Summary
The Jerusalem Perinatal Study recorded information on population-based cohorts of 92 408 live- and stillbirths in 1964–76, and their parents, with active surveillance of infant deaths and birth defects. Data on maternal conditions, obstetric complications and interventions during labour and delivery were recorded for 92% of the births. Subsets were surveyed with antenatal interviews in 1965–68 (n = 11 467), paediatric admissions to hospital (n = 17 782) and postpartum interviews in 1975–76 (n = 16 912). Data from some offspring were linked to records of a health examination at age 17. The offspring, mothers and fathers have been traced recently, their vital status assessed, and the data linked to Israel’s Cancer Registry and Psychiatric Registry. This paper describes the different types of data available, their sources, and some potential biases. Characteristics of this unique population are shown. Findings from the study are reviewed and a list of references is provided. The cohorts provide a unique source of data for a wide variety of studies.
Keywords: birth survey, longitudinal, time trends, cancer, paternal age, childhood hospitalisation, pre-eclampsia, breast feeding, BMI, diabetes, birth defects, psychiatric disorder
Introduction
The Jerusalem Perinatal Study was started in 1964 by A. M. Davies as a population-based investigation of hypertensive disorders of pregnancy. Clinical observations had shown variation in the frequency of ‘toxaemia’ in different groups of immigrants;1 verification of these associations and a search for causes required a survey of all pregnancies and their outcomes in a defined geographical area. Unlike the British national birth surveys, which were subsequently followed up,2–4 the Jerusalem study was planned to continue for several years, and in contrast with the National Collaborative Perinatal Project in the US,5 it was population-based. Data in the Jerusalem Perinatal Study spanned 13 years and two major wars, and subsequently reflected the demographic and health changes in mothers, initially refugees and immigrants, during Israel’s transition from a developing country into a modern, industrial nation.6 The methods were copied in studies in other areas in Israel.7–9 This paper summarises the characteristics of the cohort and draws attention to the main findings to date. The last overview was published in 1977.10
Methods
Definition of the cohort
The study included all births to Israeli residents of Jerusalem, regardless of place of birth. The legal definition of a birth at that time included pregnancies of 28 weeks or more, live births regardless of weight, and stillbirths weighing at least 1000 g. Residence was defined by the mother’s address at the time of the birth, as recorded on her identity card. An English version of the manual of methods was produced in 196811 and a summary of it published in 1969.12
Data available on the whole cohort
The core data were copied from the live- or stillbirth notifications sent by the hospital of delivery to the district health office: notification was compulsory and complete. Data abstracted on live- and stillborn offspring included the date of birth, birthweight, sex, birth order and status as a single or multiple birth. Data on parents included their address, of which only the census tract was retained, their ages, occupations, dates of immigration and marriage, countries of birth, and each grandfather’s country of birth. For live births, the parents’ years of education were also recorded. For record linkage, the mother’s and baby’s identity numbers were retained, with the first three letters of the mother’s surname. Surveillance of infant deaths was achieved by obtaining a copy of the notification to the district health office, augmented by active surveillance of obstetric and paediatric departments.
Birth defects
Fieldworkers (mainly nurse-midwives) made weekly visits to the three largest of West Jerusalem’s four obstetric departments, and to all three paediatric departments. They obtained data on birth defects reported at birth or during admissions to hospital. Starting in 1965, they also visited municipal well-baby clinics in order to gather information on birth defects that were reported later, and to include any of the few infants born in the smallest hospital. All death notifications were also scanned for mention of birth defects.
Data available on subsets
Maternal and obstetric conditions and obstetric interventions
In the three largest hospitals, the fieldworkers scanned the labour-ward logs, abstracting the data into precoded forms with rubrics indicating specific categories of maternal conditions, obstetric conditions, interventions and methods of delivery. Pre-eclampsia was studied from 1 January 1964 and other rubrics were added 3 months later; this surveillance of labour wards continued uninterrupted until 31 December 1976. We did not collect obstetric information systematically from the 7% of births that occurred in the fourth, smallest, obstetric unit, or from the 2% in hospitals outside of Jerusalem. Antenatal, postnatal and well-baby care were available free at a network of neighbourhood clinics; pregnant women at high risk of a poor obstetric outcome were referred to hospital-based clinics. All births took place in hospitals (or en route) and were free of charge, with an added maternity bounty. Attendants at uncomplicated deliveries were usually nursemidwives, with medical supervision as required.
Admissions to hospitals
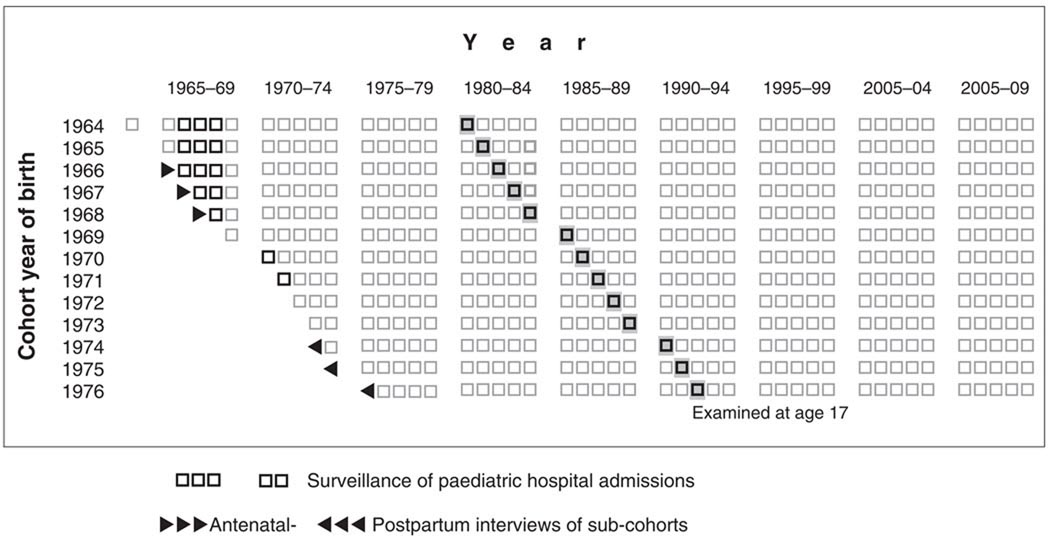
Through weekly visits of the fieldworkers to the paediatric wards in Jerusalem, the study reviewed admissions to hospitals at various times, and for various subcohorts, illustrated in Fig. 1. The dates of admission and discharge were recorded, and the discharge summary was scanned for the first three diagnoses, operation (if any), up to two in-hospital ‘events’, mention of birth defects, weight (after re-hydration) and the lowest haemoglobin. Surveillance of hospital admissions started in 1966 and continued for 4 years; this resulted in different birth cohorts being followed through different ages (Fig. 1).
Figure 1.
Schematic representation of the Jerusalem Perinatal Study main cohort and sub-cohorts. Horizontal axis represents life course; vertical represents year of birth.
Antenatal interviews
In 1965–68, we interviewed approximately 13 500 women in the municipal antenatal clinics, usually at their first antenatal visit which, in that era, was in the 4th or 5th month. These interviews covered roughly two-thirds of the area’s pregnancies. An estimated 2000 of those interviewed did not deliver; however, we kept no record of these, as we assumed that most had chosen to abort. To facilitate recruitment for the interview, the Department of Medical Ecology operated a free pregnancy-testing service for the municipality and ran the town’s main laboratory for routine antenatal blood typing and screening for syphilis; pregnant women were referred to this laboratory from all antenatal clinics. Our interview, which was voluntary, included questions about the outcome of previous pregnancies and sought information on events occurring in each of the first 4 months of pregnancy: vomiting, fever, prescribed drugs, X-ray exposure and bleeding. Other questions covered consanguinity of the parents, diabetes in the mother’s first- and second-degree relatives, smoking, religious observance and residential crowding.
Postpartum interviews
From November 1974 to the end of 1976, we interviewed 16 659 new mothers in hospital, on the first or second day postpartum. We gathered data on gynaecological history, contraceptive use since any previous pregnancy, previous obstetric history, lactation after a previous birth, fecundability and treatment to induce ovulation, bleeding and hormone treatment during pregnancy, smoking by mother and father, and observance of certain Jewish rituals. The interviews were conducted at the bedside by nurse-midwives and captured 98% of the births in the three largest obstetric units.
Institutional approvals
The Jerusalem Perinatal Study cohort was established prior to the era of written informed consent. In the 1960s and 1970s, pregnant and parturient women gave verbal consent to being interviewed, and administrative approvals for the studies were obtained from the Ministry of Health, the Jerusalem District Health Office, the Jerusalem Municipality and the individual hospitals. In the 1980s, written informed consent was obtained from the mothers and 18-year-old teenagers for the studies of long-term effects of medroxyprogesterone acetate.13–15 Since the 1990s, all work on the cohort carried out at the Hebrew University of Jerusalem, and by our collaborators in New York, has been in compliance with the Helsinki Declaration and has been approved by Institutional Review Boards at Hadassah-Hebrew University Hospital, the Hebrew University of Jerusalem, Columbia University and/or New York University. The files have always been guarded as confidential medical records, as applied both to individual citizens and to individual hospitals, and the work complies with Israeli laws for protection of the individual and of personal privacy; these laws are now in line with those of Northern Europe. In 1999 the file was registered with the Database Authority at the Israeli Ministry of Justice, its ownership being defined as the Head of the School of Public Health, ex officio. Through radio, television and newspapers, the Israeli public has been kept informed of the study’s existence including the linkage to the Cancer and Psychiatric registries, and of its results.
Results
Characteristics of the cohort and its main subsets
There were 92 408 live- and stillborn offspring in the study, in 91 248 deliveries, including 1112 sets of twins (1.22%), 22 of triplets (0.024%) and one set of quintuplets. Tables 1–3 summarise the distributions of offspring in the total cohort and in the three main subsets,i.e. the obstetric data surveyed in all years, the antenatal interview of 1965–68 and the postpartum interview of 1974–76. Table 1 shows year of birth and some basic demographic data, Table 2 focuses on maternal education and ethnic information, and Table 3 gives the distributions of birthweights and main outcomes, i.e. stillbirths and birth defects.
