract protein in a lysis buffer consisting of 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 0.1% sodium deoxycholate, 1 mM EDTA, 2 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and a protease inhibitor mixture tablet (Complete; Roche Applied Science, Indianapolis, IN) and cleared by centrifugation at 14,000 rpm at 4°C for 15 min. The other half was fixed in 4% paraformaldehyde, and embedded in paraffin. Tissue sections were subject to hematoxylin-eosin staining and immunohistochemistry.
In Vivo Bioluminescence Imaging
Bioluminescence images were acquired by using the cooled charge-coupled device camera of an in vivo imaging system (IVIS, Xenogen, Alameda, CA). A cocktail of D-luciferin (150 mg/kg of body weight)(Xenogen), Ketamine HCl (100 mg/kg)(Phoenix Scientific, St. Joseph, MO), and Xylazine (10 mg/kg) (Phoenix Scientific) was injected intraperitoneally 15–30 min before imaging. The supine mice were placed in a light-tight chamber, with a field of view set at 15 cm above the sample shelf. A grayscale reference image was obtained under low-level illumination. Photons emitted from cells implanted in the mice were collected and integrated for a period of 1 min. Images were obtained with a binning of medium (8 × 8) by using the LIVING IMAGE software (Xenogen). A whole body image was acquired. Each image was acquired at the same time, relative to the injected substrate. All of the images were shown at the same scale.
Immunohistochemistry
Immunohistochemistry was performed with the Vectastain Elite kit (Vector Laboratories, Burlingame, CA) following the manufacturer’s protocol. In brief, paraffin sections were deparaffinized with xylene, hydrated in descending ethanol solutions, and then placed in a microwave in 10 mmol/L citric acid buffer. Endogenous peroxidase was quenched using hydrogen peroxide before sections were blocked in avidin D blocking reagent and biotin blocking reagent. Sections were incubated with primary at a 1:250 dilution and with biotinylated antimouse IgG at a 1:200 dilution, and then signal was developed using the 3,3'-diaminobenzidine substrate kit for peroxidase. Anti-Ki67 (ab833, Abcam, Cambridge, MA) and anti-caspase-3 (AF835, R&D systems) were used as primary antibodies. The immunohistochemical staining was assessed independently (Klein-Szanto A.J.), and the Ki67 labeling index was determined by counting at least 600 cells per group.
Statistical analyses
Student’s t-test was used to compare data between two groups. Data represent means ± SE. p < 0.05 was considered to be statistically significant.
RESULTS
Ectopically Expressed IGFBP-3 Inhibits the IGF-IR Signaling and Prevents IGF-I from Stimulating Anchorage-Independent Cell Growth of the Ha-RasV12-Transformed Human Esophageal Cells
IGFBP-3 inhibits the mitogenic effect of IGF-1 in non-transformed human esophageal cells by restricting the G1-S cell cycle progression.26 To further delineate the biological consequences of IGFBP-3 overexpression in esophageal cancer, wild-type (WT) and Ile56Gly/Leu80Gly/Leu81Gly (GGG) mutant IGFBP-3, the latter incapable of binding IGFs9–11 were ectopically expressed in T-Te-Ras cells to a comparable extent (Fig. 1A and B). The GGG-mutant IGFBP-3 provides an ideal approach to determine the IGF-independent biological functions of IGFBP-3. WT-IGFBP-3, but not GGG-mutant IGFBP-3, profoundly prevented IGF-1 from activating IGF-1R and AKT, implying the sequestration of IGF-1 by IGFBP-3 overexpression in T-Te-Ras cells in spite of the constitutively activated status of Ras. These T-Te-Ras cell derivatives showed similar growth kinetics in monolayer culture with similar population doubling times of 23 ± 3 hrs (n = 3), indicating that growth factors other than IGF available in the culture medium are sufficient to maintain cell growth. In fact, insulin, but not serum, appeared to be an essential factor to sustain their growth (data not shown) as it can activate IGF-1R irrespective of IGFBP-3 expression.26
Figure 1.
