Abstract
Background
We reviewed cases of soft-tissue sarcoma of the foot to gain insight into the presentation, treatments and outcomes for this rare disease and to determine whether limb-salvage surgery yields reasonable functional and oncological outcomes.
Methods
We reviewed the cases of 16 patients treated by 2 of us (R.T. and M.I.) for soft-tissue sarcoma of the foot over a 15-year period. We extracted the following information from each patient’s medical record: disease status at presentation, prior treatment, histological diagnosis, American Joint Committee on Cancer (AJCC) stage, details of treatment, oncological outcome and functional outcome. Functional outcome was assessed with the Toronto Extremity Salvage Score (TESS) and the Musculoskeletal Tumor Society (MSTS 1987).
Results
Follow-up averaged 6 (range 2–15) years. Eight patients presented after unplanned excision. Histological diagnosis was synovial sarcoma for 7 of 16 patients. The tumours were evenly distributed among the hindfoot, midfoot and forefoot. Most patients (n = 13) presented with AJCC stage II or III disease. Amputation was necessary for 3 patients, whereas limb salvage was possible for the other 13. Free tissue transfer (n = 9) and radiation therapy (n = 12) were used in most cases. Surgical margins were microscopically positive in 4 of the 13 patients treated with limb salvage. Local disease recurred in 2 patients. Lung metastases occurred in 4 patients. At last follow-up, 11 of 16 patients were alive without disease, 2 with disease and 3 had died of their disease. Functional assessment with MSTS 1987 and the TESS averaged 28% and 90%, respectively, after limb salvage.
Conclusion
In this series, we found that, first, patients frequently presented after unplanned excision, and this may have led to worse oncological outcomes compared with patients who presented primarily. Second, limb salvage was usually possible, but it required accepting marginal resections, relying on free tissue transfer to obtain coverage and using radiation therapy to obtain local control. Third, this combination yielded an acceptable local control rate and very good functional outcomes.
Abstract
Contexte
Nous avons analysé des cas de sarcome des tissus mous du pied pour avoir une idée de la manifestation, des traitements et de l’issue de cette maladie rare et pour déterminer si l’intervention de sauvegarde du membre produit des résultats fonctionnels et oncologiques raisonnables.
Méthodes
Nous avons analysé les cas de 16 patients traités par 2 d’entre nous (R.T. et M.I.) pour un sarcome des tissus mous du pied au cours d’une période de 15 ans. Nous avons extrait les renseignements du dossier médical de chaque patient: état de la maladie au moment de la présentation, traitement antérieur, diagnostic histologique, stade selon l’American Joint Committee on Cancer (AJCC), détails du traitement, résultat oncologique et résultat fonctionnel. Le résultat fonctionnel a été évalué au moyen du score de préservation des membres de Toronto (Toronto Extremity Salvage Score —TESS) et du score de la Société des tumeurs de l’appareil locomoteur (MSTS 1987).
Résultats
Le suivi a atteint en moyenne 6 ans (intervalle de 2 à 15 ans). Huit patients se sont présentés après une excision non planifiée. Le diagnostic histologique a révélé un sarcome de la synovie chez 7 patients sur 16. Les tumeurs étaient réparties également entre l’arrière-pied, le milieu du pied et l’avant-pied. La plupart des patients (n = 13) étaient atteints au stade II ou III de l’AJCC. Il a fallu pratiquer une amputation chez 3 patients, tandis qu’on a pu sauver le membre chez les 13 autres. Le transfert de tissus libres (n = 9) et la radiothérapie (n = 12) ont été utilisés dans la plupart des cas. Les marges chirurgicales étaient microscopiquement positives chez 4 des 13 patients traités par préservation du membre. La maladie locale est réapparue chez 2 patients. Quatre autres ont eu des métastases aux poumons. Au dernier suivi, 11 des 16 patients étaient vivants sans maladie, 2 étaient malades et 3 étaient morts de leur maladie. L’évaluation fonctionnelle mesurée au moyen des échelles MSTS 1987 et TESS a atteint en moyenne 28 % et 90 % respectivement après l’intervention de préservation du membre.
Conclusion
Dans cette série, nous avons constaté que tout d’abord, les patients se présentaient souvent après une excision non planifiée, ce qui peut avoir produit des résultats oncologiques pires que chez les patients qui se sont présentés au stade primaire. Deuxièmement, il a été habituellement possible de sauver le membre, mais il a fallu accepter des résections marginales, compter sur le transfert de tissus libres pour couvrir la plaie et utiliser la radiothérapie pour assurer un contrôle local. Troisièmement, cette combinaison a produit un taux acceptable de contrôle local et de très bons résultats fonctionnels.
