Abstract
Background
Schizophrenia is etiologically heterogeneous. It is anticipated, but unproven, that subgroups will differ in neuropathology and that neuroimaging may reveal these differences. The optimal imaging condition may be at rest, where greater variability is observed than during cognitive tasks, which more consistently reveal hypofrontality. We previously demonstrated symptom and physiologic differences between familial and sporadic schizophrenia patients and hypothesized that the groups would show different resting regional cerebral blood flow (rCBF) patterns.
Methods
Ten familial and sixteen sporadic schizophrenia patients and nine comparison subjects had single photon emission computed tomography imaging during passive visual fixation. Images were spatially normalized into Talairach coordinates and analyzed for group rCBF differences using SPM with a Z value threshold of 2.80, p < .001.
Results
The subgroups had similar age, gender, illness duration, and medication treatment. Sporadic patients had hypofrontality (anterior cingulate, paracingulate cortices, left dorsolateral and inferior–orbitofrontal), whereas familial patients had left temporoparietal hypoperfusion; all of these regions show resting activity in healthy subjects. Both groups hyperperfused the cerebellum/pons and parahippocampal gyrus; additional hyperperfusion for sporadic patients was observed in the fusiform; familial patients also hyperperfused the hippocampus, dentate, uncus, amygdala, thalamus, and putamen.
Conclusions
Familial and sporadic schizophrenia patients had different resting rCBF profiles, supporting the hypothesis that certain subgroups have distinct neural underpinnings. Different neuropathologic processes among subgroups of schizophrenia patients may account for the prior contradictory results of resting imaging studies.
Keywords: Default state, familial, hypofrontality, resting, schizophrenia, self-representation, SPECT
Schizophrenia is an etiologically heterogeneous condition, and it is reasonable to expect that the primary pathology and corresponding neural dysfunction may differ among patients with distinct subtypes of illness. Consistent with heterogeneity, samples of schizophrenia patients show a large within-group variance on most measures, even when their mean performance differs significantly from that of a comparison group. This variability in the patients’ performance is seldom addressed, or it is attributed to confounding by such factors as medication, illness state, education, substance abuse, or other individual experiences. This within-patient variability is, however, a fundamental characteristic of the schizophrenia syndrome. The resultant and unavoidable inclusion of patients with different etiopathologies likely contributes to the frequent non-replications and contradictory results that plague schizophrenia research.
One approach to identifying more homogeneous schizophrenia subgroups is to stratify research patients based on their presumptive etiology, such as whether they have a family history of schizophrenia. Although the equivocal results for some familial–sporadic studies prompted doubts that such groups have distinct origins or pathophysiologic features (Kendler and MacLean 1990), recent studies demonstrate some consistent clinical differences between familial and sporadic subtypes including increased negative symptoms, worse outcome, and earlier age of onset in familial patients (Alda et al 1996; Keefe et al 1991; Kendler and MacLean 1990; Maier et al 1993; Verdoux et al 1996). Familial, but not sporadic, cases have treatment-resistant negative symptoms, particularly emotional withdrawal, poor rapport, and lack of spontaneity, whereas these symptoms improve with antipsychotic treatment in sporadic cases (Malaspina et al 2000). We previously demonstrated a double dissociation between familial and sporadic cases using physiologic measures, pointing toward a lateralized temporoparietal dysfunction in familial cases and significantly greater prefrontal deficits in sporadic cases (Malaspina et al 1998). Sporadic cases have significantly older mean paternal age than familial cases (Malaspina et al 2002), consistent with a role for de novo genetic events (mutations or gene methylation changes affecting parent of origin gene expression) in the etiology of some sporadic cases (Malaspina 2001). Additional evidence that familial and sporadic schizophrenia may be distinct conditions is provided by recent linkage studies that showed only familial cases were associated with the at risk haplotypes for dystrobrevin-binding protein (Van Den Bogert et al 2003) and neuregulin 1 (Williams et al 2003).
Because schizophrenia arises from aberrant brain activity, it is reasonable to examine whether functional neuroimaging would reveal metabolic differences between familial and sporadic subgroups of patients. Although scores of functional neuroimaging studies have been conducted examining schizophrenia since Ingvar and Franzen (1974) first described reduced frontal to posterior blood flow (hypofrontality) in resting patients, no study has contrasted neural activity between etiologically defined groups of schizophrenia patients. Moreover, the appropriate imaging condition for such a comparison study is not readily apparent. Although resting hypofrontality in schizophrenia was replicated by some studies, there were so many contradictory results (Weinberger and Berman, 1988) that the very presence of hypofrontality was questioned (Gur and Gur 1995). Conversely, because the vast majority of neuroimaging studies that utilize behavioral activations as the imaging condition do find hypofrontality in schizophrenia (Weinberger and Berman 1996), most current neuroimaging research in schizophrenia uses a cognitive task as the imaging condition.
