Abstract
We aimed to determine the acceptability and feasibility of a pentablet-based software program, PAINReportIt®-Plus, as a means for patients with cancer in home hospice to report their symptoms and differences in acceptability by demographic variables. Of the 131 participants (mean age = 59 ± 13, 58% women, 48.1% African American), 44% had never used a computer, but all participants easily used the computerized tool and reported an average computer acceptability score of 10.3 ± 1.8, indicating high acceptability. Participants required an average of 19.1 ± 9.5 minutes to complete the pain section, 9.8 ± 6.5 minutes for the medication section, and 4.8 ± 2.3 minutes for the symptom section. The acceptability scores were not statistically different by demographic variables but time to complete the tool differed by racial/ethnic groups. Our findings demonstrate that terminally ill patients with cancer are willing and able to utilize computer pentablet technology to record and describe their pain and other symptoms. Visibility of pain and distress is the first step necessary for the hospice team to develop a care plan for improving control of noxious symptoms.
Introduction
Computerized technology has advanced documentation of patient-reported outcomes in many clinical settings, but not hospice. Researchers demonstrated that cancer outpatients are able to use computers to report and document their psychosocial distress,1–3 quality of life,4–6 and pain.7–9 Computer documentation is a part of practice in some hospice settings, but the clinician serves as the primary recorder. To our knowledge, patients with cancer have not contributed autonomously to their hospice record by documenting their symptoms using computer technology. The purpose of our study was to determine (1) the acceptability of an interactive pentablet-based software program for cancer hospice patients and the feasibility of them using it to report and simultaneously document their symptoms and (2) the influence of age, gender, race/ethnicity, and education level on the acceptability and feasibility indicators.
There is usefulness in an instrument that will allow patients with cancer to report their pain and symptoms while remaining efficient, accurate, and acceptable in hospice settings. Patient-driven use of a computerized tool can also reduce the sources of error and bias in symptom assessment, resulting in more effective symptom management.2,3,5,10
Methods
Design
We conducted this cross-sectional comparative study as part of a larger National Institutes of Health (NIH)-funded study (RO1NR009092). The Institutional Review Board at the University of Illinois at Chicago approved the study.
Setting and subjects
Recruited from three Chicago metropolitan area hospices. Patients were eligible if they were: (1) receiving home care level of hospice service; (2) diagnosed with advanced cancer; (3) experiencing pain or taking pain medications on a daily basis; (4) able to speak, read, and write English; (5) 18 years or older; and (6) expected to live at least 2.5 to 3 weeks at the time of study enrollment, as indicated by a Palliative Performance Scale (PPS) score of 40 or higher.11 Patients were excluded if they were legally blind or had cognition or physical impairments making it impossible to complete the study instrument.
Procedures
A hospice staff member screened newly admitted patients according to the inclusion and exclusion eligibility criteria, introduced the study to eligible patients, and acquired verbal consent from the patient to be contacted by the researcher. The researcher visited all interested patients in their homes as close to admission as possible to explain study procedures and obtain signed informed consent and HIPAA forms. After being trained by the researcher and with the researcher's assistance as needed, the patient completed the PAINReportIt®-Plus.
Instrument
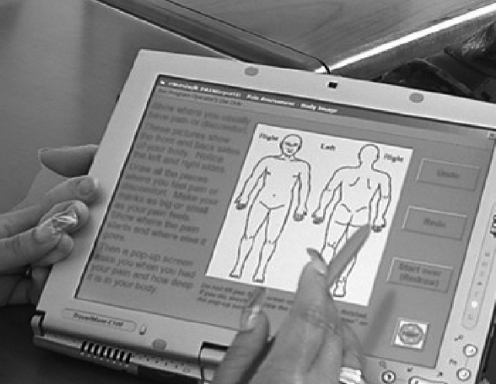
PAINReportIt®-Plus is an interactive, touch-screen computer program for multidimensional pain assessment that includes an electronic version of the valid and reliable McGill Pain Questionnaire (MPQ12; Fig. 1; Nursing Consult LLC, Seattle, WA). The 15 screens for pain assessment cover the same information as the MPQ, that is, current, worst, and least-pain intensity in past 24 hours (0–10 scale),13,14 pain location (number of pain sites),15,16 pattern,14 and quality.12 The program also includes sections for medication and demographics questions. Finally, PAINReportIt®-Plus included the valid and reliable Symptom Distress Scale (SDS)17 that measures 11 symptoms common to patients with cancer.
FIG. 1.
PAINReportIt® displayed on an Acer pentablet computer. Used with permission, Diana J. Wilkie, © 2008.