WT, but not GGG-mutant, IGFBP-3 inhibits IGF-1R signaling and anchorage independent cell growth stimulated by IGF-1 in T-Te-Ras cells. (A) IGFBP-3 and β-actin (loading control) were detected by Western blotting. (B) IGFBP-3 concentration in the conditioned media was determined. Mean ± standard error (n = 3) in a representative experiment is shown. *p = 0.0001, n.s., not significant. (C) Cells were stimulated with 20 ng/ml of recombinant human IGF-1 following growth factor deprivation (16 hrs.) for an indicated time period. Ligand-induced phosphorylation of IGF1-R (Tyr1131) and AKT (Ser473) were determined by Western blotting. (D) Macroscopic view of representative wells of colonies of T-Te-Ras cell derivatives grown in 0.67% agarose for 14 days in the presence or absence of 20 ng/ml of IGF-1. (E) Average diameter of colonies with 120 µm or larger was determined. 145–359 colonies were measured per well. Mean ± standard error (n = 3) in a representative experiment is shown. *p < 0.001, **p < 0.03. No significant differences were observed between cells treated vs. untreated with IGF-1.
We also tested how IGFBP-3 overexpression affects anchorage-independent cell growth in soft-agar colony formation assays. Average colony size appeared to be significantly larger in the presence of IGF-1 and that ectopically expressed WT, but not GGG-mutant, IGFBP-3 inhibited the growth of colony size (Fig. 1D and E). However, all of the T-Te-Ras cell derivatives formed colonies in the absence of IGF (Fig. 1E), and there was no significant difference in total number of the colonies observed between the cell lines compared (data not shown), suggesting that the IGF signaling contribute to anchorage-independent cell growth by stimulating cell proliferation but may be dispensable for the colony formation per se. Interestingly, unlike monolayer culture, serum was required for colony formation in soft agar by the Ras-transformed cell and their derivatives (data not shown). In aggregate, the above results demonstrate that IGFBP-3 negatively regulates cell proliferation in vitro in an IGF-dependent but a context dependent manner.
A Ras-Transformed Esophageal Cell Xenograft Transplantation Model Revealed the IGF-Dependent Inhibitory Effect of IGFBP-3 Upon Tumor Growth
We next carried out xenograft transplantation experiments. Interestingly, in vivo bioluminescence imaging revealed a rapid decrease in the luciferase activity in T-Te-Ras cells during first two weeks after cell injection, followed by linear tumor growth, suggesting initial loss of cell viability and tumor cell adaptation to the ectopic subcutis tissue microenvironment.29 Thus, we hypothesized that the IGF-signaling is required to foster tumor development and that IGFBP-3 interferes with such a biological process. Figure 2 demonstrates that the T-Te-Ras cells transduced with WT IGFBP-3, but not GGG-mutant IGFBP-3 or an empty vector (Bla), failed to form tumors. This is a striking result reinforcing the importance of IGF signaling in tumor formation, and that constitutive activation of Ras, as one of the essential downstream effectors for IGF-1R signaling, is not sufficient to negate the IGF-inhibitory effect of IGFBP-3 in vivo. This is also in agreement with previous studies demonstrating in vivo growth inhibitory effects of IGFBP-3.15–17 The Bla control cells and GGG-mutant IGFBP-3 overexpressing cells also underwent transient tumor regression. However, 75% of tumors eventually grew and demonstrated 10- to 100-fold increases in the luciferase activity by 6 weeks after implantation (Experiment 2 in Fig. 2B). The overall tumor formation rate was consistent with that of the parental T-Te-Ras cells.29
Figure 2.
In vivo bioluminescence imaging reveals an accelerated regression and failure of tumor formation by xenografted T-Te-Ras cells overexpressing WT IGFBP-3. T-Te-Ras cell derivatives were implanted subcutaneously in quadruplicate into the dorsal skin of athymic nude mice and tumor growth was monitored as described.29 (A) The firefly luciferase activity visualized in representative mice, imaged at one week and three weeks after injection. Tumors demonstrating the signal intensity greater than 5 × 106 photons/sec/cm2 were considered to be viable. (B) Summary of two independent experiments. In Experiment 1, mice were sacrificed to remove four tumors for histology (Fig. 3) at three weeks after injection. The luciferase activity (>1 × 107 photons/sec/cm2) was detected in remaining tumors at five weeks. †p < 0.03, ††p < 0.002 and ‡p < 0.005 vs. WT. *p < 0.01 vs. 1 week.