Soft tissue sarcoma is an uncommon form of cancer most often arising in the lower extremities, but rarely below the ankle.1 In contrast, benign soft-tissue lesions of the foot are relatively common. The infrequency of its presentation may lead to a lack of consideration of sarcoma when one is faced with a soft-tissue mass on the foot. This can lead to excision of the lesion without appropriate imaging or staging. An unplanned excision is the gross removal of tissue without preoperative staging or the consideration of the need for removal of an envelope of normal tissue around the tumour.2 Unplanned excision, even when combined with radiation therapy or chemotherapy, is inadequate for the treatment of soft-tissue sarcoma. Re-excision is necessary to lower the incidence of local recurrence.3 The rate of unplanned excision is about 40% for soft-tissue sarcoma of the extremities.2,4 For the aforementioned reasons, it is suspected that this rate is higher for soft-tissue sarcoma of the foot.
Even when correctly diagnosed, the treatment of soft-tissue sarcoma of the foot poses unique challenges. The limited amount of soft tissue puts these tumours in close proximity to neurovascular structures or bone, and resection often leaves large postresection defects relative to the surrounding soft-tissue volume. Subsequent reconstruction is made difficult by the mechanical demands that will be placed on the reconstructed foot and by the morphologic constraints that will be imposed by shoe fitting.
There has been very little written about soft-tissue sarcoma of the foot. A number of authors have included a few cases in more general reports on soft-tissue sarcoma,1,5–7 but there have been only 2 reports focused exclusively on soft-tissue sarcoma of the foot and ankle.8,9 We believe that the aforementioned factors make the diagnosis, treatment and outcome of soft-tissue sarcoma of the foot different from that of soft-tissue sarcoma in other locations, and sufficiently so to warrant an additional focused review. The goal of this study was to review all cases of soft-tissue sarcoma of the foot treated in a specialized musculoskeletal oncology practice over a 15-year period. This was done to gain insight into the presentation, treatments and outcomes for this rare disease. We hypothesized that limb salvage surgery would result in a reasonable local control rate and acceptable foot function.
Methods
We identified 16 patients (7 men and 9 women, mean age at presentation 41 yr, range 15–79 yr) from a prospectively collected database of patients with soft-tissue sarcoma treated by 2 of the authors (R.T. and M.I.) in an urban referral musculoskeletal oncology practice. Patients were eligible for inclusion if they had histologically confirmed soft-tissue sarcoma located below the tibio–talar joint, had been treated surgically and had been followed for at least 2 years postoperatively. Patients were excluded from the study if they had a histologically confirmed diagnosis of rhabdomyosarcoma, because the behaviour and treatment of this aggressive form of sarcoma is quite different than that of the others under consideration. Similarly, patients with dermatofibrosarcoma protuberans, well differentiated liposarcoma and desmoid tumour were also excluded from the study, as these tumours are either benign or rarely metastatic. We receveived approval for this study from the ethical review boards of the McGill University Health Centre and the Centre Hospitalier Maisonneuve-Rosemont.
We extracted the following information from each patient’s medical record: age at presentation, sex, disease status at presentation (primary, locally recurrent or metastatic), prior treatment, medical history, histological diagnosis, lesion characteristics (tumour size, location, depth, grade and involvement of neurovascular and vital structures), details of treatment (pre- or postoperative chemotherapy or radiation therapy, resected structures, surgical margins, reconstructive technique and complications) and condition at follow-up. Staging was performed using the system proposed by the American Joint Committee on Cancer (AJCC).10
A standardized treatment protocol was followed for all patients. At presentation, all patients completed a health assessment questionnaire, received a physical examination and were staged. Local staging included radiographs and magnetic resonance imaging (MRI) of the foot. Systemic staging consisted of computed tomography (CT) of the chest. Limb salvage was attempted whenever possible. Amputation was used only when the lesion was deemed unresectable such that limb salvage would have resulted in gross residual tumour or severely compromised foot function. The determination of resectability took into account the tumour extent and invasion of noble structures as assessed by MRI and the anatomic constraints imposed by previous surgery. Surgical margins were defined according to the system of Enneking.11 Positive margins were further classified as grossly positive when the surgeon or the pathologist could identify a tumour at the margin of the resection or microscopically positive when inspection of the margin did not reveal a tumour but it could be identified on histological examination.12
Radiation therapy was used for high-grade sarcoma or when the margins were less than wide, whether planned or not. Some of the patients recruited for this study were concurrently enrolled in a prospective study addressing the effects of preoperative versus postoperative radiotherapy.13 The timing of the radiation therapy for these patients was determined by a randomization protocol. The remainder of the participants received radiotherapy preoperatively to minimize the amount of radiation delivered to the reconstructed foot. A postoperative boost of radiation was administered when microscopically positive margins were found in the resection specimen. Postoperative radiation therapy was used in patients who underwent prior unplanned excision.