Table 1.
Percentage distribution of births in the Jerusalem Perinatal Study and its main subsets, by year, hospital of birth, age of mother and birth order
| Subsets with information on: |
||||
|---|---|---|---|---|
| Total offspring = 100% | Total cohort (N = 92 408) |
Obstetric conditions & interventions 4/64–12/76 (N = 83 493) |
Antenatal interviews 1965–68 (N = 11 467) |
Postpartum interviews 11/74–12/76 (N = 16 912) |
| Year of birth | ||||
| 1964 | 6.2 | 4.9 | – | – |
| 1965 | 6.6 | 6.6 | 3.5 | – |
| 1966 | 6.5 | 6.4 | 35.3 | – |
| 1967 | 6.4 | 6.4 | 33.3 | – |
| 1968 | 6.9 | 6.9 | 27.9 | – |
| 1969 | 7.2 | 7.3 | 0.1 | – |
| 1970 | 7.6 | 7.7 | – | – |
| 1971 | 8.2 | 8.3 | – | – |
| 1972 | 7.9 | 8.1 | – | – |
| 1973 | 8.7 | 8.7 | – | – |
| 1974 | 9.0 | 9.2 | – | 6.8 |
| 1975 | 9.3 | 9.6 | – | 46.8 |
| 1976 | 9.6 | 10.0 | – | 46.4 |
| Hospital of birth | ||||
| 1 | 34.8 | 37.9 | 35.1 | 36.7 |
| 2 | 26.9 | 29.1 | 26.1 | 21.3 |
| 3 | 30.1 | 32.9 | 30.6 | 42.0 |
| Others | 8.2 | – | 5.8 | – |
| Age of mother (years) | ||||
| <20 | 3.9 | 3.9 | 5.1 | 3.3 |
| 20–24 | 30.3 | 30.6 | 30.9 | 30.9 |
| 25–29 | 32.0 | 32.0 | 28.7 | 35.2 |
| 30–34 | 20.0 | 19.8 | 21.2 | 18.8 |
| 35–39 | 10.5 | 10.4 | 10.7 | 9.1 |
| 40–44 | 2.8 | 2.8 | 3.0 | 2.2 |
| 45+ | 0.4 | 0.4 | 0.4 | 0.2 |
| Unknown | 0.2 | 0.1 | – | 0.2 |
| Birth order | ||||
| 1 | 29.7 | 29.5 | 28.3 | 31.1 |
| 2 | 23.6 | 23.5 | 22.4 | 25.2 |
| 3 | 16.8 | 16.7 | 15.5 | 17.9 |
| 4 | 10.1 | 10.1 | 9.6 | 10.5 |
| 5–6 | 10.0 | 10.2 | 11.6 | 9.3 |
| 7+ | 9.6 | 9.9 | 12.4 | 5.9 |
| Unknown | 0.2 | 0.2 | 0.3 | 0.1 |
Table 3.
Percentage distribution of births in the Jerusalem Perinatal Study and its main subsets, by birthweight and main outcomes
| Subsets with information on: |
||||
|---|---|---|---|---|
| Total offspring = 100% | Total cohort (N = 92 408) |
Obstetric conditions & interventions 4/64–12/76 (N = 83 493) |
Antenatal interviews 1965–68 (N = 11 467) |
Postpartum interviews 1974–76 (N = 16 912) |
| Birthweight (g) | ||||
| <1000 | 0.6 | 0.6 | 0.3 | 0.5 |
| 1000–1499 | 0.7 | 0.7 | 0.5 | 0.7 |
| 1500–1999 | 1.3 | 1.3 | 1.2 | 1.3 |
| 2000–2499 | 4.3 | 4.3 | 3.9 | 4.5 |
| 2500–2999 | 19.1 | 19.2 | 20.2 | 20.2 |
| 3000–3499 | 41.5 | 41.6 | 41.7 | 42.7 |
| 3500–3999 | 25.2 | 25.2 | 24.8 | 24.0 |
| 4000–4499 | 6.0 | 6.0 | 6.0 | 5.2 |
| 4500+ | 0.9 | 0.9 | 0.9 | 0.7 |
| <2400 | 6.9 | 6.9 | 5.9 | 7.0 |
| Unknown | 0.5 | 0.3 | 0.7 | 0.3 |
| Birth defects | ||||
| None | 93.0 | 92.4 | 91.9 | 91.2 |
| Major | 3.7 | 4.0 | 4.6 | 4.8 |
| Minor only | 3.3 | 3.5 | 3.5 | 4.2 |
| Stillbirths/1000 | 10.0 | 10.1 | 10.2 | 7.9 |
Table 2.
Percentage distribution of births in the Jerusalem Perinatal Study and its main subsets, by education and ethnic origin of mother
| Subsets with information on: |
||||
|---|---|---|---|---|
| Total offspring = 100% | Total cohort (N = 92 408) |
Obstetric conditions & interventions 4/64 −12/76 (N = 83 493) |
Antenatal interviews 1965–68 (N = 11 467) |
Postpartum interviews 1974–76 (N = 16 912) |
| Education of mother (years) | ||||
| Unknown | 7.9 | 7.3 | 9.8 | 3.7 |
| 0 | 6.3 | 6.6 | 12.4 | 1.9 |
| 1–4 | 3.0 | 3.0 | 5.3 | 1.1 |
| 5–8 | 24.0 | 24.5 | 32.7 | 18.0 |
| 9–12 | 33.4 | 33.4 | 27.2 | 40.5 |
| 13+ | 25.5 | 25.2 | 12.3 | 34.8 |
| Birthplace of mother | ||||
| Israel | 45.3 | 44.9 | 46.8 | 54.2 |
| Other | 54.7 | 55.1 | 53.2 | 45.8 |
| Birthplace of mother’s father | ||||
| Israel | 15.8 | 13.5 | 13.9 | 16.9 |
| Other West Asia | 29.2 | 29.6 | 37.6 | 25.3 |
| North Africa | 22.5 | 23.0 | 28.0 | 20.5 |
| Europe etc | 32.5 | 31.9 | 20.5 | 37.3 |
| Religion of mother | ||||
| Jewish | 96.7 | 97.4 | 98.9 | 100.0 |
| Muslim | 2.0 | 1.6 | 0.8 | – |
| Christian & other | 0.3 | 0.2 | 0.3 | – |
Antenatal interviews, carried out from late 1965 until mid-1968, were linked to 6.5% of the babies born in 1965, and to 67.5%, 64.7% and 50.5% of those born in each of the subsequent years. Because the interviews were conducted in the free municipal antenatal clinics, they did not capture some of the better-off women, who used private obstetricians, or those (generally of lower education) who may have neglected antenatal care until later. Similarly, women under treatment for infertility, or with histories of serious gynaecological or obstetric complications, tended to bypass the municipal clinics, or were referred to special clinics at the hospitals prior to interview. Together, these biases are reflected in the slightly lower proportions of interviews available for the offspring of older mothers (Table 1) and for those who were better educated or of European origin (Table 2). Similarly, somewhat fewer antenatal interviews are available for mothers of low-birthweight offspring (Table 3).
Data on obstetric complications and maternal conditions were collected only from the three largest hospitals; however, they are broadly representative of the whole cohort. Postpartum interviews were conducted in the same hospitals and covered nearly 98% of the women delivering in these hospitals from November 1974 until December 1976. Only Hebrew-speaking Jewish women were targeted for the postpartum interview (Table 2). The lower parity of the women interviewed postpartum, their more restricted maternal ages (Table 1), higher education (Table 2), and the decreased proportions of higher birthweights and stillbirths (Table 3) are a reflection of the changes that had taken place in the population over time.
Marital status and family sets
The mothers of 97.4% of the offspring were known to be married at the time of the birth; 0.09% were currently divorced, 0.03% were widows, and 0.70% had never been married. Approximately four-fifths of the remaining 1.75% are assumed to have been married or in stable partnerships at the time of the offspring’s birth, based on information on the father. Preliminary work aggregating the data into family sets has been started, by linking siblings through the parents’ identity numbers. Table 4 shows an estimate of the sizes of family sets available for study in the cohort, together with some characteristics. Actual families are larger, as they may include offspring born before 1964 or after 1976.
Table 4.
Distribution of traced mothers by number of offspring and selected outcomes in mothers and offspring
| Observed offspring per mother | 1 | 2 | 3 | 4 | 5 | 6 | 7+ | Total |
| Number of mothers | 16 083 | 12 319 | 6 908 | 3 201 | 1397 | 652 | 494 | 41 054 |
| Number of offspring in cohort | 16 083 | 24 638 | 20 724 | 12 804 | 6985 | 3912 | 3730 | 88 876a |
| Percentage of families that include: | ||||||||
| 1+ offspring with major malformation | 3.6 | 6.2 | 10.0 | 13.7 | 16.2 | 19.3 | 23.1 | 7.2 |
| 1+ offspring with birthweight <2500 g | 6.1 | 10.2 | 15.8 | 21.2 | 22.7 | 26.1 | 27.3 | 8.9 |
| 1+ offspring diagnosed with cancer | 1.0 | 2.0 | 3.2 | 3.8 | 5.7 | 6.6 | 5.5 | 2.2 |
| 1+ liveborn offspring who have died | 2.5 | 5.0 | 10.0 | 14.5 | 19.8 | 21.8 | 28.9 | 6.1 |
Not including offspring whose mothers have not been traced.
Ethnic groups, countries of origin and consanguinity
Information on the birth certificate included the country of birth of the mother and father, and of each grandfather. Table 5 shows the numbers of offspring according to the specific country of birth of the paternal grandfather, together with an estimate of the numbers of fathers. Many of the immigrant parents were refugees, with approximately one-third having originated from Western Asia, North Africa or, directly or indirectly, from Europe. Note that origins in the Americas, Australasia, sub-Saharan Africa and the Far East are usually included with Europeans, because more than half of these trace their ancestry to Europe. The largest groups, apart from the second-generation Israeli-born offspring, had origins either in Morocco or in Iraq. The latter group, in Jerusalem, included many Jews of Kurdish origin.
Table 5.