The variability in the resting (default) state could be due to heterogeneous neural underpinnings among subgroups of schizophrenia patients that are better revealed at rest than during cognitive tasks. Furthermore, the mental activity occurring at rest may be relevant to the phenomenology of schizophrenia. The mental representations of the multifaceted self and of one’s goals, thoughts, memories, ideas, and speech arise through the stimulus-independent retrieval and manipulation of episodic memories, implicit knowledge, and emotional associations that occur in the default state (Greicius et al, 2003; Mazoyer et al 2001; Raichle et al 2001). Deficits in allied processes, such as monitoring of one’s own thoughts and goals and the ability to infer or represent the beliefs and intentions of other individuals (“theory of mind”), may underlie some of the phenomenology of schizophrenia (Adolphs 2001; Frith 1996; Siegal and Varley 2002). Because self-generated mental activity predominates in the absence of goal-directed activity, the question of whether schizophrenia patients show differences in self-generated mental activity may be better addressed by neuroimaging studies conducted at rest than by task-response activations. Given the wide variety of signs and symptoms associated with schizophrenia, determining whether default self-generated neural activation differs for separate subgroups may be useful in addressing whether separate neuropathologic mechanisms cause schizophrenia. It may also be important to know whether resting state heterogeneity exists, because “rest” is used as the control condition for many task-response studies and variability in this measure may confound results that are based on contrasting rest and task activities.
We hypothesized that familial and sporadic (nonfamilial) schizophrenia subgroups would show different resting regional cerebral blood flow (rCBF) patterns in comparison to healthy subjects and to one another. No previous study, to our knowledge, has compared familial and sporadic patients in a functional neuroimaging study, but there is evidence from structural imaging that the groups may differ. For example, disturbed structural brain asymmetry (posterior structures or Sylvian fissures) is reported in familial patients and in genetically vulnerable relatives (Benes et al, 2001; DeLisi et al 1997; Falkai et al 2002; Honer et al 1995; O’Callaghan et al 1995; Sharma et al 1999), but there are no such reports in patients who were defined as sporadic cases and only a single study failed to identify disturbed asymmetry in familial patients (Frangou et al 1997). Familial patients also evidence disturbed temporoparietal asymmetry on dichotic listening tone tasks (Malaspina et al 1998) and show a right lateralized gain abnormality for visual tracking that is not present in sporadic patients (Schwartz et al 1995).
Although functional magnetic resonance imaging (MRI) using the BOLD (blood oxygenation level–dependent) contrast technique can determine the small changes in blood flow linked to an activation condition relative to a baseline state, this technique cannot establish an absolute metabolism or blood flow to compare between groups of subjects. By contrast, both positron emission tomography (PET) and single photon emission computed tomography (SPECT) permit assessments of absolute cerebral blood flow (CBF). This study compared the default neural activity between familial and sporadic subgroups of schizophrenia patients using SPECT in a resting imaging condition.
Methods and Materials
We assessed resting rCBF in schizophrenia patients and in healthy subjects using passive visual fixation as the imaging condition. Passive viewing demonstrates similar neurophysiologic activity as resting with the eyes closed, aside for additional activation in extrastriate visual areas; this activation is proposed to be closer to the true resting (default) state than in the awake condition with the eyes closed (Greicius et al 2003; Gusnard and Raichle 2001; Shulman et al 1997).
Subjects
The sample included 9 healthy comparison subjects and 26 DSM-IV schizophrenia patients (10 familial and 16 sporadic cases) from the New York State Psychiatric Institute inpatient Schizophrenia Research Unit. All subjects provided written informed consent for this study, which was approved by the Institutional Review Board. The patients were physically healthy, as indicated by recent physical examinations, were right-handed, and had no active substance dependence or abuse. All patients had been either medication-free or on stable doses of antipsychotic medications for a minimum of 4 weeks. The patients’ diagnoses were based on research diagnostic interviews with the Diagnostic Interview for Genetic Studies (Nurnberger et al 1995), past records, and symptom ratings, and each represented a consensus reached among clinical and research staff. The comparison group subjects were clinically screened and had no Axis I diagnosis within the past 2 years nor any personal or family history of a psychotic disorder.
Symptoms
Symptoms were rated with the Positive and Negative Symptom Scale (PANSS) (Kay et al 1989) for 23 of the 26 patients and summarized into the positive, negative, dysphoric mood, activation, and autistic preoccupation factors (White et al 1997). Three patients were not assessed because of problems with staff availability and scheduling.
Family History
Assessments were conducted blind to patient information using the Family Interview for Genetic Studies (Maxwell 1992). At least one immediate family member provided information about psychiatric symptoms in all first- and second-degree family members so that the schizophrenia patients could be identified as either having a family history (familial) or not having a family history (sporadic). The familial cases each had a first- or second-degree relative who was afflicted with a chronic nonaffective psychosis, and sporadic cases had no family members with psychosis.