We assessed functional outcomes using 2 instruments: the Toronto Extremity Salvage Score (TESS)14 and the one proposed in 1987 by the Musculoskeletal Tumor Society (MSTS 1987).15 The TESS is a patient-completed instrument that contains 30 items that assess difficulty with daily activities such as dressing, mobility, work and sports. It is scored on a percent scale from 20 to 100. The MSTS 1987 is a physician-completed tool that contains 7 items (motion, pain, stability, deformity, strength, functional activity and emotional acceptance). Each item is scored from 0–5 for a total score of 0–35. For both instruments, a higher score corresponded to a better functional result.
Results
Eight patients had not been treated before referral, whereas the other 8 were referred after unplanned excision. At the time of presentation, 4 of these patients had had a progression of their disease and 1 had already experienced a metastasis. Follow-up averaged 6 years (range 2–15 yr).
The histological diagnosis was most often synovial sarcoma (n = 7). The other diagnoses encountered were high-grade undifferentiated sarcoma (n = 3), clear-cell sarcoma (n = 2), fibrosarcoma (n = 2), malignant fibrous histiocytoma (n = 1) and liposarcoma (n = 1). The tumours were most often located on the medial (n = 6) or dorsal (n = 6) aspect of the foot and less frequently on the plantar (n = 2) or lateral (n = 2) aspect. They were somewhat equally distributed among the hindfoot (n = 7), midfoot (n = 5) and forefoot (n = 4). Most tumours were high-grade (n = 14) and deep (n = 13). Half (n = 8) were large (> 5 cm). The AJCC stages were Ia (n = 2), IIb (n = 7), IIc (n = 1), III (n = 5) and IV (n = 1).
Surgical management consisted of resection in 9 patients, resection and arthrodesis in 4 and amputation in 3 patients. Among the 13 patients who underwent limb salvage, the resections were classified as intralesional (microscopically positive margins) in 4 patients, marginal in 7 and wide in 2. Plastic surgery reconstruction was required in 10 of 13 patients: 1 patient needed skin grafting and 9 required free tissue transfers (latissmus dorsi n = 3, lateral arm n = 5 or free fibula with its fasciocutaneous flap n = 1). The amputations were transtibial in 2 patients and through Chopart’s joint in 1 patient, yielding wide margins in all 3 patients. Radiation therapy was used preoperatively in 5 and postoperatively in 7 patients. Neoadjuvant chemotherapy was given to 2 patients in an attempt to downstage the disease to avoid amputation or treat metastatic disease. The use of radiation therapy was not associated with any substantial long-term morbidity.
There were 4 postoperative complications: 1 patient experienced a superficial wound infection that was treated with antibiotics leading to resolution; 1 with a free fibular flap had partial skin island necrosis that was treated with local wound care leading to complete healing; 1 with a free flap had perfusion problems and required a revision of the arterial anastomosis; and finally, 1 who had an amputation had an area of skin necrosis that was successfully treated with local wound care.
Local disease recurred in 2 of the patients who underwent limb salvage. One of these patients had presented after unplanned excision and was treated with marginal reresection and radiation therapy. However, his disease locally recurred, and despite subsequent amputation he experienced metastatic disease, which led to his death. The second patient presented with a mass that was deemed benign on biopsy. It was excised with micropositive margins. After subsequent local recurrence, both initial and definitive histology were reclassified as low-grade fibrosarcoma. The patient received preoperative radiation therapy, re-excision with free-flap coverage and brachytherapy. The patient remains free of disease. In total, 3 patients, including the patient discussed above, died. The second death occurred in a patient who presented with metastasis after unplanned excision with unresectable gross residual tumour. He was treated unsuccessfully with amputation and chemotherapy, which was administered both before and after surgery. The third death occurred in a patient who presented primarily with a high-grade tumour. She received preoperative radiation therapy and then wide resection. Her subsequent metastasis to the groin and the mediastinum were treated with radiation therapy, but she succumbed to her disease. At last follow-up, of the 13 limb salvage patients, 10 were alive without disease, 1 was alive with disease and 2 had died of their disease. Of the 3 patients treated with amputation, 1 was alive without disease, 1 alive with metastases and 1 had died of metastatic disease. The presentation, diagnosis, stage, treatment and oncologic outcome for all 16 patients are presented in Table 1.