Observed numbers of offspring and estimated numbers of fathers, by origin of offspring’s paternal grandfather
| Offspring (N = 92 408) |
Fathers (N = 42 724) |
Birthplace of offspring’s paternal grandfather |
|---|---|---|
| 14 862 | 6516 | Israel |
| Other Western Asia | ||
| 2 858 | 1322 | Turkey, Cyprus |
| 1 567 | 743 | Syria, Lebanon |
| 22 | 9 | Jordan, Saudi Arabia |
| 12 279 | 5565 | Iraq |
| 2 807 | 1300 | Yemen, Aden |
| 6 138 | 2780 | Iran |
| 399 | 185 | Afghanistan |
| 573 | 256 | India & South Asia |
| North Africa | ||
| 14 607 | 6457 | Morocco |
| 810 | 386 | Algeria |
| 1 282 | 609 | Tunisia |
| 246 | 124 | Libya |
| 818 | 440 | Egypt, Sudan |
| Europe etc | ||
| 4 992 | 2782 | Former USSR |
| 8 427 | 4531 | Poland |
| 3 294 | 1755 | Romania |
| 2 503 | 1132 | Hungary |
| 1 179 | 668 | Balkan States, Greece |
| 1 331 | 649 | Czechoslovakia |
| 2 293 | 1208 | Germany |
| 791 | 402 | Austria, Switzerland |
| 1 616 | 867 | Other Western Europe |
| 1 840 | 927 | North & South America, Australasia |
| 165 | 83 | Sub-Saharan Africa |
| 42 | 29 | Eastern Asia |
| 4 562 | 995 | Unknown place of birth |
There were marked differences in education, occupation and family size (offspring birth order) between parents born in the Islamic countries of Western Asia or North Africa and those born in Israel or Europe etc (data not shown). Among Israeli-born parents, such differences, while still present, were small. Table 6 shows information on consanguinity and ethnic differences or similarities between the offspring′s grandparents. Consanguinity between parents was common for offspring whose parents were born in Islamic countries (Table 6), and was strongly correlated with the parents′ social class and education.16 Fried and Davies17 reported an excess of sub-optimal pregnancy outcomes in association with uncle–niece marriages.
Table 6.
Distribution of offspring, prevalence of parental consanguinity and similarity of grandfathers’ countries of birth, by religion and by birthplace of mother and maternal grandfather (Data based on 11 467 antenatal interviews, extrapolated to other births in 1964–76 to the same parents)
| Religion & place of birth of mother |
Non-Jewish Mothers |
Jewish mothers born in Israel |
Jewish mothers born abroad |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Place of birth of Maternal grandfather |
Israel | West Asia |
North Africa |
Europe etc |
West Asia |
North Africa |
Europe etc |
Total, including unknown |
|
| Number of Offspring = 100% |
360 | 3759 | 3155 | 253 | 3036 | 8330 | 8249 | 2698 | 29 870 |
| Known to be Consanguineous |
63.9 | 2.9 | 6.7 | 2.8 | 1.6 | 17.9 | 10.5 | 1.2 | 10.0 |
| Not known to be consanguineous, country of birth of the two grandfathers is: | |||||||||
| Same | 31.9 | 46.2 | 28.4 | 13.4 | 22.1 | 54.8 | 62.3 | 27.6 | 46.9 |
| Different | 4.2 | 50.9 | 64.9 | 83.8 | 76.3 | 27.3 | 27.2 | 71.2 | 43.1 |
Occupation and social class
Occupations of parents reflected the economic base of Jerusalem in service, government and institutions of learning. Typical occupations of women included housewives (59.0% of offspring’s mothers), teachers (9.7%), secretaries (6.7%), clerks (6.6%) or domestic servants (2.4%). Compared with women employed outside the home, housewives tended to be of somewhat lower education than average with mid-or lower-status husbands. For men, occupations included clerical and managerial (14.8% of offspring’s fathers), rabbis/students in Talmud academies (12.1%), free professions (11.4%), unspecified unskilled labourers (9.3%), teachers (4.1%), and workers in transport and communications (7.5%), construction (6.9%), crafts (6.4%), agriculture (4.0%) or light industries (1.7%). There was no national classification of social class available when we started our study. A prestige-based scale was developed for Israel in 198118 but cannot be used for our data as it relies on a later system of coding occupations. We devised our own six-point scale by ranking the occupations of fathers according to their infants’ crude rates of hospital admissions, in 1966–68, for gastroenteritis.10 In recent studies, we found this scale to predict subsequent schizophrenia in offspring19 and long-term mortality in mothers.20 Earlier work had suggested effects of social class on specific complications of pregnancy and labour,21 and our scale was used as a covariate in studies of birthweight,22–24 adolescent maturation15 and cognitive ability.13 Other studies used the municipal tax level of the census tract as an indicator of socio-economic status.25
Our own scale had the advantage of allowing us to classify occupational groups peculiar to Jerusalem, such as students in Talmud academies, in a dimension that differed from education and ethnic group. On the other hand, it will be inappropriate to use it to control for social class in research that investigates the long-term consequences of infant gastroenteritis in the cohort born in 1966–68. We have therefore recently devised alternative scales that rank the 108 paternal and 81 maternal occupations according to mean education; studies are in progress to assess how these scales predict long-term outcomes in parents and offspring.
Orthodox Jewish religious observance
The population of Jerusalem includes a high proportion of ultra-orthodox Jews. The proportion of offspring with fervently orthodox parents, indicated by the fathers being rabbis or students in Talmudic academies (i.e.yeshivot), increased from under 9% in 1964 to 19% in 1976, these accounting for 60% of social class 2. At that time, the most fervently orthodox were mainly of European origin. In the sub-cohort interviewed in 1965–68, 84% of mothers reported keeping kosher at home, i.e. observing orthodox rules of nutrition, food preparation and hygiene, and 60% observed ritual monthly sexual separation (nidah). In the sub-cohort interviewed in 1975–76, the proportion observing nidah in the month of conception was 49%. Orthodox observance was correlated with early marriage, high fertility by choice, differences in contraceptive practice26 and ease of conception.27 Others have found the orthodox to have lower mortality.28
We studied 3586 women who observed nidah to investigate the timing of conception in relation to an estimate of the day of ovulation. Late conceptions were associated with an excess of males,29 unlike-sexed twins30 and Down’s syndrome (unpublished data).
Smoking
Mothers’ smoking was recorded in the interviews in 1965–68 and 1974–76, but data on alcohol and drugs were not sought; others have documented their relative rarity, compared with Western Europe or the US.31–33 Fathers of 44% of offspring were currently smoking in 1974–76, as reported by their wives, with 31% using one or more packs per day at the time of the birth, and 7% using two or more packs. Among mothers, smoking during pregnancy increased from 9% in the first sub-cohort to 14% in the second, with 0.6% and 1.9% respectively reporting that they smoked more than 20 cigarettes per day. Some 1.1% of mothers (8.6% of smokers) quit during pregnancy. Women who smoked were less educated, had husbands of lower social class, and were less religiously observant than non-smokers.23 Independently of these, smoking was associated with reduced birthweight; however, it had little effect on maternal weight gain or perinatal mortality.23 Effects of smoking on complications of pregnancy and labour were similar to others.21 Our study provided one of the earliest reports of adverse effects of passive smoking when we showed that infants whose mothers smoked were more likely to be admitted to hospital, specifically for respiratory illnesses.34 Smokers’ offspring were also injured more in infancy.34
Contraception
Data on contraceptives ever used, and on use since any previous pregnancy, were obtained at the postpartum interview in 1975–76. Barrier methods and oral contraceptives had been used less than in the UK or the US, whereas intrauterine devices were used more. Approximately half of the users relied on male methods (mainly withdrawal), and a quarter had used the pill. Religious observance was strongly related to the use of any method, and to the type of method used.26 Oral contraception was used by better-educated couples, the husband’s education being even more strongly related to the choice of this method than the wife’s.26
This subset of the study was intended to evaluate the safety of oral contraceptives in relation to subsequent births. In common with results from studies in other countries, our cohort showed no significant differences in the frequencies of obstetric complications among former oral contraceptive users and non-users21 and only minor subtle differences following oral contraceptive failures.35 Similarly, infants born to women who used oral contraceptives prior to conception were similar to others in birth defects, physical growth, haematological outcomes, and psychometric scores up to age 3.36 Multiple births were also studied among former oral contraceptive users; findings were similar to previous studies which had shown a slightly lower incidence of multiple births among users. Former users of oral contraceptives who were underweight at the time of conception had significantly fewer multiple births,37 and their offspring had more pigmented skin lesions reported at birth.38
This cohort was also used to compare conception-waits among former oral contraceptive users and women who had used other forms of birth control.39 Our data, though retrospective, suggested that women who stopped taking the pill and who achieved a pregnancy had reached the same levels of fecundability within 2–3 months as women who had stopped using other forms of birth control. Although there were effects of age and duration of use of the pill, there was no excess of long conception-waits in the group who used the pill.
Abortion
Induced abortion, though illegal throughout most of the period of the study, was more widely tolerated than in Western Europe or the US. Abortions were easily obtained from private gynaecologists, as many had been trained in Eastern European countries where abortion was legal. ‘Backstreet’ abortions were unknown. In the 1970s, abortion was made legal, subject to approval by a committee; however, political pressure from conservative groups quickly led to many restrictions. Our studies showed that abortion remained readily available from private gynaecologists for women who were ineligible for legal procedures.40 In a climate of intense debate about potential effects of abortion on future childbearing, we undertook a series of studies, with funding from the World Health Organisation.