Imaging
The SPECT imaging was done using 20mCi of Tc-HMPAO (technecium-99m-d,l-hexamethylpropyleneamine oxime; 99m Tc-HMPAO Ceretec, Amersham, Piscataway, New Jersey). The subjects were instructed to maintain their gazes at a defined spatial location by visually fixating on the intersection of a blue crosshair in their central visual field (10 by 6 inches). After 3 min, the isotope was injected through an intravenous line in the subjects’ left arm. Research staff monitored the subjects during the 15-minutes passive viewing period to ensure subject compliance with the instructions, including lack of movement. The subjects then sat quietly for an additional 20 minutes before undergoing a 20-minutes SPECT scan on a rotating triple-head Picker-PRISM 3000 camera, with a high-resolution fan beam collimator (Leuhr, Picker International, Inc., Cleveland, Ohio). Projection image data were acquired in a continuous acquisition mode at 7.5-seconds intervals using a 128 × 128 matrix for a total of 120 images over a 360° rotation orbit. The total scan time was 20 minutes duminring which four 5-minutes rapid acquisition sequence (RAS) image data sets were acquired. Motion-corrected images were reconstructed by filtered back projection, after processing with a fifth-order Butterworth filter with a cutoff frequency of .3–.4 cycles/seconds and subsequent attenuation correction using a .110 factor. Images were reconstructed in the transaxial plane, parallel to the orbitomedial line.
Image Analysis
The transaxial images (3.67 mm thickness per pixel) were reconstructed and transmitted to a Silicon Graphics workstation, where all of the calculations and image manipulations were performed. The raw image files were read into an automated three-dimensional image analysis software package (MEDX, Sensor) and subsequently saved in an ANALYZE file format. The scan files were imported into SPM96 (statistical parametric mapping) software package and transformed into the standard stereotactic space of the Talairach coordinate system using the “Normalization” function of the SPM96 software. This function employed the technique of minimizing the sum of squares between each of our images and the reference PET image provided by the software, which is already transformed into Talairach space. After the scans were normalized to the Talairach and Tournoux coordinate system they were smoothed using a Gaussian filter (with a kernel of 12-mm full width half maximum) to reduce the variance due to individual anatomical variability and to improve the signal-to-noise ratio.
Statistical Analysis
Following the stereotactic normalization and image smoothing, statistical analysis was performed. The rCBF differences between the comparison subjects and the familial and sporadic schizophrenia groups, respectively, were estimated on a pixel-by-pixel basis. Global differences in CBF were factored out as a covariate, voxel by voxel, using the analysis of covariance model because any group differences in global blood flow might otherwise obscure the differences in regional activity between the groups. We used these data to compare the mean blood flow distributions between each of the two schizophrenia groups and a healthy comparison group. We used separate contrasts to evaluate rCBF increases and decreases between the groups. Pixelwise, the mean blood flow was compared using the t test statistic, and the data were then transformed into normally distributed Z statistics. The significance of the comparisons was assessed by comparing the expected and observed distribution of the t statistic under the null hypothesis that there were no group differences in rCBF. Only activations that were significant at a Z value threshold of 2.80, p = .001, corrected for multiple comparisons with p = .05, were considered significant. The results were superimposed on a Talairach-registered T1-weighted MRI provided by SPM96 software, and significant rCBF changes were displayed as statistical parametric maps in the Talairach space, both as the probabilities of cluster size and activation magnitudes based on Z scores. The imaging data for the familial and sporadic groups were separately contrasted with that of the healthy comparison group to identify regions of significantly decreased and increased rCBF. The patient groups were also contrasted with one another.
Results
Clinical Data
The 10 familial patients, 16 sporadic patients, and 9 comparison subjects did not differ respectively in age (37.4 ± 9.1, 29.6 ± 9.2, and 29.9 ± 6.23 years, respectively; F = 2.94, p = .07) or gender proportion (male = 60%, 62.5%, 66.7%, respectively). The two patient subgroups had similar age of onset (21.0 ± 7.7 vs. 18.8 ± 4.1; t =. 93, df = 23, p = .36), but the sporadic group had more years of education (12.1, SD = 2.7 vs. 14.6, SD = 2.2: t =−2.48, df = 22, p =. 021). Medication status was similar for the familial and sporadic groups; 40% and 37.5%, respectively, had been maintained medication free for 4 weeks before the imaging and the others had been on stable medication regimens for at least 1 month. Comparable proportions of patients in each group were receiving typical neuroleptics (30% and 31.25%, respectively) and atypical antipsychotic medications (30% and 31.25%, respectively). The PANSS symptom scores obtained in the imaging treatment condition for 7 of the 10 familial patients and for the 16 sporadic patients, respectively, demonstrated significantly greater symptoms for the familial group for the negative factor (24.3, SD = 11.7 vs. 17.0, SD = 5.2: t = 2.10, df = 21, p =. 048), activation factor (14.4, SD = 5.8 vs. 8.3, SD = 2.8: t = 3.48, df = 21, p =. 002), and autistic preoccupation factor (16.0, SD = 3.3 vs. 10.2, SD = 3.1; t = 4.09, df = 21, p = .001). The groups did not differ significantly on the positive symptom factor (14.1, SD = 5.8 vs. 11.6, SD = 3.1: t = 1.36, df= 21, p = .19) or the dysphoric mood factor score (10.0, SD = 4.1 vs. 10.7, SD = 4.3; t =−.360, df = 21, p =. 72).