Table 1.
Summary of presentation, histological diagnosis, AJCC stage, treatment, progression of disease and outcome in a series of 16 patients with soft-tissue sarcoma of the foot
| Patient | Presentation | Diagnosis | Stage | Treatment | Progression | Outcome |
|---|---|---|---|---|---|---|
| 1 | Primary | Synovial | III | Amputation | N/A | Alive with no evidence of disease |
| 2 | Primary | Fibrosarcoma | Ia | Preoperative radiation therapy and limb salvage | N/A | Alive with no evidence of disease |
| 3 | Primary | Epithelioid | III | Preoperative radiation therapy and limb salvage | Metastasis | Died of disease |
| 4 | Primary | Alveolar | III | Limb salvage and postoperative radiation therapy | N/A | Alive with no evidence of disease |
| 5 | Primary | Fibrosarcoma | Ia | Limb salvage | Local recurrence | Alive with no evidence of disease |
| 6 | Primary | Synovial | III | Limb salvage and postoperative radiation therapy | Metastasis | Alive with disease |
| 7 | Primary | Not otherwise specified | IIb | Preoperative radiation therapy and limb salvage | N/A | Alive with no evidence of disease |
| 8 | Primary | Synovial | IIb | Preoperative radiation therapy and limb salvage | N/A | Alive with no evidence of disease |
| 9 | Unplanned excision/local recurrence | Liposarcoma | III | Limb salvage and postoperative radiation therapy | N/A | Alive with no evidence of disease |
| 10 | Unplanned excision/local recurrence | Malignant fibrous histiocytoma | IIb | Limb salvage and postoperative radiation therapy | Local recurrence/metastasis | Died of disease |
| 11 | Unplanned excision/metastasis | Clear cell | IV | Amputation | N/A | Died of disease |
| 12 | Unplanned excision | Synovial | IIb | Limb salvage and postoperative radiation therapy | N/A | Alive with no evidence of disease |
| 13 | Unplanned excision | Synovial | IIb | Limb salvage and postoperative radiation therapy | N/A | Alive with no evidence of disease |
| 14 | Unplanned excision/local recurrence | Synovial | III | Amputation | Metastasis | Alive with disease |
| 15 | Unplanned excision | Synovial | IIb | Limb salvage and postoperative radiation therapy | N/A | Alive with no evidence of disease |
| 16 | Unplanned excision | Clear cell | IIc | Preoperative radiation therapy and limb salvage | N/A | Alive with no evidence of disease |
AJCC = American Joint Committee on Cancer; N/A = not applicable.
Postoperative functional scores were available for 5 of the 13 patients who underwent limb salvage. The MSTS 1987 scores averaged 28 (range 16–35) out of a possible 35 points, and TESS scores averaged 90% (range 68%–100%). None of the patients who underwent limb salvage required assistive devices for ambulation.
Discussion
Our study focused exclusively on the presentation, treatments and outcomes of soft-tissue sarcoma of the foot. Most studies that have examined tumours of the foot have either included larger anatomic regions,1,16–18 have included a non-homogeneous group of diseases7–9 or have focused on a pediatric population5. There have been only 2 previous studies of soft-tissue sarcoma of the foot and ankle in adults,8,9 but both have included benign aggressive tumours (which, although locally aggressive, do not metastasize) and tumours located at the ankle. By focusing solely on soft-tissue sarcoma of the foot we hoped to better characterize the presentation, treatment and outcomes of this rare clinical entity.