Among women interviewed antenatally in 1965–68, previous abortion was reported by 3% of nulliparous women and 12.1% of those pregnant with their fourth child. It was most common among Jewish immigrants from North Africa and among older women, and was reported least by Arab women and those who had married young, were religiously observant, or were non-smokers.41 Women interviewed antenatally who reported previous induced abortions also tended to have reported more first-trimester bleeding; subsequently, we observed them to have more adverse pregnancy outcomes, including low birthweight, major and minor congenital malformations, and early and late neonatal deaths.42 Risks of adverse outcomes associated with previous induced abortion were studied in greater detail using the 16 648 women with singleton births interviewed retrospectively in 1974–76. Again, we found abortion to be associated with first-trimester bleeding and with low birthweight.43
Complications of pregnancy and interventions in labour
Maternal conditions recorded in the women delivering in the three largest hospitals included pre-existing heart disease (0.43%) and a low prevalence of diabetes (reported below). Some 0.23% and 0.36% of the new-borns had haemolytic disease due to Rhesus or ABO incompatibility, respectively; 0.14% had exchange transfusions. Placental abruption, placenta praevia and premature rupture of membranes were recorded for 0.4%, 0.4% and 3.7% of mothers respectively. There were 2.5% of deliveries assisted with forceps and 4.3% with vacuum extraction. Caesarian deliveries (5.2% overall) increased from 2.8% in 1964 to 6.8% in 1976. Demographic differences in the incidence of obstetric intervention were reported for the deliveries in 1964– 66,44 and risk factors for complications were summarised for those in 1975–76.21
Degrees of vomiting in each of the first 4 months of pregnancy were reported in the antenatal interview in 1965–68. More than half of the mothers vomited in each of the first 3 months (11–12% severely) and about 30% in the fourth month (7% of them severely). Vomiting was increased in multifetal pregnancies and decreased with maternal age and parity; it was more marked in women with ancestries in North Africa or West Asia.45
Pre-eclampsia
Women with hypertension in pregnancy were recruited to a special research clinic in 1964–66 and were followed until 3 months postpartum, when a definitive diagnosis was made. The study revealed pre-eclampsia to be rare (<2%) relative to many developing countries,46 the lowest incidence being among immigrant Jews from North Africa (1.58%) and the highest among Moslem Arabs (3.4%).47 A case–control comparison of diets showed women with pre-eclampsia to have an increased intake of sugar and sweets and fewer proteins and fats compared with matched controls; differences were thought to be a consequence of the disorder, rather than its cause.48 Later studies found a relationship of pre-eclampsia with blood groups,49 no association with use of oral contraceptives21 and a negative association with previous abortion.50 Recent analyses have revealed age of husband related to this complication,51 defined other risk factors for preeclampsia and shown their effects in nulliparae to be similar to those seen in multiparae.52 The incidence of pre-eclampsia in multiparae decreased over the 13 years, probably due to birth control.52 Our long-term follow-up studies have shown pre-eclampsia to predict mortality, especially from cardiovascular disease20 and to predict a variety of cancers.53
Birth defects
Summaries of overall proportions of birth defects were reported for offspring born in 1964–6854 and 1974–76.35 Neural tube defects were rare in this population, but frequencies of most other malformations were similar to those reported in other similar studies. Down’s syndrome was notably different, being more prevalent at birth in Jerusalem than in any other population, this high prevalence being seen in offspring of mothers of all ages55 but restricted to those born to mothers with ancestries in North Africa and Western Asia.56 There was a strong seasonal variation in Down’s syndrome, spring and autumn births being at greatest risk.57 Other malformations, particularly cardiovascular defects, were more common in offspring of immigrants from West Asia and in Arabs.54 Birth defects were reported in relation to exogenous sex hormones,58 induction of ovulation59 and oral contraception.35,38
Birthweight and gestational age
The distribution of birthweight was similar to that in other Caucasian populations (Table 3) although some determinants of birthweight differed from other studies. The proportion of live births born before 37 weeks was 7.9%; mean birthweight was 3217 g, and 6.6% of live births weighed <2500 g. An earlier report suggested unusually high birthweights in offspring of immigrants from North Africa.60 This was confirmed in the Jerusalem cohort and shown to be independent of maternal height, pre-pregnant relative weight, parity or social class,10,23 but related to the mother’s age at immigration to Israel, suggesting that some environmental difference between Israel and the country of origin during the mother’s early life was affecting birthweight of her offspring.22 Social class was unrelated to birthweight, after adjusting for maternal height.22 Mean birthweight and low birthweight showed no systematic change over the 13 years.
BMI and diabetes
The mothers had considerably lower body mass indices (BMI) than those seen today in the UK or the US. Median pre-pregnant BMI in 1975–76 was 21, with 12.4% of mothers being overweight (BMI = 25+) and 1% being obese (BMI = 30+). Diabetes was also markedly less common in the Jerusalem population than in developed countries at the same time. According to the data copied from labour-ward logs in the three largest hospitals, some 0.23% had pre-existing diabetes (presumed to be mainly insulin-dependent) and 0.55% had gestational diabetes. Of the women interviewed in 1965–68 in the low-risk municipal well-women clinics, 0.49% reported that they had diabetes and 17% knew of a blood relative with diabetes, including the interviewed woman’s sibling (1.2%), her mother (7.0%), father (4.4%), or aunt/uncle (5.5%). As expected, follow-up studies have shown diabetes to be a strong risk factor for subsequent death of the mother.20
Breast feeding
Information on the initiation and duration of breast feeding in the early and mid-1970s was provided by 8486 mothers interviewed in 1974–76, the data relating to the previous birth, if any.25 More than 79% had initiated breast feeding after a live birth. It was less frequently initiated by Israeli-born women, smokers, after a caesarian delivery or after birthweight <2500 g. It was not independently related to maternal age, birth order, social class or age at marriage, after controlling for other variables. Among those who chose to breast feed, almost half continued for 3 months or more. Longer durations were reported by older, more-educated women and by those whose husbands were rabbis or students in Talmudic academies. Smokers and Israeliborn women reported shorter durations.25 Work outside the home was not related to the initiation or duration of breast feeding, after controlling for confounders.
Changes over time
Although the numbers of births tended to increase each year (Table 1), they decreased in 1966–67, during a period of economic depression, and in 1972, following an epidemic of rubella. During these times, the decreases were most obvious in births to the moreeducated parents. The study occurred during a period of extraordinarily rapid demographic transition.6 As the pool of less-educated Immigrants became too old to reproduce, they were replaced by younger, better-educated Israeli-born couples. For example, the proportion of offspring whose mothers were born in the Islamic countries of Western Asia and North Africa decreased from 26% and 23% respectively in 1964, to 11% and 15% in 1976, while the proportion with mothers born and educated in Israel increased from 37% in 1964 to 55%in 1976. Family size of the newborns decreased: some 28% were fifth-born or later in 1964, compared with only 15% in 1976. Over time, more births were to mothers aged 20–39 years, with the proportion aged under 20 years falling from 4.4% in 1964 to 3.3% in 1976 and the proportion aged 40 years or more decreasing from 3.5% to 2.5%. These changes were typical of those occurring throughout Israel.
Follow-up studies based on examination by the military
In the late 1980s, Seidman and colleagues linked part of the cohort to records of medical examinations done at age 17. All Jewish males are required to undergo this examination, to determine eligibility for compulsory military service. Females may be exempted if they are religiously observant, so that the proportion of females who undergo medical examinations is lower than that for males. The outcomes studied by Seidman’s group included height,61,62 weight,63–65 blood pressure,64,66,67 asthma68 and a summary measure of cognitive ability.66,69–72 These were investigated in relation to pre-eclampsia,66 birthweight,61,63,64,67,68,71 small-for-dates,62,72,73 forceps and vacuum deliveries,70 and macrosomia in the offspring of diabetic mothers.65 In one of the hospitals in 1975–76, plasma bilirubin levels had been recorded systematically in newborns with jaundice: these were studied in relation to cognitive ability at age 17.69,74
The Israel Defence Force is currently allowing researchers in Israel to study data on anthropometry and blood pressure for 81% of our cohort’s males and 52% of the females. Malaspina’s group linked the cohort to more detailed measure of cognitive assessment at age 17. They showed independent effects of maternal and paternal ages on offspring IQ scores, with specific effects of late paternal age on the scores for PRM-R (Progressive Raven’s Matrices-Revised) and Arithmetic.75
Other follow-up studies of teenagers
A sub-cohort was followed up at age 18–19 years by tracing, visits to the home, and interviews with the teenager and the mother. This study of 1005 subjects born to mothers interviewed antenatally in 1965–68 evaluated the safety of medroxyprogesterone acetate (MPA), a hormone used in Israel for pregnancy maintenance and used in other countries as an injectable long-acting contraceptive. A few minor differences were found comparing MPA-exposed and non-exposed offspring, most being due to the more favourable distribution of social variables in the exposed group. The study provided data on the prevalence of various health conditions in teenagers, on factors associated with puberty, and on gender-dimorphic measures of cognitive ability, aggression and behaviour in school.13–15,76
Tracing in 2000 and 2005
To allow the database to be linked with Israeli disease registries, we traced the offspring and mothers in 2000 and 2005 and the fathers in 2005. For the 91 459 offspring born alive, we matched the identity number (ID) and date of birth to Israel’s Population Registry, tracing 98.5% and obtaining the date of death or current address of all 90 079 with active files in the registry. The remainder, for which the law allows no information, are presumed to be emigrants or individuals assigned new identities (e.g. after adoption). Of the 1380 individuals remaining untraced, 47% were from out-of-wedlock births, therefore likely to have been adopted. Among liveborn offspring, the proportions of male and female offspring traced were 98.6% and 98.3%, respectively, and were close to 98% in almost all demographic subgroups including those with birthweight <2500 g (97.5%) or <1000 g (99.0%). Tracing was less complete, however, for first-born offspring (97.1%) and those whose mothers were aged <20 years (90.5%), Muslim (96.1%) or Christian (73.8%); the last of these are likely to have included tourists and exchange students.
At the time of writing, we have traced 96.9% of the mothers and 93.6% of the fathers of the cohort’s 92 408 births. For offspring whose parents were married at the time of their birth, 97.6% of mothers and 94.8% of fathers were traced by January 2005. Tracing was less complete for the parents of offspring who died in infancy or who had low birthweight, or those whose spouses were very young or elderly, unmarried or not Jewish. Tracing of offspring and parents was easiest for the most recent births: for the cohort born in 1976, 99.9% of liveborn offspring with married parents were traced, with 98.5% of their mothers and 96.4% of their fathers. For the cohort of 1964, these proportions were 97.3%, 94.7% and 91.8% respectively.