Decreased rCBF in Sporadic Patients Contrasted with the Comparison Subjects
Sporadic patients had lower rCBF in three significant clusters that included the prefrontal cortex, anterior cingulate, and paracingulate cortices (Table 1, Figure 1). The hypoperfusion was maximally significant in the left hemisphere at the inferior frontal gyrus (Brodmann’s area [BA] 47), dorsolateral prefrontal cortex (BA 46), and middle frontal gyrus (BA 9) and in the right hemisphere at the medial frontal gyrus (BA 10) and middle frontal gyrus (BA 6 and BA9). The region of significant hypoperfusion included the bilateral middle, inferior, and frontal gyri, anterior cingulate, and insula.
Table 1.
Regional Cerebral Blood Flow in Prefrontal, Anterior Cingulate, and Paracingulate Cortices in Sporadic Patients Is Lower Compared with Control Subject Group
| Cluster: Cluster Size (voxels), Z Value |
Coordinates |
Z Score |
||
|---|---|---|---|---|
| x | y | z | ||
| 453 Voxels, 3.96 | ||||
| Left inferior frontal gyrus (BA46) (principal sulcus) |
−48 | 26 | 16 | 3.96 |
| Left inferior frontal gyrus (BA47) (lateral orbitofrontal cortex) |
−42 | 22 | −8 | 3.58 |
| Left middle frontal gyrus (BA9) (paracingulate cortex) |
−42 | 8 | 32 | 3.38 |
| 622 Voxels, 3.92 | ||||
| Right medial frontal gyrus (BA10) (anterior cingulate cortex) |
2 | 44 | 12 | 3.92 |
| Left anterior cingulate gyrus | −4 | 34 | 0 | 3.41 |
| 603 Voxels, 3.56 | ||||
| Right middle frontal gyrus (BA6) |
46 | 10 | 48 | 3.56 |
| Right middle frontal gyrus (BA9) (paracingulate cortex) |
44 | 32 | 32 | 3.47 |
| Right middle frontal gyrus (BA9) (paracingulate cortex) |
36 | 32 | 36 | 3.45 |
Figure 1.

Areas of decreased resting regional cerebral blood flow for sporadic schizophrenia patients versus healthy subjects.
Decreased rCBF in Familial Patients Contrasted with the Comparison Subjects
Familial patients had significantly lower rCBF in the left parietal cortex (Table 2, Figure 2). The hypoperfusion was maximally significant at the superior parietal lobule (BA 7) and the inferior parietal lobule, which includes the supramarginal and angular gyri (BA 39 and 40). The region of significant hypoperfusion also included the gyri of the middle and superior temporal lobe.
Table 2.
Regional Cerebral Blood Flow in Left Parietal Cortex in Sporadic Patients Is Lower Compared with Control Subject Group
| Cluster: Cluster Size (voxels), Z Value |
Coordinates |
Z Score |
||
|---|---|---|---|---|
| x | y | z | ||
| 523 Voxels, 5.03 | ||||
| Left superior parietal lobule (BA7) |
−42 | −66 | 52 | 5.03 |
| Left inferior parietal lobule (BA39) |
−46 | −68 | 44 | 4.40 |
| Left inferior parietal lobule (BA40) |
−50 | −56 | 52 | 4.23 |
Figure 2.
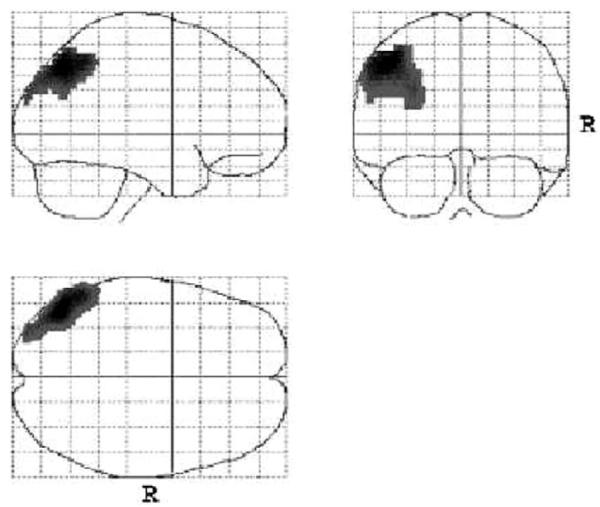
Areas of decreased resting regional cerebral blood flow for familial schizophrenia patients versus the healthy subjects.