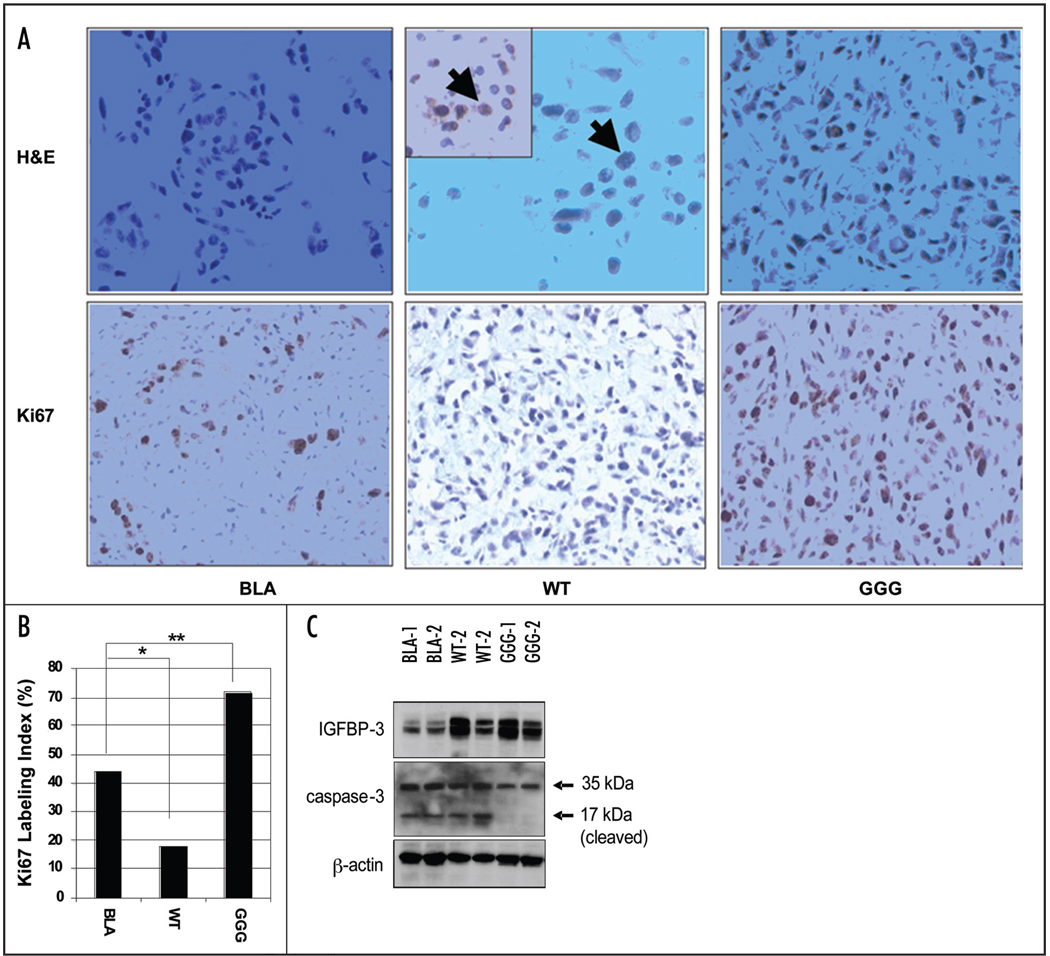
Upon necropsy, tumors were to be much softer and smaller in size, and the surrounding vasculature was less abundant in WT IGFBP-3 transduced cells than GGG-mutant IGFBP-3 or Bla transduced cells (data not shown). Histology documented active tumor growth by the Bla and GGG-mutant IGFBP-3 expressing cells (Fig. 3). Interestingly enough, the Bla cells showed circumscribed local growth within the remaining matrigel that was coinjected while GGG-mutant IGFBP-3 overexpressing tumors appeared to be more invasive (Fig. 3A). By contrast, WT IGFBP-3 expressing tumors were the least viable accompanied with an increased number of apoptotic cells (Fig. 3A). Corroborating the above findings, the GGG-mutant IGFBP-3 expressing tumors showed more Ki67 positive cells (Fig. 3A and B), an unexpected finding. The tumor volume tended to be greater upon tissue harvest in the GGG-mutant IGFBP-3 expressing tumors than Bla tumors (data not shown). However, there was no significant correlation between tumor volume and the luciferase activity, suggesting a possible difference in the tumor microenvironment, such as the level of blood supply and the availability of energy sources, and the extent of energy consumption by tumor cells. The histological data suggest that the xenotransplanted tumor cells may require IGF-1R ligands for adaptation to the microenvironment in the host tissue, while IGFBP-3 may antagonize such a process in an IGF-dependent fashion in vivo. Thus, it is tempting to speculate that the mutant IGFBP-3 contributed to enhance cell viability and promote the process of cancer cell adaptation to the microenvironment in an IGF-independent fashion. Such IGF-independent effects upon tumor cells themselves should be carefully interpreted as such mutation of IGFBP-3 has not been found in nature. Nonetheless, it is possible that IGFBP-3 may influence tumor progression, analogous to the dichotomous role of TGF-β in cancer biology: loss of tumor-suppressor functions may contribute to tumor development in early stages, whereas activation of its signaling pathway may lead to tumor cell invasion and metastasis at later stages.32
Figure 3.