In the present study, 50% of patients were referred after unplanned excision. Previous studies of soft-tissue sarcoma located elsewhere in the body have reported a somewhat lower rate (34%–43%),2,4 whereas Colterjohn and colleagues8 and Thacker and colleagues9 have found similarly high rates (67% and 56%, respectively). Despite our limited numbers, we suspect that the relative rarity of soft-tissue sarcoma of the foot compared with its benign counterparts and the inconspicuous nature of these lesions contributes to this higher rate. Patients who presented after unplanned excision fared worse with respect to oncological outcome than those who presented primarily. Half of the patients who presented after unplanned excision had documented progression of local disease (Fig. 1) and 1 had metastasized at the time of presentation. Moreover, 2 of the 3 patients who died, 2 of the 3 who had amputations (Fig. 2) and 1 of 2 who experienced local recurrences presented after unplanned excision. This finding is corroborated in previous studies; most notably, Thacker and colleagues9 found that unplanned excision more often necessitated free tissue transfer and more often lead to local recurrence. Despite the many publications and years of education regarding the importance of correctly assessing soft-tissue masses, it is apparent that there is still a need to educate clinicians that manage these patients primarily.
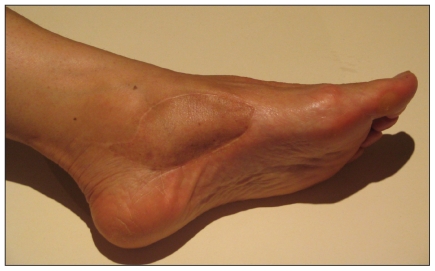
Fig. 1.
Unplanned excision of synovial sarcoma of the tarsal tunnel in a 25-year-old woman that necessitated extensive reresection. This picture was taken 4 years after reresection (including posterior tibial tendon), lateral arm free flap and adjuvant radiotherapy.
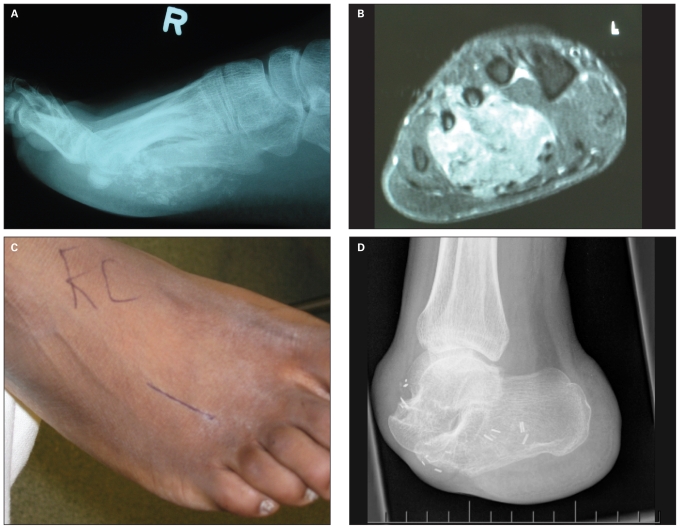
Fig. 2.
Unplanned excision frequently leads to subsequent limitations in the options for reconstruction. This case of a synovial sarcoma on the plantar aspect of the foot in a 28-year-old man illustrates this point. (A) A plain lateral roentgenogram of the foot showing typical ossification. (B) This axial T2-weighted magnetic resonance image demonstrates extensive involvement of the soft tissue and bones of the forefoot. (C) Photograph of the dorsal aspect of the foot showing a dorsal intermetatarsal incision done for biopsy of this plantar tumour. The placement of this biopsy tract unnecessarily limited the reconstructive options. (D) As the lesion was felt to be unresectable, a forefoot amputation was elected. The patient has developed slowly progressive lung disease.
Limb salvage has become the standard of treatment for most soft-tissue sarcoma of the extremities. There are a number of differences between our experience with limb salvage in the foot and what is reported for limb salvage elsewhere in the extremities. First, recent studies report an amputation rate of 6%–8% for soft-tissue sarcoma.1,4 In the present study, limb salvage was attempted whenever possible. Not surprisingly, owing to the anatomy of the foot, the amputation rate remained high (19%). Second, limb salvage resulted in a microscopically positive resection margin in 4 of 13 patients (31%). This can be compared with an 11% rate from a previous study of lower-limb sarcoma.1 Finally, after resection, free tissue transfer was used to restore both the soft-tissue envelope and the mechanical stability of the foot (Figs. 3 and 4). This also was required more often (69%) compared with a previous study of lower-extremity soft-tissue sarcoma (16%).1 These differences are likely owing to the limited amount of soft tissue in the foot and, thus, the proximity of the tumours to the bones, joints and neurovascular structures. Our experience also differs substantially from that reported by the 2 other studies of soft-tissue sarcoma of the foot8,9 (Table 2). We more frequently accepted microscopically positive margins and used free tissue transfer in combination with radiation therapy. Despite these differences, our oncologic outcomes (local recurrence rate and disease free survival) are comparable to the 2 aforementioned studies.