Increased rCBF for Sporadic Patients Contrasted with the Comparison Subjects
Sporadic patients had significantly greater rCBF than the comparison subject group in the left anterior cerebellum (Table 3, Figure 3). The region of significant hyperperfusion included the bilateral cerebellar culmen and pons and the left parahippocampal and fusiform gyrus.
Table 3.
Regional Cerebral Blood Flow in Cerebellum of Sporadic Patients Is Greater Compared with Control Subject Group
| Cluster: Cluster Size (voxels), Z Value |
Side | Coordinates |
Z Score |
||
|---|---|---|---|---|---|
| x | y | z | |||
| 795 Voxels, 5.10 | |||||
| Cerebellum, Anterior Lobe | L | −24 | −42 | −24 | 4.46 |
| Culmen | −6 | −20 | −24 | 5.10 | |
| −12 | −34 | −32 | 3.19 | ||
Figure 3.

Areas of increased resting regional cerebral blood flow for sporadic schizophrenia patients versus the healthy subjects.
Increased rCBF for Familial Patients Contrasted with the Comparison Subjects
Familial patients had greater metabolism that was maximally significant in the bilateral pons and right brainstem (Table 4, Figure 4). This region of significant hyperperfusion extended to include the bilateral amygdalae, parahippocampal gyri, and cerebellum, the left hemispheric hippocampus, dentate gyrus, and uncus (olfactory cortex; BA28), and the right hemispheric thalamus and striatum (putamen lentiform).
Table 4.
Familial Patients Had Increased Regional Cerebral Blood Flow in the Bilateral Pons and Right Brainstem Compared with Control Subject Group
| Cluster: Cluster Size (voxels), Z Value |
Coordinates |
Z Score |
||
|---|---|---|---|---|
| x | y | z | ||
| 1286 Voxels, 4.24 | ||||
| Right brainstem, pons | 6 | −20 | −24 | 4.24 |
| Left brainstem, pons | −8 | −20 | −24 | 4.23 |
| Right brainstem, midbrain | 18 | −18 | −8 | 3.85 |
Figure 4.

Areas of increased resting regional cerebral blood flow for familial schizophrenia patients versus the healthy subjects.
Comparison Between the Schizophrenia Subgroups
Contrasts between the two schizophrenia groups demonstrated significantly lower rCBF for sporadic patients in the two clusters where familial patients had significantly greater rCBF. Sporadic patients had significantly lower rCBF in the left anterior cingulate (BA 32: coordinates x, y, z = −10, 44, −4, and Z = 3.13, and coordinates x, y, z = −10, 36, 0, and Z = 3.08) and the right medial frontal gyrus (BA 9: coordinates x, y, z = 6, 40, 20, and Z = 2.96). Familial patients had significantly greater rCBF in the right striatum at the sublobar claustrum (coordinates x, y, z = 24, 16, 16 and Z = 3.12) and putamen (coordinates x, y, z = −18, 10, −4, and Z = 2.79). There were no regions where familial patients had significantly lower rCBF and, equivalently, where sporadic patients had significantly greater rCBF.
Discussion
Groups of familial and sporadic schizophrenia patients demonstrated different resting rCBF patterns. The sporadic patients showed hypofrontality (left frontal gyrus at the principal sulcus, orbitofrontal cortex, anterior cingulate, and paracingulate cortices), whereas familial patients had left temporoparietal hypoperfusion (posterior Sylvian fissure at the superior and inferior parietal lobules, angular and supramarginal gyri). Both schizophrenia subgroups had increased rCBF in the parahippocampal gyrus, which has been strongly associated with positive schizophrenia symptoms (Bogerts 1997) and in cerebellum and pons. The hypofrontal sporadic patients showed increased perfusion in the fusiform area, whereas the parietotemporal hypoperfusion in familial patients was accompanied by widespread subcortical hyperperfusion, including in the brainstem, amygdala, hippocampus, dentate gyrus, uncus, thalamus, and striatum. Comparisons between the two schizophrenia groups also showed significantly lower anterior cingulate and paracingulate rCBF for sporadic patients and increased sublobar claustrum and putamen rCBF for familial patients.
Negative schizophrenia symptoms have previously been associated with both left dorsolateral prefrontal and with left superior parietal association areas hypoperfusion, although they are more frequently associated with hypofrontality (Lahti et al 2001; Liddle et al 1990). The greater negative symptoms in the familial cases bolster a role for parietal dysfunction as a source of negative symptoms (Lahti et al 2001). Resting and behavioral activation conditions engage different neural circuitry and cannot be readily compared. It is worth noting that resting hypofrontality may paradoxically produce greater task-related increases in rCBF, such that lower resting activity may translate into apparently greater activation for behavioral conditions. Perhaps this may contribute to the association between increased task related perfusion and poor performance observed in some schizophrenia studies that has been attributed to inefficiency (Callicott et al 2000; Siegel et al 1995).