Tumor growth is suppressed by WT IGFBP-3 but stimulated by GGG-mutant IGFBP-3. (A) Tumor tissues at three weeks after injection (experiment 1 in Fig. 2B) were stained with hematoxylin-eosin (upper panel, ×400), anti-caspase-3 (Company, ×400, inset of WT), or Ki67 (lower panel, ×200). Note Caspase-3 positive apoptotic cells indicated by arrows. (b) Labeling index for Ki67 was determined by counting at least 600 cells. *p < 0.001, **p < 0.001. (C) IGFBP-3 and caspase-3 were detected in representative tumor samples one week after injection. Note the IGFBP-3 (DSL-R00536) and caspase-3 (9662, Cell Signaling Technology) antibodies are human specific and not crossreactive with mouse tissues.
IGFBP-3 May Induce Apoptosis by Antagonizing the Pro-Survival Effect of IGF in Xenografted Tumors
To further explore the possible mechanisms for the IGFBP-3-mediated tumor growth inhibition, we examined the tumor tissues by Western blotting. Both WT and GGG-mutant IGFBP-3 overexpressing tumors showed a comparable level of IGFBP-3 one week after injection (Fig. 3C). IGFBP-3 was not detected in T-Te-Ras parental cells or empty vector transduced cells in monolayer culture (Fig. 1A). Nonetheless, endogenous IGFBP-3 appeared to be induced in the Bla control tumor tissues (Fig. 3C), suggesting that IGFBP-3 may be induced in vivo through a mechanism that can overcome the Ras-mediated transcriptional inhibition.26
A cleaved form of caspase-3 was observed at one week after injection in at least two independent tumors formed by cells with either endogenously induced IGFBP-3 (Bla) or ectopically overexpressed WT IGFBP-3 (Fig. 3C). By contrast, it was barely, albeit not absent, detectable in the GGG-mutant IGFBP-3 transduced cells (Fig. 3C), suggesting induction of apoptosis as a consequence of IGF sequestration by endogenous or WT IGFBP-3 in vivo. Furthermore, caspase-3 cleavage was no longer detectable in either control or GGG-IGFBP-3 expressing solid tumors at three weeks after injection (data not shown).
DISCUSSION
We demonstrate that IGFBP-3 inhibits IGF-signaling, leading to suppression of anchorage- independent cell growth in vitro and tumor formation in vivo by oncogenic Ha-Ras-transformed human esophageal cells. Although we have reported previously that IGFBP-3 negatively regulates cell proliferation of non-tumorigenic primary and immortalized human esophageal cells in vitro,26 this is the first report showing the effect of IGFBP-3 overexpression in genetically defined tumorigenic human esophageal cells. Importantly, we show that IGFBP-3 may exert IGF-independent functions that have not been appreciated in vitro in human esophageal cells,26 a critical finding given frequent IGFBP-3 overexpression in primary esophageal cancers.25 Therefore, IGFBP-3 functions may depend upon IGF as well as coexisting factors in the tumor microenvironment.
Our findings imply a pivotal role of the IGF system in the esophageal tumorigenesis. The cellular actions of IGF are in general mediated by the type I IGF receptor (IGF-IR).33 IGF-IR not only transmits mitogenic signals but protects cells from apoptosis, promotes growth in cell size, and regulates cell adhesion and cell motility. IGF-IR also plays an essential role in malignant transformation.34 IGF-IR is required for transformation of mouse embryonic fibroblasts by cellular and viral oncogenes such as EGFR,35 Ha-Ras36 and SV40T antigen.37 Thus, inhibition of tumor formation by wild-type IGFBP-3 observed in the T-Te-Ras cells expressing SV40T antigen and Ha-Ras reinforces the role of IGF-IR signaling in esophageal carcinogenesis.
