Abstract
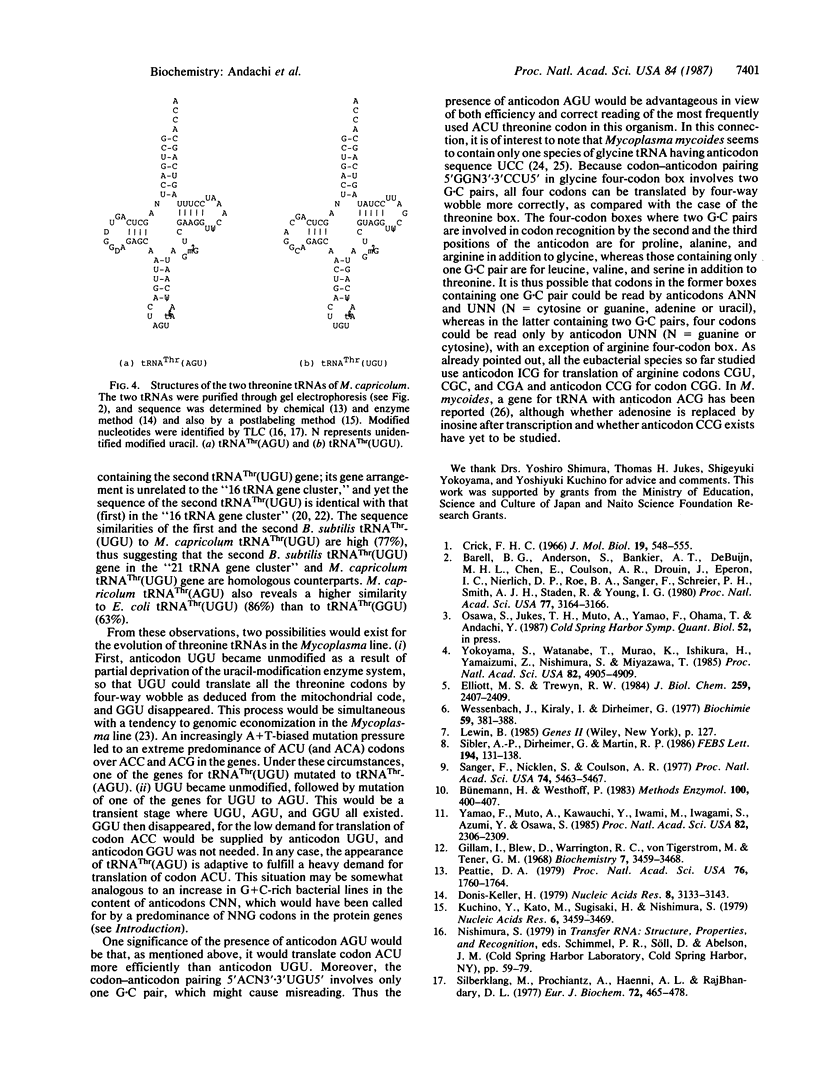
Codon usage pattern in the threonine four-codon (ACN) box in Mycoplasma capricolum is strongly biased towards adenine and uracil for the third base of codons. Codons ending in uracil or adenine, especially ACU, predominate over ACC and ACG. This bacterium contains two isoacceptor threonine tRNAs having anticodon sequences AGU and UGU, both with unmodified first nucleotides. It would thus appear that ACN codons are translated in an unusual way; tRNA(Thr)(AGU) would translate the most abundantly used codon ACU exclusively, because adenine at the first anticodon position can, according to the wobble rule, pair only with uracil of the third codon position. The tRNA(Thr)(UGU) would mainly be responsible for translation of three other codons, ACA, ACG, and ACC. Anticodon UGU would also be used for reading codon ACU as a redundancy of tRNA(Thr)-(AGU), as deduced from the mitochondrial code where unmodified uracil at the first anticodon position can pair with adenine, cytosine, guanine, and uracil by four-way wobble. The tRNA(Thr)(AGU) has much higher sequence homology to tRNA(Thr)(UGU) from M. capricolum (88%), Bacillus subtilis (77%) and Escherichia coli (86%) than to tRNA(Thr)(GGU) from B. subtilis (66%) and E. coli (63%), suggesting that tRNA(Thr)-(AGU) has been derived from tRNA(Thr)(UGU), but not from tRNA(Thr)(GGU).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrell B. G., Anderson S., Bankier A. T., de Bruijn M. H., Chen E., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann H., Westhoff P. Hybrid selection of specific RNAs using DNA covalently coupled to macroporous supports. Methods Enzymol. 1983;100:400–407. doi: 10.1016/0076-6879(83)00069-5. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. S., Trewyn R. W. Inosine biosynthesis in transfer RNA by an enzymatic insertion of hypoxanthine. J Biol Chem. 1984 Feb 25;259(4):2407–2410. [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Sequence analysis of a cluster of twenty-one tRNA genes in Bacillus subtilis. Nucleic Acids Res. 1983 Aug 25;11(16):5763–5774. doi: 10.1093/nar/11.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi Y., Muto A., Osawa S. The protein composition of Mycoplasma capricolum. Mol Gen Genet. 1982;188(1):7–11. doi: 10.1007/BF00332989. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of glycine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1980 Jun 25;8(12):2783–2786. doi: 10.1093/nar/8.12.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Kato M., Sugisaki H., Nishimura S. Nucleotide sequence of starfish initiator tRNA. Nucleic Acids Res. 1979 Aug 10;6(11):3459–3469. doi: 10.1093/nar/6.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Kawauchi Y., Yamao F., Osawa S. Preferential use of A- and U-rich codons for Mycoplasma capricolum ribosomal proteins S8 and L6. Nucleic Acids Res. 1984 Nov 12;12(21):8209–8217. doi: 10.1093/nar/12.21.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Osawa S. The guanine and cytosine content of genomic DNA and bacterial evolution. Proc Natl Acad Sci U S A. 1987 Jan;84(1):166–169. doi: 10.1073/pnas.84.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Axberg T., Borén T., Lagerkvist U. Unconventional reading of the glycine codons. J Biol Chem. 1983 Nov 10;258(21):13178–13184. [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett. 1986 Jan 1;194(1):131–138. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Weissenbach J., Kiraly I., Dirheimer G. Structure primaire des tRNA Thr 1a et b de levure de bière. Biochimie. 1977;59(4):381–391. doi: 10.1016/s0300-9084(77)80314-3. [DOI] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]