We measured the acceptability of the PAINReportIt®-Plus with the Computer Acceptability Scale (CAS).9 It was a 13-item dichotomous scale that queried subjects about the ease of using the touch screen, the understandability of directions and questions, and the environmental conditions (lighting, screen glare, etc.) that might impact on one's ability to complete the program. In this sample, the CAS internal consistency was 0.62.
Analysis
The program code automatically calculated the time-to-complete PAINReportIt®-Plus using the computer's internal clock, which allowed calculation of elapsed time for each section. We exported data from the Microsoft Access (Microsoft, Redmond, WA) database to SPSS (SPSS, Chicago, IL) for statistical analysis. We examined acceptability scores for differences by age (<50, 51–60, and > 61), gender, race/ethnic (Caucasian, African American, Hispanic, and Other), and education level (high school or less, vocational or associate degree, baccalaureate or higher degree) groups using χ2 and analysis of variance (ANOVA).
Results
Characteristics of the 131 participants appear in Table 1. Noteworthy is the fact that 48.1% of the participants were African Americans.
Table 1.
Demographic Characteristics (n = 131)
| Variable | Category | Number (%) | Mean ± SD (Min-Max) |
|---|---|---|---|
| Gender | Male | 55 (42) | |
| Female | 76 (58) | ||
| Age | Male | 59.8 ± 13.0 (27–85) | |
| Female | 58.5 ± 13.6 (20–92) | ||
| Age group | 18–50 years old | 31 (23.7) | |
| 51–60 years old | 42 (32.1) | ||
| ≥61 years old | 58 (44.3) | ||
| Education level | ≤9th grade | 14 (10.7) | |
| High school | 52 (39.7) | ||
| Vocational/Associate degree | 24 (39.7) | ||
| ≥Some college | 34 (26.0) | ||
| Missing | 7 (5.3) | ||
| Race/Ethnicity | African American | 63 (48.1) | |
| Caucasian | 51 (38.9) | ||
| Hispanic | 9 (6.9) | ||
| Other | 8 (6.1) | ||
| Marital status | Living with spouse | 44 (33.6) | |
| Living alone | 80 (61.1) | ||
| Missing | 7 (5.3) | ||
| Computer use | Daily | 44 (33.6) | |
| Weekly | 9 ( 6.9) | ||
| Monthly | 11 ( 8.4) | ||
| Never use | 57 (43.5) | ||
| Missing | 10 ( 7.6) | ||
| Computer access | Yes | 88 (67.2) | |
| No | 33 (25.2) | ||
| Missing | 10 ( 7.6) | ||
| Computer acceptability scale | 10.3 ± 1.8 (4–12) | ||
| Time taken to complete each part of the PAINReportIt®-Plus tool | 1. Demographic Data | 127a | 10.1 ± 4.9 min (0.2–30) |
| 2. McGill Pain Questionnaire | 129a | 19.1 ± 9.5 min (3–56) | |
| 3. Medications | 128a | 9.8 ± 6.5 min (0.3–46) | |
| 4. Symptom Distress Scale | 131 | 4.8 ± 2.3 min (0.9–13) | |
| All four components (total min) | 125a | 44.1 ± 16.7 min (17–99) | |
| 5. Computer Acceptability Scale | 96b | 9.2 ± 5.4 min (2–34) | |
| All five components (total min) | 95b | 53.2 ± 19.4 min (22–116) |
Missing: some participants skipped the item.
Subjects excluded with extreme minimum time (0).
Subjects excluded with extreme maximum time >60 min for 13-item scale.
SD, standard deviation.
Acceptability
Although nearly half had never used a computer and one fourth had no access to computers, all participants easily used this tool (Table 1). There were no discernible gender or race/ethnic group differences in those reporting that they never used a computer. The proportion of participants who reported they never used a computer was 28% for those 50 years old or younger, 35% for those between 51 and 60 years, and 67% of those over 61 years, a statistical difference (χ2(2) = 15.31, p = 0.000). Similarly, the proportion was 55% for those who completed high school or less education, 46% for those who had vocational education, and 24% for those with a baccalaureate or higher degree, a statistical difference (χ2(2) = 6.4, p = 0.05). On the 0–13 CAS, participants reported an average score of 10.3 ± 1.8, indicating high acceptability. Despite the differences in computer use, the CAS scores were not statistically different based on age, gender, race/ethnic, or education level groups.