Tracing of the parents has been more difficult than that of the offspring. We had recorded only the first three letters of the family name and made frequent errors in transcribing the mother’s identity numbers. For 1.6% of the births, including many stillbirths, no maternal identity number was given; moreover, we could not distinguish between Israeli numbers and foreign passport numbers. The Population Registry has no record of stillbirths, and may be less complete in the early years of the study with regard to early neonatal deaths. Parents in some ultra-religious sects did not recognise the State of Israel, so presented their foreign passports when registering the birth, instead of their identity card. We had recorded 44 070 individual identity numbers for mothers; however, we have found a few to be the numbers for fathers, for which we had no rubric. Consequently, the total number of individual fathers and mothers is unknown.
Long-term follow-up studies
Among those traced, outcomes studied in offspring over the 24–36 years since their birth include mortality,24 haematological77,78 and other78 malignancies; schizophrenia and other major psychiatric disease;19,79,80 and cognitive outcomes.75 In traced mothers, the long-term studies include malignancies53,78 and mortality.20 In offspring, we have found lower birthweight to be a predictor of increased deaths of young adult males.24 In contrast, higher birthweights were a predictor of acute myeloid leukaemia in children and young adults, especially females; similarly, birthweights of siblings predicted this outcome as well as lymphatic leukaemia.77 The birth of one or more offspring weighing <2500 g was a risk factor for subsequent mortality in the mothers, independent of pre-eclampsia.20 The latter complication was also associated with an increase in the mothers’ mortality, especially from cardiovascular disease, after more than two decades of follow-up.20 An unexpected finding was that pre-eclampsia was associated with an increased incidence of several malignancies;53 studies by others have suggested that this complication of pregnancy is associated with no change in cancer incidence, or a reduced risk of cancer.
In separate studies, the cohort of offspring was linked to data from Israel’s Psychiatric Registry, and an anonymous database used for studies of hospitalised cases of schizophrenia and of other conditions. The incidence of schizophrenia was 0.96% by age 34,19 i.e. similar to the incidence recorded in most other countries. We showed, for the first time, that paternal age was a strong risk factor for this outcome, accounting for 25% of cases of schizophrenia in this cohort.19 This finding, since replicated by many other research groups, prompted our further studies of paternal age in the cohort; these include pre-eclampsia,51 cognitive75 and behavioural outcomes and miscarriages.81 Other studies for this cohort include those on offspring admissions to hospital,9,82 child injuries and neglect,83 consequences of early gestational bleeding84 and coitus in late pregnancy.85
Discussion
Features of the Jerusalem Cohort include the wealth of available data from birth, the completeness of follow-up over 27–40 years, the varied ethnic origins and aggregation into families. These allow the cohort to be used currently for research in long-term studies of mortality, cancer and psychiatric disease, with the outcomes related to events at birth or to intergenerational effects. While the core data are population-based and the postnatal interviews are broadly representative of the population, information from the antenatal interviews is more limited. Nevertheless, the presence of core data on those not interviewed allows an assessment of possible biases.
An added feature is the unusual exposure to war, stress and famine in the past. The State of Israel was established in 1948 and over the next few years absorbed large numbers of refugees. Those from Europe included survivors of the Second World War and the Nazi camps for mass extermination. Those from Islamic countries included survivors of pogroms, riots and other violence associated with the wars of 1948 and 1956.86 Mass immigration to Israel of refugees from Iraq and neighbouring Islamic countries in 1949–51 led to housing many families in tent cities, while the refugees from Morocco and nearby countries arrived in the early 1960s and were often accommodated in asbestos huts, sometimes for several years. Some of these privations and stresses were shared by the native population of Israel; for example, Western Jerusalem came under siege and experienced a 6-month famine in 1948, during the first Arab–Israeli war.
The population of Israel is also known for some interesting genetic characteristics, with numerous founder mutations or polymorphisms identified in Jews originating from different areas (reviewed by Zlotogora et al.,87 Ostrer88); these have given great insights into disease. In cancer, notable examples include the genes for BRCA1, BRCA2 (i.e. Fanconi D1), Fanconi C, BLM and APC, each of which is associated with an unusual incidence of cancer in Jews of European (Ashkenazi) origin, relative to non-Jews living in similar environments in Western Europe, Australia or the US.89 In other fields, notable polymorphisms exist in CYP21A2 (steroid hormone synthesis, carried by 1/3); F11 (coagulation Factor-X1, carried by 1/20); and for the lysozymal enzymes GBA, HEXA and SMPD1 (for Gaucher’s, Tay-Sachs and Niemann-Pick diseases, carried by 1/13, 1/25 and 1/90 Ashkenazi Jews respectively). Numerous other founder mutations are known for Ashkenazim.87,88
In Jews who are not Ashkenazim, specific patterns of other diseases are known, many founder mutations identified or unique haplotypes linked to disease.87,88 Descendents of families who were expelled from the Iberian Peninsula in 1492 are known as ‘Sephardim’ (i.e. from Spain). This term usually includes all Jews from North Africa and is often applied generally to all Jews who are not Ashkenazim; however, those from Western Asia are also known as ‘Mizrahim’ (i.e. Eastern). The groups were separated from each other for many centuries, and subgroups from countries such as Afghanistan and Iran have in many senses been ‘genetic isolates’.90 The history of migrations is known for many, confirmed by genetic studies88 or inferred from, for example, Y chromosome haplotypes.91 Notable Mendelian disorders in specific Sephardic communities include familial mediterranean fever (MEFV, carried by 1:5–1:13 depending on country of origin), ataxia telangiectasia (AT, 1:80 from Morocco), limb girdle muscular dystrophy (DYSF, 1:10 from Libya) and fragile X syndrome (FMR1). For Mizrahi communities, prevalent disorders include the X-linked glucose-6-phosphate deficiency (G6PD, 1:2– 1:7), pseudocholinesterase deficiency (BCHE, 1:9–1:11), Dubin–Johnson syndrome (1:20 from Iran) and various haemoglobinopathies.87
Regarding risk factors for chronic disease, numerous studies in Israel have established that there are differences between groups defined by ethnicity, religion or education for smoking,92–96 alcohol,31,97–99 diet,92,100–105 exercise,106 stature, weight and BMI,107–110 and diabetes.111 In the cancer field, some limited information is available on the prevalence of mutations in genes such as Fanconi A112 or RET (MEN2),113 or polymorphisms in the p53 gene (TP53114,115), the androgen receptor116 and MTHFR.117 Also, there are limited reports for the prevalence of specific mutations in tumours, tumour subtypes or polymorphisms in other genes as modifiers of genetic risk.118,119 However, in contrast to a strong body of literature relevant to the genetics of cardiovascular disease in Israel,87,107,120–123 very little information is available yet on common genetic variants likely to modify the risk of cancer (e.g. variants of metabolic enzymes or genes contributing to DNA repair pathways or to radiation sensitivity).
There are unusual opportunities for epidemiological research in Israel. There is universal health insurance. Every resident, newborn and immigrant has a unique identity number, as in the Scandinavian countries; this number is used for personal documents, administrative data such as births and deaths, as well as for medical records. Because of compulsory military service, the authorities keep an up-to-date record of the whereabouts of Jewish teenagers; and for most of the males who do serve, this surveillance continues for many years. These, together with well-managed census records, facilitate the tracing and ascertainment of vital status. Data management systems in most hospitals are sophisticated, and there are growing numbers of disease registries. Some, such as the registries of cancer and psychiatric hospitalisations mentioned here, both of which have existed for many years, are managed from within the Ministry of Health. Others came into existence more recently and are managed from epidemiological units in the university teaching hospitals, under contracts with the Ministry of Health or the sick funds.124 Yet others, such as the registers of diabetes,125 multiple sclerosis126 and blindness, rely mainly on non-governmental sources of support.
Conclusions
The Jerusalem Perinatal Study is a unique cohort. The linkage of offspring and mothers provides a wealth of data on demographic and social characteristics, pregnancy and obstetric outcomes. Data on fathers allow us to investigate the role of the male in reproduction. The cohort serves as a source of data for follow-up studies, especially as applied to the fetal and neonatal origins of adult diseases, and to associations between obstetric outcomes and the development of chronic diseases in later life.
Acknowledgements
Supported by grants from the National Institutes of Health: 1R01 CA080197 (S.H.); 1R01 MH059114 (D.M.); 2K24 MH001699 (D.M.); 1K07 CA090685 (MBT); and from the National Alliance for Research on Schizophrenia and Depression (S.H., D.M.). We thank the subjects born in Jerusalem, and their parents.