Our data also indicate that cancer cells that fail to undergo adaptation to the microenvironment are likely to be eliminated by apoptosis. In fact, c-Myc transformed human esophageal cells were not tumorigenic unless Bcl-XL gene was simultaneously transduced to inhibit apoptosis.29 The inability of tumor formation by the T-Te-Ras cells overexpressing wild-type IGFBP-3 implies an essential role of the IGF-signaling in cell survival. Earlier studies using tumor cell xenografts have demonstrated growth inhibitory effects of IGFBP-3 in vivo. In NCI-H23 non-small cell lung cancer cell line, IGFBP-3 overexpression resulted in growth inhibition when xenotransplanted into nude mice.15 Injection of IGFBP-3 expression adenovirus into H1299 non-small cell lung cancer cell-xenografted tumors led to massive tumor cell death.16 IGFBP-3 expression in the M12 prostate cancer cell line also resulted in decreased tumor formation in nude mice.17 In the present study, we demonstrated clearly the IGF-dependency in the proapoptotic effects of the wild-type IGFBP-3 overexpressed in xenografted tumors by using the GGG-mutant IGFBP-3 as a critical control. Recently, Silha et al reported the anticancer functions of IGFBP-3 in a prostate cancer model where transgenic mice expressing SV40T antigen under a prostate specific promoter were crossbred with mice expressing either wild-type or GGG-mutant IGFBP-3.14 They found that wild-type IGFBP-3, but not the GGG-mutant IGFBP-3, inhibited prostate tumor growth through inhibition of the AKT activity and induction of apoptosis in the early stage of tumor development. Interestingly, the GGG-mutant IGFBP-3 also appeared to induce apoptosis in the tumors at the later stage, apparently in an IGF-independent manner. The lack of apoptosis or growth inhibition in the GGG-mutant IGFBP-3 overexpressing esophageal cells in vitro as well as in vivo indicate that esophageal cells are unlikely to be susceptible to IGF-independent antiproliferative or proapoptotic effects of IGFBP-3, implying a cell-type specific functions of IGFBP-3.
Unexpectedly, we observed increased cell proliferation and more aggressive tumor growth by the T-Te-Ras cells overexpressing GGG-mutant IGFBP-3 than empty vector transduced control cells. This can be interpreted as an IGF-independent growth stimulatory or pro-survival effect, which may be accounted for by the barely detectable caspase-3 cleavage in the tumors examined at one week after injection. However, even the GGG-mutant IGFBP-3 cells underwent a transient tumor regression as indicated by a decrease in the luciferase activity measured by in vivo bioluminescence imaging (Fig. 2). Thus, it is tempting to speculate that the mutant IGFBP-3 contributed to enhance cell viability and promote the process of cancer cell adaptation to the microenvironment in an IGF-independent fashion. Such IGF-independent effects upon tumor cells themselves should be carefully interpreted as such mutation of IGFBP-3 has not been found in nature. Nonetheless, it is possible that IGFBP-3 may influence tumor progression after cancer cells are adapted to the microenvironment, analogous to the dichotomous role of TGF-β in cancer biology: loss of tumor-suppressor functions may contribute to tumor development in early stages, whereas activation of its signaling pathway may lead to tumor cell invasion and metastasis at later stages.32 Interestingly, a recent study demonstrated that TGF-β directly suppresses cytotoxic T-cell function in vivo, permitting tumor evasion of immune surveillance.38 Given the ability of IGFBP-3 to stimulate the TGF-β signaling pathways,1 it is conceivable that the IGFBP-3 may affect T-cell function in the tumor microenvironment.
In our xenograft transplantation experiments, the cleaved form of caspase-3 was detected in the tumors formed by empty vector transduced cells at one week after injection (Fig. 3). Since the extent of caspase-3 cleavage was comparable to that observed in the wild-IGFBP-3 overexpressing cells, the IGFBP-3 level induced in the empty vector carrying tumors may be sufficient to induce apoptosis. Yet, it appeared to be permissive for a subset of tumor cells to undergo adaptation to the microenvironment and permit continuous tumor growth.