Feasibility: Time taken on the PAINReportIt®-Plus
The patients required an average of 44.1 ± 16.7 minutes to complete the four sections of PAINReportIt®-Plus. Table 1 presents time required to complete each section. Time taken to complete the entire tool was not statistically different by age, gender, or education level groups, but differed statistically by race/ethnic group (F(3,121) = 3, p < 0.05). African Americans required the shortest amount of time to complete the tool (40.0 ± 15.6) compared to Hispanics (43.9 ± 13.6), Caucasians (47.6 ± 17.5), and others (54.7 ± 16.5). Although there was a main significant effect for race/ethnic group, no pairwise comparison was statistically significant.
Discussion
We discovered that terminally ill patients are willing and able to utilize computer pentablet technology to record their pain and other symptoms and there were no differences in their acceptability by age, gender, race/ethnic, or education level groups. These findings have significant implications for hospice and palliative care as well as the field of medical eschatology. For those people involved in end-of-life care, contribution to eschatological study is sometimes difficult because the balance between compassion and detachment is hard to find. To gain empirical knowledge, one must consider this balance yet stand at a distance and record. Although challenging, there is a growing recognition that to be effective caregivers for the dying, we need a full spectrum of research to generate knowledge. Since the modern hospice movement has focused so intently on caregiving to the exclusion of data collection, research has been a more recent phenomenon, steadily increasing over the past two decades.18
Research informs practice, and from our study, there is promise of a more creative and effective management of time, the most precious of commodities in hospice care. Our participants were comfortable reporting intense pain directly to a computer rather than to a data collector. Furthermore, participants were very adept at utilizing technology, requiring two minutes more on average to self-report at home than patients with cancer required to describe their pain in a hospital setting.9 Allowing hospice patients to provide patient-reported pain and symptom outcomes can increase patient self-efficacy and free the clinicians and caregivers to focus on interpreting the data rather than collecting it, and then on managing the symptoms. Furthermore, if the home hospice nurse is not busy entering the patient's data on a paper form or computer, he or she can use the time saved to attend more closely to the needs of the loved ones and caregivers while the patient is entering his/her own data.
This study also deepens our understanding of the phenomenon of dying by expanding the options for patient reported outcomes. Some patients have difficulty describing their symptoms to health care professionals, or they may feel compelled to maintain a veneer of stoicism for family and loved ones. For such individuals, the use of a computerized program relieves inhibitions and allows for protected subjective disclosure. This method of patient reporting is clinically promising as it may increase visibility of symptoms and lessen the pervasiveness of unrelieved and uncontrolled symptoms experienced at the end-of-life. Our findings enlarge the clinical inventory of knowledge-gathering strategies, a benefit to both patient and clinician.
Advances in clinical research are important for evidence-based practice, which enhances compassionate caregiving. While informatics and hospice care may seem an unlikely coupling, our desire to understand the phenomena of dying and to generate new knowledge in the ancient study of eschatology, spurs us toward creativity. These findings testify to the delicate balance obtainable between detachment and compassion. Further research will reveal the extent to which technology can expand the potential for patient-generated outcomes, enlightening and informing practice.
In summary, we reported previously19 some initial comments from study participants that indicated their enthusiasm for the novel way to report their symptoms. We now provide quantitative evidence of the high acceptability and feasibility and demonstrate that dying people are able to use computer technology to report their symptom experience without differences in acceptability based on age, gender, race/ethnic or education level. Technology thus offers patients dying of cancer an efficient instrument to communicate with clinicians the evidence about their symptoms that only the dying have to offer.