References
- 1.Brzezinski A, Davies AM, Serr DM, Koren Z. [Toxemias of pregnancy among the different communities of Israel.] Harefuah. 1964;67:105–107. [PubMed] [Google Scholar]
- 2.Butler NR, Bonham DG. Perinatal Mortality: The First Report of the 1958 British Perinatal Mortality Survey. Edinburgh: Livingston; 1963. [Google Scholar]
- 3.Golding J, Porter C. National cohort studies – the facts about Britain’s children. Health Visitor. 1982;55:639–643. [PubMed] [Google Scholar]
- 4.Power C. A review of child health in the 1958 birth cohort: National Child Development Study. Paediatric and Perinatal Epidemiology. 1992;6:81–110. doi: 10.1111/j.1365-3016.1992.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 5.Niswander KR, Gordon M. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke. Washington, DC: US Department of Health Education and Welfare, Public Health Service, National Institutes of Health; 1972. The Women and Their Pregnancies; pp. 73–379. DHEW Publication No. (NIH) [Google Scholar]
- 6.Davies AM. Demography, morbidity and mortality in Israel: changes over 30 years. Israel Journal of Medical Science. 1979;15:959–964. [PubMed] [Google Scholar]
- 7.Harlap S, Prywes R, Grover NB, Davies AM. Fertility and perinatal and infant mortality in the Jewish population of Beersheba and the Negev, 1972. Israel Journal of Medical Science. 1976;12:1418–1431. [PubMed] [Google Scholar]
- 8.Harlap S, Prywes R, Grover NB, Davies AM. Maternal, perinatal and infant health in Bedouin and Jews in southern Israel. Israel Journal of Medical Science. 1977;13:514–528. [PubMed] [Google Scholar]
- 9.Winter ST, Davies AM, Harlap S. Scoring newborns for the risk of subsequent hospital admissions: comparative findings in Jerusalem and Haifa. Israel Journal of Medical Science. 1981;17:399–402. [PubMed] [Google Scholar]
- 10.Harlap S, Davies AM, Grover NB, Prywes R. The Jerusalem Perinatal Study: the first decade 1964–73. Israel Journal of Medical Science. 1977;13:1073–1091. [PubMed] [Google Scholar]
- 11.Davies AM, Tzur B, Prywes R, Weiskopf P. The Jerusalem Perinatal Study Methodology Manual. Jerusalem: Department of Medical Ecology, Hebrew University of Jerusalem – Hadassah Medical School; 1968. [Google Scholar]
- 12.Davies AM, Prywes R, Tzur B, Weiskopf P, Sterk VV. The Jerusalem Perinatal Study: 1. Design and organization of a continuing, community-based, record-linked survey. Israel Journal of Medical Science. 1969;5:1095–1106. [PubMed] [Google Scholar]
- 13.Jaffe B, Harlap S, Baras M, Gordon L, Lieblich A, Magidor S, et al. Long-term effects of MPA on human progeny: intellectual development. Contraception. 1988;37:607–619. doi: 10.1016/0010-7824(88)90007-8. [DOI] [PubMed] [Google Scholar]
- 14.Jaffe B, Shye D, Harlap S, Baras M, Lieblich A. Aggression, physical activity levels and sex role identity in teenagers exposed in utero to MPA. Contraception. 1989;40:351–363. doi: 10.1016/0010-7824(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe B, Shye D, Harlap S, Baras M, Belmaker E, Gordon L, et al. Health, growth and sexual development of teenagers exposed in utero to medroxyprogesterone acetate. Paediatric and Perinatal Epidemiology. 1990;4:184–195. doi: 10.1111/j.1365-3016.1990.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen T, Vardi-Saliternik R, Friedlander Y. Consanguinity, intracommunity and intercommunity marriages in a population sample of Israeli Jews. Annals of Human Biology. 2004;31:38–48. doi: 10.1080/0301446032000159255. [DOI] [PubMed] [Google Scholar]
- 17.Fried K, Davies AM. Some effects on the offspring of uncle-niece marriage in the Moroccan Jewish community in Jerusalem. American Journal of Human Genetics. 1974;26:65–72. [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus V. Perception of the occupational structures in Israel. Megamot. 1981;26:283–294. [Google Scholar]
- 19.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, et al. Advancing paternal age and the risk of schizophrenia. Archives of General Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 20.Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, et al. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–215. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- 21.Harlap S, Davies AM, Baras M. Complications of pregnancy and labor in former oral contraceptive users. Contraception. 1981;24:1–13. doi: 10.1016/0010-7824(81)90064-0. [DOI] [PubMed] [Google Scholar]
- 22.Yudkin PL, Harlap S, Baras M. High birthweight in an ethnic group of low socioeconomic status. British Journal of Obstetrics and Gynaecology. 1983;90:291–296. doi: 10.1111/j.1471-0528.1983.tb08912.x. [DOI] [PubMed] [Google Scholar]
- 23.Rush D, Cassano P, Harlap S. Perinatal outcome, maternal weight gain, cigarette smoking and social status in Jerusalem. Revue d’Epidémiologie et de Santé Publique. 1988;36:186–195. [PubMed] [Google Scholar]
- 24.Friedlander Y, Paltiel O, Deutsch L, Knaanie A, Massalha S, Tiram E, et al. Birthweight and relationship with infant, child and adult mortality in the Jerusalem perinatal study. Paediatric and Perinatal Epidemiology. 2003;17:398–406. doi: 10.1046/j.1365-3016.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- 25.Ever-Hadani P, Seidman DS, Manor O, Harlap S. Breast feeding in Israel: maternal factors associated with choice and duration. Journal of Epidemiology and Community Health. 1994;48:281–285. doi: 10.1136/jech.48.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harlap S. Contraceptive use by Jerusalem mothers with special reference to orthodoxy, ethnic group and husband’s and wife’s education. Jewish Population Studies. 1977;12:329–339. [Google Scholar]
- 27.Harlap S, Baras M, Slater PE. Conception-waits in Jerusalem Jewesses. Human Fertility Factors. 1981;103:409–419. [Google Scholar]
- 28.Kark JD, Shemi G, Friedlander Y, Martin O, Manor O, Blondheim SH. Does religious observance promote health? Mortality in secular vs religious kibbutzim in Israel. American Journal of Public Health. 1996;86:341–346. doi: 10.2105/ajph.86.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harlap S. Gender of infants conceived on different days of the menstrual cycle. New England Journal of Medicine. 1979;300:1445–1448. doi: 10.1056/NEJM197906283002601. [DOI] [PubMed] [Google Scholar]
- 30.Harlap S, Shahar S, Baras M. Overripe ova and twinning. American Journal of Human Genetics. 1985;37:1206–1215. [PMC free article] [PubMed] [Google Scholar]
- 31.Baras M, Harlap S, Eisenberg S. Alcohol drinking in Jerusalem. Alcohol. 1984;1:435–439. doi: 10.1016/0741-8329(84)90018-1. [DOI] [PubMed] [Google Scholar]
- 32.Kandel D. Substance abuse by adolescents in Israel and France: a cross-cultural perspective. Public Health Reports. 1984;99:277–283. [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein L, Tamir A. Health-related behavior of adolescents: change over time. Journal of Adolescent Health Care. 1984;5:91–95. doi: 10.1016/s0197-0070(84)80005-4. [DOI] [PubMed] [Google Scholar]
- 34.Harlap S, Davies AM. Infant admissions to hospital and maternal smoking. Lancet. 1974;1:529–532. doi: 10.1016/s0140-6736(74)92714-7. [DOI] [PubMed] [Google Scholar]
- 35.Harlap S, Eldor J. Births following oral contraceptive failures. Obstetrics and Gynecology. 1980;55:447–452. [PubMed] [Google Scholar]
- 36.Magidor S, Palti H, Harlap S, Baras M. Long-term follow-up of children whose mothers used oral contraceptives prior to conception. Contraception. 1984;29:203–214. doi: 10.1016/s0010-7824(84)80001-3. [DOI] [PubMed] [Google Scholar]
- 37.Harlap S. Multiple births in former oral contraceptive users. British Journal of Obstetrics and Gynaecology. 1979;86:557–562. doi: 10.1111/j.1471-0528.1979.tb10809.x. [DOI] [PubMed] [Google Scholar]
- 38.Harlap S. Pigmented skin lesions in babies born to underweight former oral-contraceptive users. Lancet. 1978;2:39. doi: 10.1016/s0140-6736(78)91342-9. [DOI] [PubMed] [Google Scholar]
- 39.Harlap S, Baras M. Conception-waits in fertile women after stopping oral contraceptives. International Journal of Fertility. 1984;29:73–80. [PubMed] [Google Scholar]
- 40.Slater PE, Weiner D, Davies AM. Requests for abortion and outcomes of pregnancy in Jerusalem, Israel. Journal of Reproductive Medicine. 1978;21:279–282. [PubMed] [Google Scholar]
- 41.Harlap S, Davies AM. Characteristics of pregnant women who report previous induced abortions. Bulletin of the World Health Organization. 1975;52:149–154. [PMC free article] [PubMed] [Google Scholar]
- 42.Harlap S, Davies AM. Late sequelae of induced abortion: complications and outcome of pregnancy and labor. American Journal of Epidemiology. 1975;102:217–224. doi: 10.1093/oxfordjournals.aje.a112150. [DOI] [PubMed] [Google Scholar]
- 43.Slater PE, Davies AM, Harlap S. The effect of abortion method on the outcome of subsequent pregnancy. Journal of Reproductive Medicine. 1981;26:123–128. [PubMed] [Google Scholar]
- 44.Harlap S, Kaufman R, Prywes R, Davies AM, Sterk VV, Weiskopf P. Patterns of obstetric intervention in a total population. A report from the Jerusalem perinatal study. Israel Journal of Medical Science. 1971;7:1115–1127. [PubMed] [Google Scholar]
- 45.Sterk VV, Prywes R, Davies AM, Ever-Hadani P, Lilos P. Vomiting during pregnancy in Jerusalem women. Israel Journal of Medical Science. 1971;7:1248–1255. [PubMed] [Google Scholar]
- 46.Davies AM. Geographical epidemiology of the toxemias of pregnancy. Israel Journal of Medical Science. 1971;7:751–821. [PubMed] [Google Scholar]
- 47.Davies AM, Czaczkes JW, Sadovsky E, Prywes R, Weiskopf P, Sterk VV. Toxemia of pregnancy in Jerusalem. I. Epidemiological studies of a total community. Israel Journal of Medical Science. 1970;6:253–266. [PubMed] [Google Scholar]