The results of this study are unlikely to follow from methodologic limitations of SPECT imaging technology, and the groups were well matched for medication treatment and chronicity. The hypofrontal sporadic cases had significantly more years of education and lower negative symptoms ratings, showing that their hypofrontality was not explained by negative symptom severity. Similar educational and symptom differences between familial and sporadic groups have been observed in larger patient samples (Malaspina et al 2001). Although SPM96 software carries with it some downside risk in terms of spatial localization of regions, similar widespread dysfunction in cortico-striato-thalamo-cortical and limbic circuitry as in the familial group was reported from a PET study of resting neuroleptic-naive schizophrenia patients (Andreasen et al 1997). In addition to having decreased left parietal and increased thalamic and cerebellar rCBF, those patients also had lower rCBF in lateral, orbital, and medial prefrontal regions, similar to our sporadic group, as might happen if the groups in our study were combined. Andreasen proposed a “Unitary Model” for schizophrenia in which all of its phenomena resulted from such cortical-cerebellar disconnectivity (mediated by the thalamus), which interfered with the processes linking thought and action (Andreasen et al 1999). A widespread disruption in cortical–subcortical circuitry, as seen in the familial cases, could arise from diverse factors affecting excitatory or inhibitory neuroregulation, including neuroreceptor abnormalities or neurodevelopmental pathology. Familial schizophrenia is a polygenic disorder, and individual relevant loci may each have only a minor effect on neurocircuitry function. A pattern of hypofrontality accompanied by increased medial temporal lobe (limbic) rCBF, as observed in these sporadic patients, is also commonly observed in schizophrenia imaging studies. A recent PET study of medication-free schizophrenia patients found that increased parahippocampal and cerebellar and decreased dorsolateral prefrontal and anterior cingulate rCBF accounted for the significant differences between schizophrenia patients and comparison subjects (Meyer-Lindenberg et al 2001).
These results further suggest that subgroup differences in default neural activity may contribute to the conflicting findings concerning resting metabolism in patients with schizophrenia. The variable inclusion of subjects with disparate default metabolic profiles may explain such diverse prior imaging results as relative hyperfrontality, global hypoperfusion, only lateralized abnormalities and absent hypofrontality. These discrepant results have not been explained by age, gender, medication status, illness state, chronicity, or other methodologic differences and were largely attributed to the use of “resting” baseline conditions that did not adequately control cognitive activity. The prefrontal hypometabolism for cognitive tasks found in most studies may reflect a final common pathway related to the schizophrenia syndrome but be less indicative of heterogeneous subgroups than default neural activity. There are many analogous situations in medicine, such as congestive heart failure. Just as decreased cardiac output, particularly with a physical challenge, may define the congestive heart failure syndrome without indicating etiology, low prefrontal perfusion, particularly with mental challenge, may comparably characterize the schizophrenia syndrome without differentiating between the subgroups.
Three of the four general regions that are normally active in the default state were hypoperfused by the schizophrenia groups: the ventral medial prefrontal cortex (VMPFC) and dorsal medial prefrontal cortex (DMPFC) by the sporadic group and the posterior lateral cortices by the familial group. Neither group differed from the comparison group in posterior cingulate rCBF, a region that shows the highest default activity because it monitors the environment and allocates attention to relevant stimuli. Because default processing supports self-generated mental operations, including goal formation and conscious self-awareness (Binder et al 1999; Frith 1996; McGuire et al 1996), this study bolsters the hypotheses that these processes are important in explaining schizophrenia (Raichle et al 2001). The VMPFC is densely interconnected to the limbic system and participates in monitoring the environment and the internal milieu with respect to stimuli with emotional salience. The DMPFC includes the anterior cingulate and paracingulate cortices, whose activity accompanies monitoring or reporting one’s own mental state, such as self-generated thoughts, intended speech, and emotions, and which may represent the multifaceted self (Frith and Frith 1999). The posterior lateral region, which includes the inferior parietal lobe and temporal and occipital areas at the posterior end of the Sylvain fissure and superior temporal sulcus, is involved in the intentional or unintentional recall of episodic memories and its relationship to language areas, consistent with providing language and thought concerning these representations. The region is active when the actions of others are imitated, which may be related to the development of empathy (Decety et al 2002).