Acknowledgment
This publication was made possible by Grant Number R01 NR009092 from the National Institutes of Health, National Institute of Nursing Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Nursing Research. We are grateful to Kevin Grandfield for editorial assistance, Jan Durham, Glendra Smith, Gloria Booth, Lisa Fernandez, Valerie Ralston, Vivian Moore, Carolyn Rainer, Edith Ransom, and Donna Schiller for patient screening, Noreen Hoenig and Hope Engeseth for participant recruitment and data collection, and the hospice patients who ventured into the technology world as study participants. We also thank Dr. Ruth McCorkle for permission to computerize the Symptoms Distress Scale for use in our research.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Allenby A. Matthews J. Beresford J. McLachlan SA. The application of computer touch-screen technology in screening for psychosocial distress in an ambulatory oncology setting. Eur J Cancer Care (Engl) 2002;11:245–253. doi: 10.1046/j.1365-2354.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 2.Cull A. Gould A. House A. Smith A. Strong V. Velikova G. Wright P. Selby P. Validating automated screening for psychological distress by means of computer touchscreens for use in routine oncology practice. Br J Cancer. 2001;85:1842–1849. doi: 10.1054/bjoc.2001.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLachlan SA. Allenby A. Matthews J. Wirth A. Kissane D. Bishop M. Beresford J. Zalcberg J. Randomized trial of coordinated psychosocial interventions based on patient self-assessments versus standard care to improve the psychosocial functioning of patients with cancer. J Clin Oncol. 2001;19:4117–4125. doi: 10.1200/JCO.2001.19.21.4117. [DOI] [PubMed] [Google Scholar]
- 4.Carlson LE. Speca M. Hagen N. Taenzer P. Computerized quality-of-life screening in a cancer pain clinic. J Palliat Care. 2001;17:46–52. [PubMed] [Google Scholar]
- 5.Velikova G. Brown JM. Smith AB. Selby PJ. Computer-based quality of life questionnaires may contribute to doctor–patient interactions in oncology. Br J Cancer. 2002;86:51–59. doi: 10.1038/sj.bjc.6600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright EP. Selby PJ. Crawford M. Gillibrand A. Johnston C. Perren TJ. Rush R. Smith A. Velikova G. Watson K. Gould A. Cull A. Feasibility and compliance of automated measurement of quality of life in oncology practice. J Clin Oncol. 2003;21:374–382. doi: 10.1200/JCO.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Huang HY. Wilkie DJ. Zong SP. Berry D. Hairabedian D. Judge MK. Farber S. Chabal C. Developing a computerized data collection and decision support system for cancer pain management. Comput Inform Nurs. 2003;21:206–217. doi: 10.1097/00024665-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Wilkie DJ. Huang HY. Berry DL. Schwartz A. Lin YC. Ko NY. Chen A. Gralow J. Lindsley SK. Fitzgibbon D. Cancer symptom control: Feasibility of a tailored, interactive computerized program for patients. Fam Community Health. 2001;24:48–62. [PubMed] [Google Scholar]
- 9.Wilkie DJ. Judge MK. Berry DL. Dell J. Zong S. Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manage. 2003;25:213–224. doi: 10.1016/s0885-3924(02)00638-3. [DOI] [PubMed] [Google Scholar]
- 10.Velikova G. Wright EP. Smith AB. Cull A. Gould A. Forman D. Perren T. Stead M. Brown J. Selby PJ. Automated collection of quality-of-life data: A comparison of paper and computer touch-screen questionnaires. J Clin Oncol. 1999;17:998–1007. doi: 10.1200/JCO.1999.17.3.998. [DOI] [PubMed] [Google Scholar]
- 11.Weng LC. Huang HL. Wilkie DJ. Hoenig NA. Suarez ML. Marschke M. Durham J. Predicting survival with the palliative performance scale in a minority-serving hospice and palliative care program. J Pain Symptom Manage. 2009;37:642–648. doi: 10.1016/j.jpainsymman.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melzack R. The McGill pain questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 13.Wilkie D. Lovejoy N. Dodd M. Tesler M. Cancer pain intensity measurement: Concurrent validity of three tools—finger dynamometer, pain intensity number scale, visual analogue scale. Hospice J. 1990;6:1–13. doi: 10.1080/0742-969x.1990.11882662. [DOI] [PubMed] [Google Scholar]
- 14.Wilkie DJ. Kim YO. Molokie RE. Suarez ML. Wang ZJ. Ngamkham S. Zong S. Composite Pain Index (CPI): Reliability, validity, sensitivity of a new multidimensional outcome measure. in press.
- 15.Wilkie DJ. Keefe FJ. Coping strategies of patients with lung cancer-related pain. Clin J Pain. 1991;7:292–299. doi: 10.1097/00002508-199112000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Wilkie DJ. Keefe FJ. Dodd MJ. Copp LA. Behavior of patients with lung cancer: Description and associations with oncologic and pain variables. Pain. 1992;51:231–240. doi: 10.1016/0304-3959(92)90264-C. [DOI] [PubMed] [Google Scholar]
- 17.McCorkle R. Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 18.Buchholz W. Combined role for caregiver and scientist. Am J Hosp Palliat Care. 1985;2:22. doi: 10.1177/104990918500200101. [DOI] [PubMed] [Google Scholar]
- 19.Gorman G. Forrest J. Stapleton SJ. Hoenig NA. Suarez M. Marschke M. Durham J. Suarez ML. Wilkie DJ. Massage for cancer pain: A study with university and hospice collaboration. J Hosp Palliat Nurs. 2008;10:191–197. doi: 10.1097/01.njh.0000319160.89854.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]