- 48.Davies AM, Poznansky R, Weiskopf P, Prywes R, Sadovsky E, Czaczkes W. Toxemia of pregnancy in Jerusalem. II. The role of diet. Israel Journal of Medical Science. 1976;12:509–518. [PubMed] [Google Scholar]
- 49.Harlap S, Davies AM. Letter: Maternal blood group A and pre-eclampsia. British Medical Journal. 1974;3:171–172. doi: 10.1136/bmj.3.5924.171-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seidman DS, Ever-Hadani P, Stevenson DK, Gale R. The effect of abortion on the incidence of pre-eclampsia. European Journal of Obstetrics, Gynecology and Reproductive Biology. 1989;33:109–114. doi: 10.1016/0028-2243(89)90203-7. [DOI] [PubMed] [Google Scholar]
- 51.Harlap S, Paltiel O, Deutsch L, Knaanie A, Masalha S, Tiram E, et al. Paternal age and preeclampsia. Epidemiology. 2002;13:660–667. doi: 10.1097/00001648-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Funai EF, Paltiel OB, Malaspina D, Friedlander Y, Deutsch L, Harlap S. Risk factors for pre-eclampsia in nulliparous and parous women: the Jerusalem Perinatal Study. Paediatric and Perinatal Epidemiology. 2005;19:59–68. doi: 10.1111/j.1365-3016.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 53.Paltiel O, Friedlander Y, Tiram E, Barchana M, Xue X, Harlap S. Cancer after pre-eclampsia: follow up of the Jerusalem perinatal study cohort. British Medical Journal. 2004;328:919. doi: 10.1136/bmj.38032.820451.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harlap S, Davies AM, Haber M, Rossman H, Prywes R, Samueloff N. Congenital malformations in the Jerusalem perinatal study. An overview with special reference to maternal origin. Israel Journal of Medical Science. 1971;7:1520–1528. [PubMed] [Google Scholar]
- 55.Harlap S. Down’s syndrome in west Jerusalem. American Journal of Epidemiology. 1973;97:225–232. doi: 10.1093/oxfordjournals.aje.a121503. [DOI] [PubMed] [Google Scholar]
- 56.Hook EB, Harlap S. Differences in maternal age-specific rates of Down syndrome between Jews of European origin and of North African or Asian origin. Teratology. 1979;20:243–248. doi: 10.1002/tera.1420200209. [DOI] [PubMed] [Google Scholar]
- 57.Harlap S. A time-series analysis of the incidence of Down’s syndrome in West Jerusalem. American Journal of Epidemiology. 1974;99:210–217. doi: 10.1093/oxfordjournals.aje.a121604. [DOI] [PubMed] [Google Scholar]
- 58.Harlap S, Prywes R, Davies AM. Letter: Birth defects and oestrogens and progesterones in pregnancy. Lancet. 1975;1:682–683. doi: 10.1016/s0140-6736(75)91784-5. [DOI] [PubMed] [Google Scholar]
- 59.Harlap S. Ovulation induction and congenital malformations. Lancet. 1976;2:961. doi: 10.1016/s0140-6736(76)90921-1. [DOI] [PubMed] [Google Scholar]
- 60.Grossman S, Handlesman Y, Davies AM. Birth weight in Israel, 1968–70. I. Effects of birth order and maternal origin. Journal of Biosocial Science. 1974;6:43–58. doi: 10.1017/s0021932000009494. [DOI] [PubMed] [Google Scholar]
- 61.Seidman DS, Gale R, Stevenson DK, Laor A, Bettane PA, Danon YL. Is the association between birthweight and height attainment independent of the confounding effect of ethnic and socioeconomic factors? Israel Journal of Medical Science. 1993;29:772–776. [PubMed] [Google Scholar]
- 62.Paz I, Seidman DS, Danon YL, Laor A, Stevenson DK, Gale R. Are children born small for gestational age at increased risk of short stature? American Journal of Diseases of Children. 1993;147:337–339. doi: 10.1001/archpedi.1993.02160270099030. [DOI] [PubMed] [Google Scholar]
- 63.Seidman DS, Laor A, Gale R, Stevenson DK, Danon YL. A longitudinal study of birth weight and being overweight in late adolescence. American Journal of Diseases of Children. 1991;145:782–785. [PubMed] [Google Scholar]
- 64.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Birth weight, current body weight, and blood pressure in late adolescence. British Medical Journal. 1991;302:1235–1237. doi: 10.1136/bmj.302.6787.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seidman DS, Laor A, Stevenson DK, Sivan E, Gale R, Shemer J. Macrosomia does not predict overweight in late adolescence in infants of diabetic mothers. Acta Obstetricia et Gynecologica Scandinavica. 1998;77:58–62. doi: 10.1034/j.1600-0412.1998.770113.x. [DOI] [PubMed] [Google Scholar]
- 66.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Pre-eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. British Journal of Obstetrics and Gynaecology. 1991;98:1009–1014. doi: 10.1111/j.1471-0528.1991.tb15339.x. [DOI] [PubMed] [Google Scholar]
- 67.Laor A, Stevenson DK, Shemer J, Gale R, Seidman DS. Size at birth, maternal nutritional status in pregnancy, and blood pressure at age 17: population based analysis. British Medical Journal. 1997;315:449–453. doi: 10.1136/bmj.315.7106.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seidman DS, Laor A, Gale R, Stevenson DK, Danon YL. Is low birth weight a risk factor for asthma during adolescence? Archives of Disease in Childhood. 1991;66:584–587. doi: 10.1136/adc.66.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seidman DS, Paz I, Stevenson DK, Laor A, Danon YL, Gale R. Neonatal hyperbilirubinemia and physical and cognitive performance at 17 years of age. Pediatrics. 1991;88:828–833. [PubMed] [Google Scholar]
- 70.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Long-term effects of vacuum and forceps deliveries. Lancet. 1991;337:1583–1585. doi: 10.1016/0140-6736(91)93273-c. [DOI] [PubMed] [Google Scholar]
- 71.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Birth weight and intellectual performance in late adolescence. Obstetrics and Gynecology. 1992;79:543–546. [PubMed] [Google Scholar]
- 72.Paz I, Laor A, Gale R, Harlap S, Stevenson DK, Seidman DS. Term infants with fetal growth restriction are not at increased risk for low intelligence scores at age 17 years. Journal of Pediatrics. 2001;138:87–91. doi: 10.1067/mpd.2001.110131. [DOI] [PubMed] [Google Scholar]
- 73.Paz I, Gale R, Laor A, Danon YL, Stevenson DK, Seidman DS. The cognitive outcome of full-term small for gestational age infants at late adolescence. Obstetrics and Gynecology. 1995;85:452–456. doi: 10.1016/0029-7844(94)00430-l. [DOI] [PubMed] [Google Scholar]
- 74.Seidman DS, Paz I, Stevenson DK, Laor A, Danon YL, Gale R. Effect of phototherapy for neonatal jaundice on cognitive performance. Journal of Perinatology. 1994;14:23–28. [PubMed] [Google Scholar]
- 75.Malaspina D, Reichenberg A, Weiser M, Fennig S, Davidson M, Harlap S, et al. Paternal age and intelligence: implications for age-related genomic changes in male germ cells. Psychiatric Genetics. 2005;15:117–125. doi: 10.1097/00041444-200506000-00008. [DOI] [PubMed] [Google Scholar]
- 76.Shye D, Jaffe B. Prevalence and correlates of perimenstrual symptoms: a study of Israeli teenage girls. Journal of Adolescent Health. 1991;12:217–224. doi: 10.1016/0197-0070(91)90014-d. [DOI] [PubMed] [Google Scholar]
- 77.Paltiel O, Harlap S, Deutsch L, Knaanie A, Massalha S, Tiram E, et al. Birth weight and other risk factors for acute leukemia in the Jerusalem Perinatal Study cohort. Cancer Epidemiology, Biomarkers and Prevention. 2004;13:1057–1064. [PubMed] [Google Scholar]
- 78.Paltiel O, Friedlander Y, Deutsch L, Yanetz R, Calderon-Margalit R, Tiram E, et al. Time interval between the diagnosis of cancer in mothers and offspring in the Jerusalem Perinatal Study (JPS) cohort. American Journal of Epidemiology. 2005;161 Suppl:S16. [Google Scholar]
- 79.Malaspina D, Harlap S, Fennig S, Corcoran C, Susser E. Maternal psychophysiological stress and offspring schizophrenia risk. Biological Psychiatry. 2003;53(8S):168–169. [Google Scholar]
- 80.Kimhy D, Harlap S, Fennig S, Deutsch L, Draiman BG, Corcoran C, et al. Maternal household crowding during pregnancy and the offspring’s risk of schizophrenia. Schizophrenia Research. 2006;86:23–29. doi: 10.1016/j.schres.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleinhaus K, Perrin M, Friedlander Y, Paltiel O, Malaspina D, Harlap S. Paternal age and spontaneous abortion. Obstetrics and Gynecology. 2006;108:369–377. doi: 10.1097/01.AOG.0000224606.26514.3a. [DOI] [PubMed] [Google Scholar]
- 82.Harlap S, Stenhouse NN, Davies AM. A multiple regression analysis of admission of infants to hospital. A report from the Jerusalem perinatal study. British Journal of Preventive and Social Medicine. 1973;27:182–187. doi: 10.1136/jech.27.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harlap S, Drapkin I. Child injury in West Jerusalem. In: Drapkin I, Viano E, editors. Violence and Its Victims. Lexington, MA: Lexington Books; 1975. [Google Scholar]
- 84.Berkowitz GS, Harlap S, Beck GJ, Freeman DH, Baras M. Early gestational bleeding and pregnancy outcome: a multivariable analysis. International Journal of Epidemiology. 1983;12:165–173. doi: 10.1093/ije/12.2.165. [DOI] [PubMed] [Google Scholar]
- 85.Mills JL, Harlap S, Harley EE. Should coitus late in pregnancy be discouraged? Lancet. 1981;2:136–138. doi: 10.1016/s0140-6736(81)90311-1. [DOI] [PubMed] [Google Scholar]
- 86.Gilbert M. Atlas of the Arab–Israeli Conflict. New York: Oxford University Press; 1993. [Google Scholar]
- 87.Zlotogora J, Bach G, Munnich A. Molecular basis of mendelian disorders among Jews. Molecular Genetics and Metabolism. 2000;69:169–180. doi: 10.1006/mgme.2000.2969. [DOI] [PubMed] [Google Scholar]
- 88.Ostrer H. A genetic profile of contemporary Jewish populations Nature Reviews. Genetics. 2001;2:891–898. doi: 10.1038/35098506. [DOI] [PubMed] [Google Scholar]