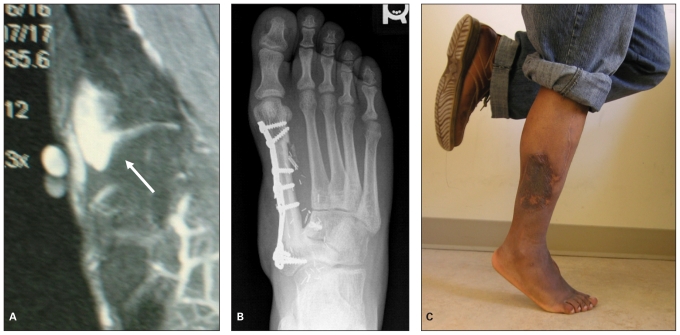
Fig. 3.
Limb salvage surgery with reconstruction using free tissue transfer and adjuvant radiation therapy can yield excellent functional results, as demonstrated in this case of synovial sarcoma in a 21-year-old man. He was treated with neoadjuvant radiotherapy then resection with reconstruction of the first ray using a free vascularized fibular graft with an island of skin. (A) T2-weighted magnetic resonance image shows the lesion involving the first metataso–cuneiform joint (arrow). (B) Radiograph taken 3 years after surgery. (C) Photograph taken at 3-year follow-up showing good plantar flexion and excellent strength.
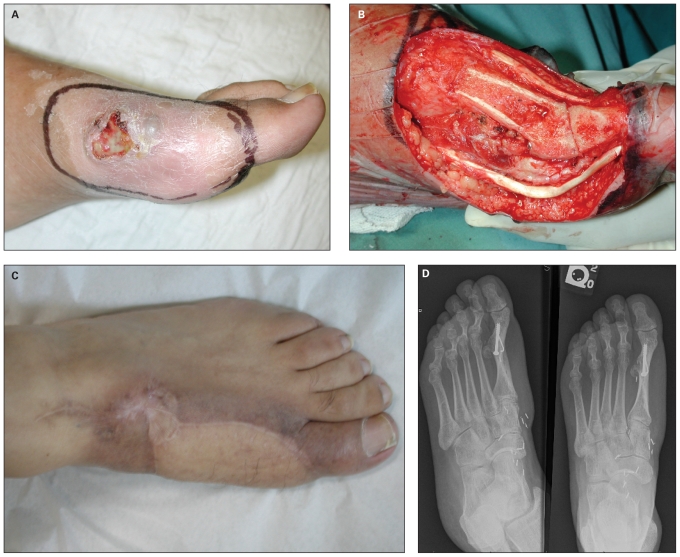
Fig. 4.
Successful treatment of a recurrent fungating sarcoma of the medial side of the forefoot in a 46-year-old man using neoadjuvant radiation, marginal resection, arthrodesis and free tissue transfer. (A) Photograph taken at the time of surgery after the completion of neoadjuvant radiotherapy. (B) Intraoperative photograph showing hemiresection of the medial aspect of the proximal phalanx of great toe and first metatarsal with fusion of the first metatarsophalangeal joint. (C) Photograph taken 2 years after coverage with a lateral arm flap. (D) Anteroposterior and oblique radiographs of the foot showing healed arthodesis of the first metatarsophalangeal joint.
Table 2.
Comparison of treatment and oncologic outcomes among studies of soft-tissue sarcoma of the lower extremity
| Outcome, % | Popov et al.1 | Colterjohn et al.8 | Thacker et al.9 | Current series |
|---|---|---|---|---|
| Amputation | 8 | 17 | 27 | 19 |
| Positive margin | 11 | 13 | 3 | 25 |
| Radiation therapy | 39 | 86 | 35 | 92 |
| Free tissue transfer | 16 | 28 | 37 | 69 |
| Local recurrence | 16 | 7 | 14 | 15 |
| Disease-free survival (duration) | 63 (est 5 yr) | 80 (52 mo) | 73 (99 mo) | 77 (72 mo) |
Est = estimated.
Soft-tissue sarcoma is best managed with wide surgical resection. Radiation therapy is often added to enhance local control of the disease. With this management, the local recurrence rate is reported to be below 10%.19 The finding of tumour cells at the edge of the resected tumour specimen (the so-called positive margin) has been linked with increased local recurrence and limited survival.20–22 One study found a recurrence rate of 32% for patients who underwent unplanned excision.12 In contrast, excision with a planned positive margin in the context of radiation therapy was shown to yield a very low local recurrence rate of 3.6%.12 In the current study, this treatment strategy was used in 70% of the patients, leading to a local recurrence rate of 15%. Whereas this rate exceeds what is usually targeted for sarcoma management, it lies at the low end of the range of recurrence rates (10%–38%) reported in other studies recently reviewed.1,19 Moreover, this number seems reasonable given that half of the patients in our study presented after unplanned excision.