Although the presence of underlying regional structural abnormalities cannot be concluded from this functional imaging data, it is of interest that prominent volume reductions are reported in frontomedial and frontoorbital (paralimbic) cortices, middle frontal gyrus, anterior cingulate and paracingulate gyri, insula, and supramarginal gyrus for schizophrenia patients (Goldstein et al 1999). Volume reductions in the inferior parietal cortex and prefrontal cortex are each found in about 60% of relevant morphometric studies (Shenton et al 2001). Neuropathologic studies show increased neuronal density, consistent with volume loss, in the medial prefrontal and paracingulate cortices (Selemon et al 1995), the principal sulcus (Selemon et al 1998), and the anterior cingulate (Benes et al 2001), although none of these have discriminated among subgroups of patients. The lateralized hypoperfusion we observed in the familial patients is congruent with the results of other studies that have categorized schizophrenia patients by family history. Some familial cases may derive from processes that disrupt lateralized neural functioning. In fact, genes controlling human brain asymmetries in language centers are purported to underlie genetic schizophrenia susceptibility (Crow et al 1998).
Our findings are particularly striking given that these familial and sporadic subgroups are also likely to be heterogeneous, in part due to misclassification biases from family history methods. We minimized this problem by requiring the presence of chronic nonaffective psychoses in first- or second-degree relatives to define a case as familial. Such family history methods are vulnerable to underreporting of affective illness, substance abuse and personality disorders but are sensitive to the presence of broadly defined psychotic illness in relatives. Defined in this way, the familial and sporadic subgroups are expected to contain, respectively, greater proportions of cases with penetrable genes for psychosis versus de novo etiologies.
Resting neural activity may better indicate homogeneous subgroups within the schizophrenia syndrome that provide clues to disparate pathways than do activation studies. These resting neuroimaging results suggest that a significant portion of familial schizophrenia patients have a lateralized parietotemporal dysfunction and that a significant portion of sporadic patients have greater prefrontal and cingulate dysfunction. If these findings are replicated, particularly using other neuroimaging techniques, they may have indications for stratifying schizophrenia patients to yield more homogeneous subgroups for neuropathologic, genetic, and pharmaceutical studies.
Acknowledgments
This work was supported by National Alliance for Research on Schizophrenia and Depression, the G. Harold and Leila Y. Mathers Foundation, and Grant Nos. 1K24 MH01699 (DM) and National Institute of Mental Health 5P20MH50727 (JG).
References
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Alda M, Ahrens B, Lit W, Dvorakova M, Labelle A, Zvolsky P, Jones B. Age of onset in familial and sporadic schizophrenia. Acta Psychiatr Scand. 1996;93:447–450. doi: 10.1111/j.1600-0447.1996.tb10676.x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Ponto LL Boles, Hichwa RD. Hypofrontality in schizophrenia: Distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bogerts B. The temporolimbic system theory of positive schizophrenic symptoms. Schizophr Bull. 1997;23:423–435. doi: 10.1093/schbul/23.3.423. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Crow LR, Done DJ, Leask S. Sex-related effects of cerebral asymmetry on cognition: Further evidence relating to the genetic predisposition to psychosis. Schizophr Res. 1998;29:6919. [Google Scholar]
- Decety J, Chaminade T, Grezes J, Meltzoff AN. A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage. 2002;15:265–272. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Kushner M, Finer DL, Hoff AL, Crow TJ. Anomalous cerebral asymmetry and language processing in schizophrenia. Schizophr Bull. 1997;23:255–271. doi: 10.1093/schbul/23.2.255. [DOI] [PubMed] [Google Scholar]
- Falkai P, Honer WG, Alfter D, Schneider-Axmann T, Bussfeld P, Cordes J, et al. The temporal lobe in schizophrenia from uni- and multiply affected families. Neurosci Lett. 2002;325:25–28. doi: 10.1016/s0304-3940(02)00224-0. [DOI] [PubMed] [Google Scholar]
- Frangou S, Sharma T, Sigmudsson T, Barta P, Pearlson G, Murray RM. Normal planum temporale asymmetry in familial schizophrenia. A volumetric MRI study. Br J Psychiatry. 1997;170:328–333. doi: 10.1192/bjp.170.4.328. [DOI] [PubMed] [Google Scholar]
- Frith C. The role of the prefrontal cortex in self-consciousness: The case of auditory hallucinations. Philos Trans R Soc Lond B Biol Sci. 1996;351:1505–1512. doi: 10.1098/rstb.1996.0136. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gur RE. Hypofrontality in schizophrenia: RIP. Lancet. 1995;345:1383–1385. doi: 10.1016/s0140-6736(95)92591-0. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Brain Nat Rev Neurosc. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Honer WG, Bassett AS, Squires-Wheeler E, Falkai P, Smith GN, Lapointe JS, et al. The temporal lobes, reversed asymmetry and the genetics of schizophrenia. Neuroreport. 1995;7:221–224. doi: 10.1097/00001756-199512290-00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar DH, Franzen G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand. 1974;50:425–462. doi: 10.1111/j.1600-0447.1974.tb09707.x. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): Rationale and standardization. Br J Psychiatry Suppl. 1989;7:59–67. [PubMed] [Google Scholar]