- 89.Feldman GE. Do Ashkenazi Jews have a higher than expected cancer burden? Implications for cancer control prioritization efforts. Israel Medical Association Journal. 2001;3:341–346. [PubMed] [Google Scholar]
- 90.Cohen T, Simhai B, Steinberg AG, Levene C. Genetic polymorphisms among Iranian Jews in Israel. American Journal of Medical Genetics. 1981;8:181–190. doi: 10.1002/ajmg.1320080209. [DOI] [PubMed] [Google Scholar]
- 91.Nebel A, Filon D, Brinkmann B, Majumder PP, Faerman M, Oppenheim A. The Y chromosome pool of Jews as part of the genetic landscape of the Middle East. American Journal of Human Genetics. 2001;69:1095–1112. doi: 10.1086/324070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Friedlander Y, Kark JD, Kaufmann NA, Stein Y. Coronary heart disease risk factors among religious groupings in a Jewish population sample in Jerusalem. American Journal of Clinical Nutrition. 1985;42:511–521. doi: 10.1093/ajcn/42.3.511. [DOI] [PubMed] [Google Scholar]
- 93.Shahar Y, Carel RS. Changes in smoking patterns in young military recruits in relationship to psychosocial characteristics. Military Medicine. 1991;156:455–461. [PubMed] [Google Scholar]
- 94.Kark JD, Laor A. Cigarette smoking and educational level among young Israelis upon release from military service in 1988 – a public health challenge. Israel Journal of Medical Science. 1992;28:33–37. [PubMed] [Google Scholar]
- 95.Green MS, Harari G. Past and present smoking behaviour and its association with health-related habits in selected Israeli working populations. The Cordis Study. International Journal of Epidemiology. 1992;21:494–501. doi: 10.1093/ije/21.3.494. [DOI] [PubMed] [Google Scholar]
- 96.Sperber AD, Peleg A, Friger M, Shvartzman P. Factors associated with daily smoking among Israeli adolescents: a prospective cohort study with a 3-year follow-up. Preventive Medicine. 2001;33:73–81. doi: 10.1006/pmed.2001.0836. [DOI] [PubMed] [Google Scholar]
- 97.Weiss S. Adult women’s drinking in Israel: a review of the literature. Alcohol. 1991;26:277–283. doi: 10.1093/oxfordjournals.alcalc.a045113. [DOI] [PubMed] [Google Scholar]
- 98.Aharonovich E, Hasin D, Rahav G, Meydan J, Neumark Y. Differences in drinking patterns among Ashkenazic and Sephardic Israeli adults. Journal of Studies on Alcohol. 2001;62:301–305. doi: 10.15288/jsa.2001.62.301. [DOI] [PubMed] [Google Scholar]
- 99.Neumark YD, Rahav G, Jaffe DH. Socio-economic status and binge drinking in Israel. Drug and Alcohol Dependence. 2003;69:15–21. doi: 10.1016/s0376-8716(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 100.Kaufmann NA, Kark JD, Friedlander Y, Dennis BH, McClish D, Stein Y. Nutrient intake in Jerusalem – effects of origin, social class and education. Israel Journal of Medical Science. 1982;18:1198–1209. [PubMed] [Google Scholar]
- 101.Kaufmann NA, Friedlander Y, Halfon ST, Slater PE, Dennis BH, McClish D, et al. Nutrient intake in Jerusalem - consumption in 17-year-olds. Israel Journal of Medical Science. 1982;18:1167–1182. [PubMed] [Google Scholar]
- 102.Kaufmann NA, Friedlander Y, Halfon ST, Slater PE, Dennis BH, McClish D, et al. Nutrient intake in Jerusalem - consumption in adults. Israel Journal of Medical Science. 1982;18:1183–1197. [PubMed] [Google Scholar]
- 103.Lubin F, Lusky A, Chetrit A, Modan M. Differential nutritional habits in distinct ethnic groups in the Israel population. Public Health Reviews. 1998;26:79–85. [PubMed] [Google Scholar]
- 104.Lubin F, Chetrit A, Lusky A, Modan M, Modan B. Dietary assessment in Israel: past experience. Public Health Reviews. 1998;26:23–29. [PubMed] [Google Scholar]
- 105.Kaluski DN, Meir C, Rotem N, Zadka P. Sources of nutritional data in Israel. Public Health Reviews. 1998;26:73–77. [PubMed] [Google Scholar]
- 106.Slater PE, Kark JD, Friedlander Y, Kaufmann NA, Eisenberg S, Stein Y. Reported physical activity and blood lipids in Jerusalem adults. Israel Journal of Medical Science. 1982;18:1144–1149. [PubMed] [Google Scholar]
- 107.Friedlander Y, Kark JD, Kaufmann NA, Berry EM, Stein Y. Familial aggregation of body mass index in ethnically diverse families in Jerusalem. The Jerusalem Lipid Research Clinic. International Journal of Obesity. 1988;12:237–247. [PubMed] [Google Scholar]
- 108.Green MS, Peled I. Differences in the prevalence of hypertension by ethnic origin and age at immigration in a cohort of 5,146 Israelis. American Journal of Epidemiology. 1992;135:1237–1250. doi: 10.1093/oxfordjournals.aje.a116230. [DOI] [PubMed] [Google Scholar]
- 109.Kertzman H, Livshits G, Green MS. Ethnic differences in indices of body mass and body fat distribution in Israel. International Journal of Obesity and Related Metabolic Disorders. 1994;18:69–77. [PubMed] [Google Scholar]
- 110.Kaluski DN, Chinich A, Leventhal A, Ifrah A, Cohen-Mannheim I, Merom D, et al. Overweight, stature, and socioeconomic status among women - cause or effect: Israel National Women’s Health Interview Survey, 1998. Journal of Gender-Specific Medicine. 2001;4:18–24. [PubMed] [Google Scholar]
- 111.Stern E, Raz I, Weitzman S. Prevalence of diabetes mellitus among workers in Israel: a nation-wide study. Acta Diabetologia. 1999;36:169–172. doi: 10.1007/s005920050162. [DOI] [PubMed] [Google Scholar]
- 112.Tamary H, Bar-Yam R, Shalmon L, Rachavi G, Krostichevsky M, Elhasid R, et al. Fanconi anaemia group A (FANCA) mutations in Israeli non-Ashkenazi Jewish patients. British Journal of Haematology. 2000;111:338–343. doi: 10.1046/j.1365-2141.2000.02323.x. [DOI] [PubMed] [Google Scholar]
- 113.Peretz H, Luboshitsky R, Baron E, Biton A, Gershoni R, Usher S, et al. Cys 618 Arg mutation in the RET proto-oncogene associated with familial medullary thyroid carcinoma and maternally transmitted Hirschsprung’s disease suggesting a role for imprinting. Human Mutation. 1997;10:155–159. doi: 10.1002/(SICI)1098-1004(1997)10:2<155::AID-HUMU7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 114.Yair D, Ben Baruch G, Chetrit A, Friedman T, Hirsh YG, Gotlieb WH, et al. p53 and WAF1 polymorphisms in Jewish-Israeli women with epithelial ovarian cancer and its association with BRCA mutations. British Journal of Obstetrics and Gynaecology. 2000;107:849–854. doi: 10.1111/j.1471-0528.2000.tb11082.x. [DOI] [PubMed] [Google Scholar]
- 115.Arbel-Alon S, Menczer J, Feldman N, Glezerman M, Yeremin L, Friedman E. Codon 72 polymorphism of p53 in Israeli Jewish cervical cancer patients and healthy women. International Journal of Gynecological Cancer. 2002;12:741–744. doi: 10.1046/j.1525-1438.2002.01124.x. [DOI] [PubMed] [Google Scholar]
- 116.Yaron M, Levy T, Chetrit A, Levavi H, Sabah G, Schneider D, et al. The polymorphic CAG repeat in the androgen receptor gene in Jewish Israeli women with endometrial carcinoma. Cancer. 2001;92:1190–1194. doi: 10.1002/1097-0142(20010901)92:5<1190::aid-cncr1437>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 117.Rosenberg N, Murata M, Ikeda Y, Opare-Sem O, Zivelin A, Geffen E, et al. The frequent 5,10-methylenetetrahydrofolate reductase C677T polymorphism is associated with a common haplotype in whites, Japanese, and Africans. American Journal of Human Genetics. 2002;70:758–762. doi: 10.1086/338932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kislitsin D, Lerner A, Rennert G, Lev Z. K-ras mutations in sporadic colorectal tumors in Israel: unusual high frequency of codon 13 mutations and evidence for nonhomogeneous representation of mutation subtypes. Digestive Diseases and Sciences. 2002;47:1073–1079. doi: 10.1023/a:1015090124153. [DOI] [PubMed] [Google Scholar]
- 119.Gershoni-Baruch R, Dagan E, Israeli D, Kasinetz L, Kadouri E, Friedman E. Association of the C677T polymorphism in the MTHFR gene with breast and/or ovarian cancer risk in Jewish women. European Journal of Cancer. 2000;36:2313–2316. doi: 10.1016/s0959-8049(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 120.Friedlander Y, Kark JD, Stein Y. Family history of myocardial infarction as an independent risk factor for coronary heart disease. British Heart Journal. 1985;53:382–387. doi: 10.1136/hrt.53.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Friedlander Y, Kark JD, Stein Y. Complex segregation analysis of low levels of plasma high-density lipoprotein cholesterol in a sample of nuclear families in Jerusalem. Genetic Epidemiology. 1986;3:285–297. doi: 10.1002/gepi.1370030502. [DOI] [PubMed] [Google Scholar]
- 122.Friedlander Y, Elkana Y, Sinnreich R, Kark JD. Genetic and environmental sources of fibrinogen variability in Israeli families: the Kibbutzim Family Study. American Journal of Human Genetics. 1995;56:1194–1206. [PMC free article] [PubMed] [Google Scholar]
- 123.Friedlander Y, Kark JD, Sinnreich R, Basso F, Humphries SE. Combined segregation and linkage analysis of fibrinogen variability in Israeli families: evidence for two quantitative-trait loci, one of which is linked to a functional variant (−58G > A) in the promoter of the alpha-fibrinogen gene. Annals of Human Genetics. 2003;67:228–241. doi: 10.1046/j.1469-1809.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 124.Central Bureau of Statistics. (in Hebrew) http://www.cbs.gov.il/briut/briut2.htm.
- 125.Laron-Kenet T, Shamis I, Weitzman S, Rosen S, Laron ZV. Mortality of patients with childhood onset (0–17 years) Type I diabetes in Israel: a population-based study. Diabetologia. 2001;44 Suppl. 3:B81–B86. doi: 10.1007/pl00002959. [DOI] [PubMed] [Google Scholar]
- 126.Karni A, Kahana E, Zilber N, Abramsky O, Alter M, Karussis D. The frequency of multiple sclerosis in Jewish and Arab populations in greater Jerusalem. Neuroepidemiology. 2003;22:82–86. doi: 10.1159/000067101. [DOI] [PubMed] [Google Scholar]