Abstract
Objective
Previous work has demonstrated that corticospinal facilitation from 20Hz repetitive transcranial magnetic stimulation (rTMS) was greater during a second rTMS session 24 hours after the first. We sought to determine whether such metaplasticity is dependent on a particular phase of the sleep-wake/circadian cycle.
Methods
Twenty healthy participants received two sessions of 20 Hz rTMS over the hand motor cortex (M1) spaced 12 hours apart, either over-day or overnight.
Results
Baseline corticospinal excitability did not differ by group or session. The time-of-day of Session 1 did not influence the relative increase in excitability following rTMS. However, the increase in excitability from the second rTMS session was 2-fold greater in the overnight group.
Conclusions
When a night with sleep follows rTMS to M1, the capacity to induce subsequent plasticity in M1 is enhanced, suggesting sleep-wake and/or circadian-dependent modulation of processes of metaplasticity.
Significance
TMS treatment of neuropsychiatric disorders entails repeated sessions of rTMS. Our findings suggest that the timing of sessions relative to the sleep-wake/circadian cycle may be a critical factor in the cumulative effect of treatment. Future studies using this paradigm may provide mechanistic insights into human metaplasticity, leading to refined strategies to enhance non-invasive stimulation therapies.
Keywords: TMS, transcranial magnetic stimulation, plasticity, metaplasticity, sleep, circadian
Introduction
Repetitive transcranial magnetic stimulation (rTMS), or the repeated application of TMS pulses to one scalp location at a particular frequency, has modulatory effects on the excitability of cortical networks that outlast the duration of stimulation (Pascual-Leone et al., 1998). High-frequency stimulation at 20 Hz to the motor cortex generally increases corticospinal excitability in humans, which can be measured as augmented motor evoked potentials (MEPs) (Maeda et al., 2000b, Maeda et al., 2000a) or other electrophysiological measures such as the silent period or the slope of the input-output recruitment curve (Khedr et al., 2007). This facilitating effect of high-frequency rTMS may be mediated by enhancing synaptic efficacy through mechanisms of long-term potentiation (LTP) (Tokay et al., 2009), though the underlying neurobiological substrate remains unresolved. In an animal model, repeated sessions of high-frequency rTMS across several days increased the capacity to induce LTP by subsequent electrical stimulation (Ogiue-Ikeda et al., 2003). In humans, 20 Hz rTMS induced a significantly greater increase in MEPs when performed 24 hours following a prior rTMS session using the same stimulation parameters (Maeda et al., 2000b). These data suggest that rTMS may prime neuroplastic mechanisms, such that the capacity to induce plasticity by stimulation increases as a result of repeated prior stimulation sessions. This change in the ability of a synapse to undergo neuroplastic changes as a result of its recent history is known metaplasticity, a process that regulates plasticity and may serve a synaptic homeostatic function (Abraham, 2008, Mockett and Hulme, 2008).
Human behavioral studies of memory consolidation, which is presumed to be a marker for cellular processes of plasticity (Martin and Morris, 2002), have suggested that sleep may provide a unique physiological environment to promote processes of plasticity (Diekelmann and Born, 2010). Sleep shortly following learning has been associated with improved memory performance for verbal material (Plihal and Born, 1997, Ellenbogen et al., 2006), motor skills (Walker et al., 2002, Fischer et al., 2002, Robertson et al., 2004), perceptual judgments (Karni et al, 1994, Stickgold et al., 2000), extracting a hidden rule (Wagner et al., 2004), and spatial navigation (Peigneux et al., 2004, Ferrara et al., 2008). A contemporary hypothesis suggests that waking activity causes synapses to undergo net potentiation, challenging the finite capacity of space, energy, and metabolic resources in the brain (Vyazovskiy et al., 2008). Sleep, and in particular slow wave activity (SWA) during sleep, is hypothesized to promote synaptic homeostasis by causing a general downscaling of synaptic efficacy so that subsequent potentiation may be possible (Tononi and Cirelli, 2003). SWA can increase in specific cortical regions during sleep that follows learning, suggesting a tight coupling between local synaptic activity and sleep homeostasis (Huber et al., 2004). Similarly, non-invasive stimulation techniques to induce LTP-like changes in the motor cortex also lead to local sleep SWA changes (De Gennaro et al., 2008). However, challenging the idea that sleep uniquely benefits memory and plasticity, some neuronal circuits may be selectively potentiated during the waking day (Cohen et al., 2005, Cohen and Robertson, 2006). Therefore, we hypothesized that the metaplasticity induced from rTMS, previously demonstrated over a 24-hour interval (Maeda et al., 2000b), was dependent on a particular phase of the sleep-wake/circadian cycle.