- Keefe RS, Lobel DS, Mohs RC, Silverman JM, Harvey PD, Davidson M, et al. Diagnostic issues in chronic schizophrenia: Kraepelinian schizophrenia, undifferentiated schizophrenia and state-independent negative symptoms. Schizophr Res. 1991;4:71–79. doi: 10.1016/0920-9964(91)90026-n. [DOI] [PubMed] [Google Scholar]
- Kendler KS, MacLean CJ. Estimating familial effects on age at onset and liability to schizophrenia. Genet Epidemiol. 1990;7:409–417. doi: 10.1002/gepi.1370070603. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Weiler MA, Tamminga CA, Carpenter WT., Jr Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. Am J Psychiatry. 2001;158:1797–1808. doi: 10.1176/appi.ajp.158.11.1797. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RSJ. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1990;60:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Maier W, Lichtermann D, Minges J, Heun R, Hallmayer J. The impact of gender and age at onset on the familial aggregation of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1993;242:279–285. doi: 10.1007/BF02190387. [DOI] [PubMed] [Google Scholar]
- Malaspina D. Paternal factors and schizophrenia risk: De novo mutations and imprinting. Schizophr Bull. 2001;3:379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S. Paternal age and sporadic schizophrenia: Evidence for de novo mutations. Am J Med Genet. 2002;114:299–303. doi: 10.1002/ajmg.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Friedman JH, Kaufmann C, Bruder G, Amador X, Strauss D, et al. Psychobiological heterogeneity of familial and sporadic schizophrenia. Biol Psychiatry. 1998;43:489–496. doi: 10.1016/s0006-3223(97)00527-1. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Goetz RR, Yale S, Berman A, Friedman JH, Tremeau F, et al. Relation of familial schizophrenia to negative symptoms but not to the deficit syndrome. Am J Psychiatry. 2000;157:994–1003. doi: 10.1176/appi.ajp.157.6.994. [DOI] [PubMed] [Google Scholar]
- Maxwell ME. Family Interview for Genetic Studies (FIGS): A Manual for FIGS. Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; Bethesda, MD: 1992. [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. Neuroreport. 1996;7:2095–2099. [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SC, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies: Rationale, unique features and training. NIMH genetics initiative. Arch Gen Psychiatry. 1995;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- O’Callaghan E, Buckley P, Madigan C, Redmond O, Stack JP, Kinsella A, et al. The relationship of minor physical anomalies and other putative indices of developmental disturbance in schizophrenia to abnormalities of cerebral structure on magnetic resonance imaging. Biol Psychiatry. 1995;38:516–524. doi: 10.1016/0006-3223(94)00381-C. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BD, O’Brien BA, Evans WJ, Sautter FJ, Jr, Winstead DK. Smooth pursuit eye movement differences between familial and nonfamilial schizophrenia. Schizophr Res. 1995;17:211–219. doi: 10.1016/0920-9964(94)00089-q. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: Application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- Sharma T, Lancaster E, Sigmundsson T, Lewis S, Takei N, Gurling H. Lack of normal pattern of cerebral asymmetry in familial schizophrenic patients and their relatives—The Maudsley Family Study. Schizophr Res. 1999;40:111–120. doi: 10.1016/s0920-9964(99)00143-7. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE. Top-down modulation of early sensory cortex. Cereb Cortex. 1997;7:193–206. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]
- Siegel BV, Jr, Nuechterlein KH, Abel L, Wu JC, Buchsbaum MS. Glucose metabolic correlates of continuous performance test performance in adults with a history of infantile autism, schizophrenics and controls. Schizophr Res. 1995;17:85–94. doi: 10.1016/0920-9964(95)00033-i. [DOI] [PubMed] [Google Scholar]
- Siegal M, Varley R. Neural systems involved in “theory of mind”. Nat Rev Neurosci. 2002;3:463–471. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- Van Den Bogert AJ. Exotendons for assistance of human locomotion. Biomed Eng Online. 2003;2:17. doi: 10.1186/1475-925X-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoux H, van Os J, Sham P, Jones P, Gilvarry K, Murray R. Does familiality predispose to both emergence and persistence of psychosis? Br J Psychiatry. 1996;168:620–626. doi: 10.1192/bjp.168.5.620. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull. 1988;14:157–168. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Prefrontal function in schizophrenia: Confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996;29:1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. The PANSS Study Group. Psychopathology. 1997;30:263–274. doi: 10.1159/000285058. [DOI] [PubMed] [Google Scholar]
- Williams NM, Preece A, Spurlock G, Norton N, Williams HJ, Zammit S, et al. Support for genetic variation in neuregulin 1 and susceptibility to schizophrenia. Mol Psychiatry. 2003;8:485–487. doi: 10.1038/sj.mp.4001348. [DOI] [PubMed] [Google Scholar]