EGF suppresses IGFBP-3 at the mRNA level in primary and immortalized epithelial cells.39–41 We have recently found the Ras-MAPK signaling pathway mediates the transcriptional inhibition of IGFBP-3 by EGF.26 Consistent with such a notion, IGFBP-3 was not detectable in the T-Te-Ras cells expressing constitutively active mutant Ha-RasV12 in vitro (Fig. 1A). What factors in the tumor microenvironment permit in vivo induction of IGFBP-3? It is likely that xenografted tumor cells are exposed to substantive stresses such as lack of nutrients and oxygen until they are adapted to the new microenvironment. In fact, we have observed disorganized vascular structures and focal upregulation of glucose transporter Glut1 in the T-Te-Ras cell tumors, suggesting tumor hypoxia and activation of hypoxia-inducible factor (HIF)-1α.29 Since IGFBP-3 is induced by hypoxia,42 and HIF-1α is implicated in hypoxic induction of IGFBP-3 mRNA in embryonic stem cells,43 it is plausible that HIF-1α plays a role in IGFBP-3 induction in the tumors. In agreement, IGFBP-3 is induced by hypoxia in the presence of EGF (Nakagawa et al, unpublished observations). Cellular stress can activate p53 tumor suppressor protein. However, p53 is unlikely to contribute to IGFBP-3 induction in the T-Te-Ras cell tumors since p53 is inhibited by SV40T antigen in spite of the fact that IGFBP-3 is one of the transcriptional target genes of p53.44,45
In conclusion, the balance between IGF and IGFBP-3 may determine the tumor cell fate in vivo through regulation of the maintenance of cell viability and the process of cancer cell adaptation to tissue microenvironment for tumor formation. Once adapted to the microenvironment, IGFBP-3 may rather promote tumor growth in an IGF-independent manner, thus supporting a notion that IGFBP-3 may exert differential biological functions in the early and late stages of carcinogenesis.14,46
Acknowledgements
This study was supported in part by National Institutes of Health (NIH) Grant K01-DK-066205 (to HN), the American Gastroenterological Association/Foundation for Digestive Health and Nutrition Research Scholar Award (to HN and CDA), NIH Grant P01-CA-098101 (to HN, MT, SK, TO, CZM, CDA, DS, AJPK, and WSE), the NIH Center for Molecular Studies in Digestive and Liver Diseases, and its core facilities. We thank Dr. Yvette Liu (In-vivo Bioluminescence Molecular Imaging Core), Shukriyyah Mitchell and Dr. Gary Swain (Morphology Core), Momo Nakagawa, Azal A. Dezfuli, and Mark J. Bowser for technical assistance, Drs. Therese B. Deramaudt and Melanie P. Wescott for helpful discussions. Finally, we are grateful to Drs. Volker H. Haase, Meenhard M. Herlyn, and Anil K. Rustgi for the support and advice and critical review of the manuscript by Dr. Rustgi.
ABBREVIATIONS
- IGFBP
insulin-like growth factor binding protein
- IGF
insulin-like growth factor
References
- 1.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 2.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 3.Butt AJ, Fraley KA, Firth SM, Baxter RC. IGF-binding protein-3-induced growth inhibition and apoptosis do not require cell surface binding and nuclear translocation in human breast cancer cells. Endocrinology. 2002;143:2693–2699. doi: 10.1210/endo.143.7.8876. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein S, Moerman EJ, Jones RA, Baxter RC. Insulin-like growth factor binding protein 3 accumulates to high levels in culture medium of senescent and quiescent human fibroblasts. Proc Natl Acad Sci USA. 1991;88:9680–9684. doi: 10.1073/pnas.88.21.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grillari J, Hohenwarter O, Grabherr RM, Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp Gerontol. 2000;35:187–197. doi: 10.1016/s0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 6.Wraight CJ, Murashita MM, Russo VC, Werther GA. A keratinocyte cell line synthesizes a predominant insulin-like growth factor-binding protein (IGFBP-3) that modulates insulin-like growth factor-I action. J Invest Dermatol. 1994;103:627–631. doi: 10.1111/1523-1747.ep12397667. [DOI] [PubMed] [Google Scholar]