Methods
Subjects
Twenty healthy volunteers participated in this study (12 males, 8 females; mean age 28.8 years, range 24 to 41). All were right-handed according to the Oldfield Questionnaire (Oldfield, 1971). Participants had no significant psychiatric disorders, medical conditions, or contraindications for TMS (Rossi et al., 2009). The protocol consisted of two rTMS sessions spaced approximately 12 hours apart, and participants were randomly and equally distributed into two groups in which the 12-hour interval spanned either: 1) over-day; or 2) overnight. The morning sessions occurred approximately 3 hours after usual waking. The timing of sessions was based on each participants’ habitual sleep-wake schedule rather than specific clock times in order to minimize variability in the length of time awake and approximate circadian phase at the time of each rTMS session (Cohen et al., 2010). Participants were encouraged to get at least six hours of sleep prior to the morning session and were discouraged from napping during the day. All participants gave written informed consent to the study, which had been approved by the Beth Israel Deaconess Medical Center Committee of Clinical Investigation and was conducted at the Harvard-Thorndike Clinical Research Center.
Experimental procedures
Detailed methods used in this study have been previously reported (Maeda et al., 2000b, Maeda et al., 2000a). Briefly, participants were seated comfortably and relaxed in a reclining chair, and monitoring electrodes were placed on the right abductor pollicis brevis (APB) muscle belly and tendon for recording of motor evoked potentials (MEPs). The electromyogram (EMG) signals were amplified (Dantec Counterpoint EMG, Dantec, Denmark) with a gain of 1.0 mV/division and band pass filtered at 20–1000 Hz. EMG signals were digitized with a sampling rate of 2 kHz and stored on a computer for off-line analysis. A commercially available stimulator (Magstim Super Rapid, Magstim Inc., UK) and a 70mm figure-of-eight coil (Magstim Inc.) were used. The handle of the coil was oriented posteriorly to the participants’ head and rotated 135 degrees parallel to the midsaggital plane. The optimal scalp site in the left primary motor cortex (M1) for eliciting maximal amplitude MEPs of the right APB was determined, and the minimal intensity required to induce at least a 50µV peak-to-peak amplitude in at least 6 out of 10 trials was defined as the resting motor threshold (RMT).
Experimental paradigm
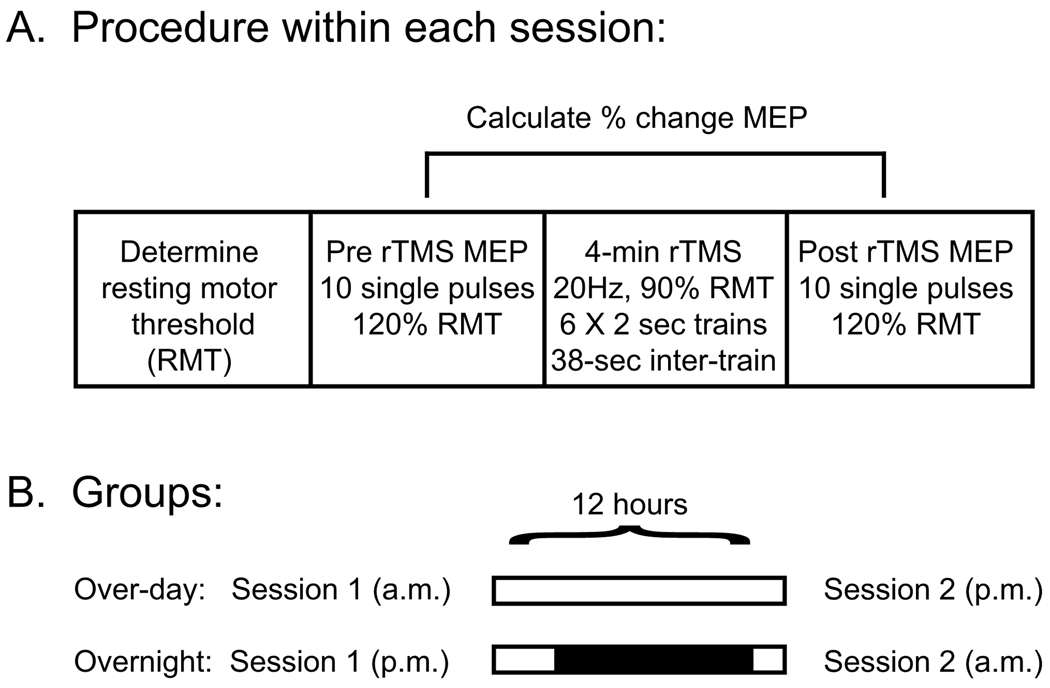
For a baseline measure of corticospinal excitability, 10 single pulses spaced approximately 10 seconds apart were applied to the optimal scalp position at 120% RMT, and the averaged area-under-the-curve (AUC) was calculated. Each participant then underwent a 4-minute rTMS protocol consisting of 20Hz stimulation to the left M1 at 90% RMT; trains lasted two seconds each (40 pulses), and six trains were delivered with a 38-second inter-train interval (240 pulses total). Following rTMS, evoked MEPs were again measured in the same manner as the baseline MEPs. The percent change in the AUC of evoked MEPs pre and post rTMS, which is a measure of corticospinal facilitation, was the primary outcome variable (Figure 1).