The impact of these local recurrences on the metastatic spread of disease and consequently on survival is a subject of considerable controversy. The traditional view was that the risks of both local recurrence and metastatic progression were increased in the setting of inadequate resection. The implication of this view is that resections ought to be wide to prevent recurrence and minimize subsequent metastasis. Recently, some authors have challenged this notion by suggesting that metastases are due to the inherent tumour biology rather than inadequate resection. As such, a patient with an aggressive tumour may suffer metastasis regardless of the local treatment.23–26 Conversely, reduction in local recurrence rates does not translate into improved survival.20,21,27,28 Moreover, patients who have a local recurrence, but without concurrent metastasis, may have a prognosis equal to those who remained free of local disease after treatment.29 The implication of this view on treatment is that if preventing metastasis and mortality is the goal, then accepting nothing less than wide resection may not always be necessary. The results of the current study seem to support the latter view: first, patients who were treated with wide resection or amputation had a lower recurrence rate but did not always survive, and, second, patients who suffered a local recurrence did not always die. In other words, local recurrence may not always need to be prevented at all costs: a balance should be found between adequate oncologic resection and limb reconstruction.
The average postoperative functional scores in the current study, (28% with MSTS 1987 and 90% with TESS) suggest very good function and compare favourably with figures reported by others for limb salvage in soft-tissue sarcoma (30% with MSTS 1987 and 83%–88% with TESS).6,30 We recognize the very limited number of patients for whom results were available, but all had free flaps and/or arthrodesis.
Limitations
There are a number of limitations to this study. First, this is a retrospective review with a small number of patients. This is an inherent limitation in the study of a rare disease, and it precludes drawing any statistically significant conclusions. Second, we chose to limit our review to a homogeneous group of soft-tissue sarcomas occurring in the foot. In so doing, we had a smaller number of patients on whom to report, but we believe that this homogeneity has enabled us to show something quite different than what is known about sarcoma in other locations. Finally, the small numbers of patients who had amputations and of patients who completed the functional assessments severely limited our ability to make conclusions about oncological and functional differences between amputation and limb salvage. In the future, larger series of soft-tissue sarcoma of the foot will be needed to validate our findings.
Footnotes
Previously presented at the combined meeting of the American Orthopaedic Association and the Canadian Orthopaedic Association, Québec, Que., June 4–7, 2008; the International Symposium on Limb Salvage, Hamburg, Germany, Sept. 11–14, 2007; and the Meeting of the Musculoskeletal Tumor Society, St. Louis, Mo., May 11, 2007.
Competing interests: None declared.
Contributors: Drs. Latt, Turcotte and Isler designed the study. Drs. Latt and Wong acquired the data. Drs. Latt and Turcotte analyzed the data and wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Popov P, Tukiainen E, Asko-Seljaavaara S, et al. Soft tissue sarcomas of the lower extremity: surgical treatment and outcome. Eur J Surg Oncol. 2000;26:679–85. doi: 10.1053/ejso.2000.0980. [DOI] [PubMed] [Google Scholar]
- 2.Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66:81–7. doi: 10.1002/(sici)1096-9098(199710)66:2<81::aid-jso2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol. 1985;3:1344–8. doi: 10.1200/JCO.1985.3.10.1344. [DOI] [PubMed] [Google Scholar]
- 4.Ghert MA, Abudu A, Driver N, et al. The indications for and the prognostic significance of amputation as the primary surgical procedure for localized soft tissue sarcoma of the extremity. Ann Surg Oncol. 2005;12:10–7. doi: 10.1007/s10434-004-1171-3. [DOI] [PubMed] [Google Scholar]
- 5.Gross E, Rao BN, Bowman L, et al. Outcome of treatment for pediatric sarcoma of the foot: a retrospective review over a 20-year period. J Pediatr Surg. 1997;32:1181–4. doi: 10.1016/s0022-3468(97)90678-3. [DOI] [PubMed] [Google Scholar]