- 7.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: Interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–E976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 8.Baxter RC. Signalling pathways involved in antiproliferative effects of IGFBP-3: A review. Mol Pathol. 2001;54:145–148. doi: 10.1136/mp.54.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckway CK, Wilson EM, Ahlsen M, Bang P, Oh Y, Rosenfeld RG. Mutation of three critical amino acids of the N-terminal domain of IGF-binding protein-3 essential for high affinity IGF binding. J Clin Endocrinol Metab. 2001;86:4943–4950. doi: 10.1210/jcem.86.10.7936. [DOI] [PubMed] [Google Scholar]
- 10.Longobardi L, Torello M, Buckway C, O’Rear L, Horton WA, Hwa V, Roberts CT, Jr, Chiarelli F, Rosenfeld RG, Spagnoli A. A novel insulin-like growth factor (IGF)-independent role for IGF binding protein-3 in mesenchymal chondroprogenitor cell apoptosis. Endocrinology. 2003;144:1695–1702. doi: 10.1210/en.2002-220959. [DOI] [PubMed] [Google Scholar]
- 11.Silha JV, Gui Y, Mishra S, Leckstrom A, Cohen P, Murphy LJ. Overexpression of gly56/gly80/gly81-mutant insulin-like growth factor-binding protein-3 in transgenic mice. Endocrinology. 2005;146:1523–1531. doi: 10.1210/en.2004-0905. [DOI] [PubMed] [Google Scholar]
- 12.Modric T, Silha JV, Shi Z, Gui Y, Suwanichkul A, Durham SK, Powell DR, Murphy LJ. Phenotypic manifestations of insulin-like growth factor-binding protein-3 overexpression in transgenic mice. Endocrinology. 2001;142:1958–1967. doi: 10.1210/endo.142.5.8165. [DOI] [PubMed] [Google Scholar]
- 13.Edmondson SR, Thumiger SP, Kaur P, Loh B, Koelmeyer R, Li A, Silha JV, Murphy LJ, Wraight CJ, Werther GA. Insulin-like growth factor binding protein-3 (IGFBP-3) localizes to and modulates proliferative epidermal keratinocytes in vivo. Br J Dermatol. 2005;152:225–230. doi: 10.1111/j.1365-2133.2004.06350.x. [DOI] [PubMed] [Google Scholar]
- 14.Silha JV, Sheppard PC, Mishra S, Gui Y, Schwartz J, Dodd JG, Murphy LJ. Insulin-like growth factor (IGF) binding protein-3 attenuates prostate tumor growth by IGF-dependent and IGF-independent mechanisms. Endocrinology. 2006;147:2112–2121. doi: 10.1210/en.2005-1270. [DOI] [PubMed] [Google Scholar]
- 15.Hochscheid R, Jaques G, Wegmann B. Transfection of human insulin-like growth factor-binding protein 3 gene inhibits cell growth and tumorigenicity: A cell culture model for lung cancer. J Endocrinol. 2000;166:553–563. doi: 10.1677/joe.0.1660553. [DOI] [PubMed] [Google Scholar]
- 16.Lee HY, Chun KH, Liu B, Wiehle SA, Cristiano RJ, Hong WK, Cohen P, Kurie JM. Insulin-like growth factor binding protein-3 inhibits the growth of non-small cell lung cancer. Cancer Res. 2002;62:3530–3537. [PubMed] [Google Scholar]
- 17.Devi GR, Sprenger CC, Plymate SR, Rosenfeld RG. Insulin-like growth factor binding protein-3 induces early apoptosis in malignant prostate cancer cells and inhibits tumor formation in vivo. Prostate. 2002;51:141–152. doi: 10.1002/pros.10068. [DOI] [PubMed] [Google Scholar]
- 18.Ali O, Cohen P, Lee KW. Epidemiology and biology of insulin-like growth factor binding protein-3 (IGFBP-3) as an anti-cancer molecule. Horm Metab Res. 2003;35:726–733. doi: 10.1055/s-2004-814146. [DOI] [PubMed] [Google Scholar]
- 19.Enzinger PC, Mayer RJ. Esophageal cancer. Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa H, Katzka D, Rustgi AK. Biology of esophageal cancer. In: Rustgi AK, editor. Gastrointestinal Cancers. London: Elsevier; 2003. pp. 241–251. [Google Scholar]
- 21.Itakura Y, Sasano H, Shiga C, Furukawa Y, Shiga K, Mori S, Nagura H. Epidermal growth factor receptor overexpression in esophageal carcinoma: An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer. 1994;74:795–804. doi: 10.1002/1097-0142(19940801)74:3<795::aid-cncr2820740303>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Yacoub L, Goldman H, Odze RD. Transforming growth factor-alpha, epidermal growth factor receptor, and MiB-1 expression in Barrett’s-associated neoplasia: Correlation with prognosis. Mod Pathol. 1997;10:105–112. [PubMed] [Google Scholar]
- 23.Liu YC, Leu CM, Wong FH, Fong WS, Chen SC, Chang C, Hu CP. Autocrine stimulation by insulin-like growth factor I is involved in the growth, tumorigenicity and chemoresistance of human esophageal carcinoma cells. J Biomed Sci. 2002;9:665–674. doi: 10.1159/000067282. [DOI] [PubMed] [Google Scholar]