- 6.Gerrand CH, Wunder JS, Kandel RA, et al. The influence of anatomic location on functional outcome in lower-extremity soft-tissue sarcoma. Ann Surg Oncol. 2004;11:476–82. doi: 10.1245/ASO.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita G, Matsumoto M, Maruoka T, et al. Bone and soft tissue tumours of the foot: review of 83 cases. J Orthop Surg (Hong Kong) 2002;10:173–8. doi: 10.1177/230949900201000212. [DOI] [PubMed] [Google Scholar]
- 8.Colterjohn NR, Davis AM, O’Sullivan B, et al. Functional outcome in limb-salvage surgery for soft tissue tumors of the foot and ankle. Sarcoma. 1997;1:67–74. doi: 10.1080/13577149778326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thacker MM, Potter BK, Pitcher JD, et al. Soft tissue sarcomas of the foot and ankle: impact of unplanned excision, limb salvage, and multimodality therapy. Foot Ankle Int. 2008;29:690–8. doi: 10.3113/FAI.2008.0690. [DOI] [PubMed] [Google Scholar]
- 10.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York (NY): Springer; 2002. Soft tissue sarcoma; pp. 221–8. [Google Scholar]
- 11.Enneking WF. Musculoskeletal tumor surgery. Vol. 1. New York (NY): Churchill Livingstone; 1983. [Google Scholar]
- 12.Gerrand CH, Wunder JS, Kandel RA, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br. 2001;83:1149–55. doi: 10.1302/0301-620x.83b8.12028. [DOI] [PubMed] [Google Scholar]
- 13.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 14.Davis AM, Wright JG, Williams JI, et al. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508–16. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 15.Enneking WF. Limb salvage in musculoskeletal oncology. New York (NY): Churchill-Livingston; 1987. Modification of the system for functional evaluation in the surgical management of musculoskeletal tumors; pp. 626–39. [Google Scholar]
- 16.Davis AM, Devlin M, Griffin AM, et al. Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: a matched case-control study. Arch Phys Med Rehabil. 1999;80:615–8. doi: 10.1016/s0003-9993(99)90161-2. [DOI] [PubMed] [Google Scholar]
- 17.Gerrand CH, Bell RS, Wunder JS, et al. The influence of anatomic location on outcome in patients with soft tissue sarcoma of the extremity. Cancer. 2003;97:485–92. doi: 10.1002/cncr.11076. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld GS, Morris CG, Scarborough MT, et al. Adjuvant radiotherapy in the management of soft tissue sarcoma involving the distal extremities. Am J Clin Oncol. 2006;29:62–5. doi: 10.1097/01.coc.0000197660.23734.24. [DOI] [PubMed] [Google Scholar]
- 19.Davis AM, O’Sullivan B, Turcotte R, et al. Canadian Sarcoma Group, NCI Canada Clinical Trial Group Randomized Trial. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JJ, Leung D, Heslin M, et al. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–52. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 21.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–89. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 22.Stojadinovic A, Leung DH, Hoos A, et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–34. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafson P. Soft tissue sarcoma. Epidemiology and prognosis in 508 patients. Acta Orthop Scand Suppl. 1994;259:1–31. [PubMed] [Google Scholar]
- 24.Trovik CS, Bauer HC. Local recurrence of soft tissue sarcoma a risk factor for late metastases. 379 patients followed for 0.5–20 years. Acta Orthop Scand. 1994;65:553–8. doi: 10.3109/17453679409000913. [DOI] [PubMed] [Google Scholar]
- 25.Trovik CS, Bauer HC, Alvegård TA, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36:710–6. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 26.Trovik CS, Gustafson P, Bauer HC, et al. Consequences of local recurrence of soft tissue sarcoma: 205 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand. 2000;71:488–95. doi: 10.1080/000164700317381199. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe KK, Pollock RE, Ellis LM, et al. Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer. 1994;73:1652–9. doi: 10.1002/1097-0142(19940315)73:6<1652::aid-cncr2820730617>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Yang JC, Rosenberg SA. Surgery for adult patients with soft tissue sarcomas. Semin Oncol. 1989;16:289–96. [PubMed] [Google Scholar]
- 29.Gustafson P, Dreinhöfer KE, Rydholm A. Metastasis-free survival after local recurrence of soft-tissue sarcoma. J Bone Joint Surg Br. 1993;75:658–60. doi: 10.1302/0301-620X.75B4.8331127. [DOI] [PubMed] [Google Scholar]
- 30.Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol. 2000;73:206–11. doi: 10.1002/(sici)1096-9098(200004)73:4<206::aid-jso4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]