- 24.Mori M, Inoue H, Shiraishi T, Mimori K, Shibuta K, Nakashima H, Mafune K, Tanaka Y, Ueo H, Barnard GF, Sugimachi K, Akiyoshi T. Relaxation of insulin-like growth factor 2 gene imprinting in esophageal cancer. Int J Cancer. 1996;68:441–446. doi: 10.1002/(SICI)1097-0215(19961115)68:4<441::AID-IJC7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Takaoka M, Harada H, Andl CD, Oyama K, Naomoto Y, Dempsey KL, Klein-Szanto AJ, El-Deiry WS, Grimberg A, Nakagawa H. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 2004;64:7711–7723. doi: 10.1158/0008-5472.CAN-04-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takaoka M, Smith CE, Mashiba MK, Okawa T, Andl CD, El-Deiry WS, Nakagawa H. EGF-mediated regulation of IGFBP-3 determines esophageal epithelial cellular response to IGF-I. Am J Physiol Gastrointest Liver Physiol. 2006;290:G404–G416. doi: 10.1152/ajpgi.00344.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qureshi FG, Tchorzewski MT, Duncan MD, Harmon JW. EGF and IGF-I synergistically stimulate proliferation of human esophageal epithelial cells. J Surg Res. 1997;69:354–358. doi: 10.1006/jsre.1997.5080. [DOI] [PubMed] [Google Scholar]
- 28.Simmons JG, Hoyt EC, Westwick JK, Brenner DA, Pucilowska JB, Lund PK. Insulin-like growth factor-I and epidermal growth factor interact to regulate growth and gene expression in IEC-6 intestinal epithelial cells. Mol Endocrinol. 1995;9:1157–1165. doi: 10.1210/mend.9.9.7491108. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Nakagawa H, Navaraj A, Naomoto Y, Klein-Szanto AJ, Rustgi AK, El-Deiry WS. Tumorigenic conversion of primary human esophageal epithelial cells using oncogene combinations in the absence of exogenous ras. Cancer Res. 2006;66:10415–10424. doi: 10.1158/0008-5472.CAN-06-2104. [DOI] [PubMed] [Google Scholar]
- 30.Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 31.Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B, von Werder A, Enders GH, Opitz OG, Rustgi AK. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res. 2003;1:729–738. [PubMed] [Google Scholar]
- 32.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 33.Butt AJ, Firth SM, Baxter RC. The IGF axis and programmed cell death. Immunol Cell Biol. 1999;77:256–262. doi: 10.1046/j.1440-1711.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 34.Valentinis B, Baserga R. IGF-I receptor signalling in transformation and differentiation. Mol Pathol. 2001;54:133–137. doi: 10.1136/mp.54.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppola D, Ferber A, Miura M, Sell C, D’Ambrosio C, Rubin R, Baserga R. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994;14:4588–4595. doi: 10.1128/mcb.14.7.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci USA. 1993;90:11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Edmondson SR, Murashita MM, Russo VC, Wraight CJ, Werther GA. Expression of insulin-like growth factor binding protein-3 (IGFBP-3) in human keratinocytes is regulated by EGF and TGFbeta1. J Cell Physiol. 1999;179:201–207. doi: 10.1002/(SICI)1097-4652(199905)179:2<201::AID-JCP10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Hembree JR, Agarwal C, Eckert RL. Epidermal growth factor suppresses insulin-like growth factor binding protein 3 levels in human papillomavirus type 16-immortalized cervical epithelial cells and thereby potentiates the effects of insulin-like growth factor 1. Cancer Res. 1994;54:3160–3166. [PubMed] [Google Scholar]
- 41.Wraight CJ, Werther GA. Insulin-like growth factor-I and epidermal growth factor regulate insulin-like growth factor binding protein-3 (IGFBP-3) in the human keratinocyte cell line HaCaT. J Invest Dermatol. 1995;105:602–607. doi: 10.1111/1523-1747.ep12323716. [DOI] [PubMed] [Google Scholar]
- 42.Grimberg A, Coleman CM, Burns TF, Himelstein BP, Koch CJ, Cohen P, El-Deiry WS. p53-Dependent and p53-independent induction of insulin-like growth factor binding protein-3 by deoxyribonucleic acid damage and hypoxia. J Clin Endocrinol Metab. 2005;90:3568–3574. doi: 10.1210/jc.2004-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- 44.Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 45.Grimberg A. P53 and IGFBP-3: Apoptosis and cancer protection. Mol Genet Metab. 2000;70:85–98. doi: 10.1006/mgme.2000.3008. [DOI] [PubMed] [Google Scholar]
- 46.Cohen P. Insulin-like growth factor binding protein-3: Insulin-like growth factor independence comes of age. Endocrinology. 2006;147:2109–2111. doi: 10.1210/en.2006-0195. [DOI] [PubMed] [Google Scholar]