Figure 1. Schematic representation of the protocol.
(A) Testing procedures within each session are shown. For the determination of MEPs and for the 4-minute rTMS protocol, stimulation was applied to the left M1 with the intensity determined by each participants’ resting motor threshold (RMT). The primary outcome measure was the change in size of the averaged MEPs following rTMS relative to the baseline within each session (B) Individuals in the over-day group remained awake during the 12 hours between sessions; individuals in the overnight group were asked to maintain their habitual sleep schedule (denoted black shading).
Statistical analysis
All analyses were done using SPSS version 17. Two-factor repeated measures ANOVA was used to test for differences in the response to rTMS as a function of session (1 versus 2) and group (over-day versus overnight). Significant effects were followed by planned comparisons: paired t-tests were used to test for within group differences in the amount of MEP facilitation induced during Session 1 compared to Session 2. Separate unpaired t-tests were used to determine between group differences in the amount of facilitation induced within each session. Bonferroni corrected alpha (0.05/2=0.025) was used as the threshold for significance to correct for multiple comparisons within each set of tests. To exclude a possible confounding influence from differences in baseline corticospinal excitability, baseline MEPs were similarly compared within and between the groups.
Results
Baseline MEPs
A 2 × 2 repeated measures ANOVA with a within-participant factor [SESSION] and a between-participant factor [GROUP] revealed non-significant main effects of SESSION (F(1,18) = 0.26, p = 0.617) and GROUP (F(1,18) = 0.004, p = 0.949) and a non-significant SESSION × GROUP interaction (F(1,18) = 0.414, p = 0.528). Therefore, baseline MEPs measured prior to rTMS did not differ by the time-of-day of testing, and baseline MEPs during Session 2 were not influenced by the prior rTMS procedure from Session 1.
Effects of rTMS
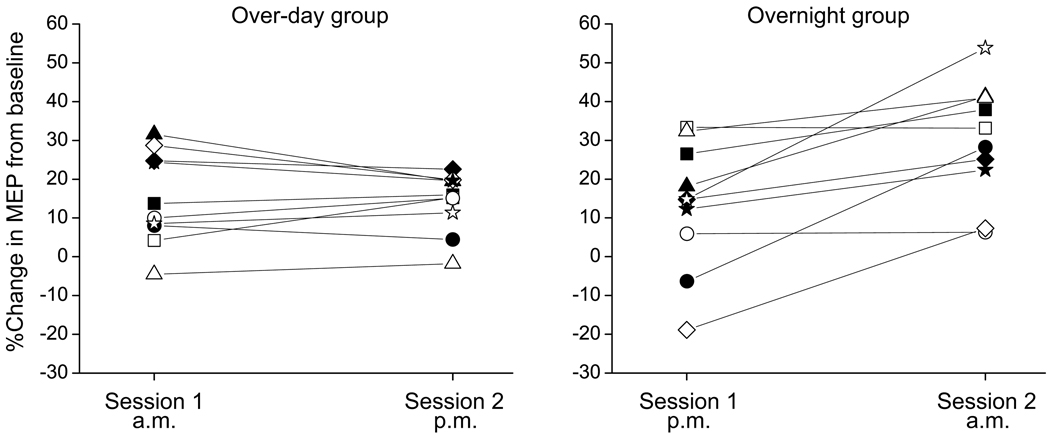
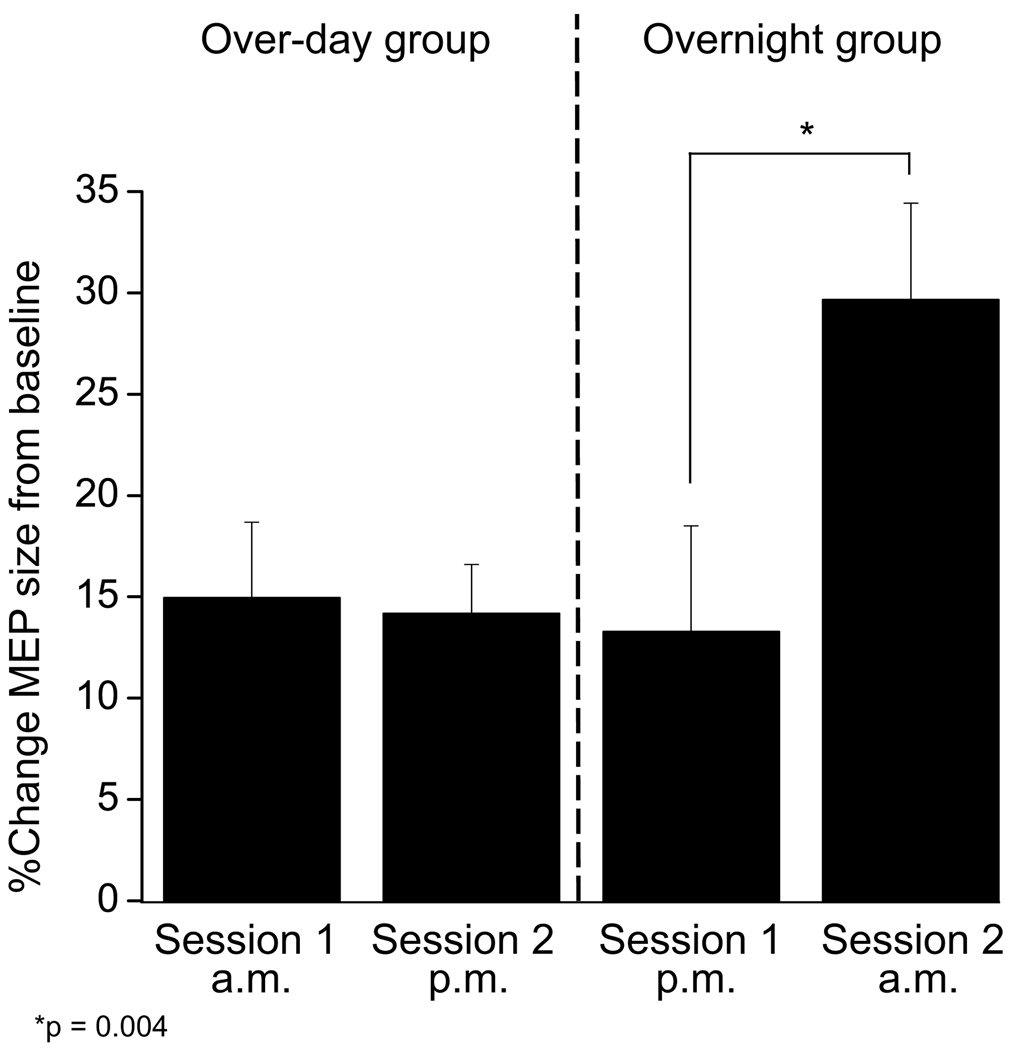
The effect of rTMS was calculated within each session as the percent change in MEP AUC following rTMS relative to the baseline MEP prior to rTMS. A 2 × 2 repeated measures ANOVA with a within-participant factor [SESSION] and a between-participant factor [GROUP] revealed a significant main effect of SESSION (F(1,18) = 10.41, p = 0.005) and a significant SESSION × GROUP interaction (F(1,18) = 12.54, p = 0.002). There was a non-significant main effect of GROUP (F(1,18) = 1.64, p = 0.22). Within the over-day group, the 4-minute 20 Hz rTMS protocol induced a 14.94 % increase in MEP size compared to baseline during Session 1 and a 14.18% increase during Session 2; the magnitude of increase was not significantly different between these sessions (paired t-test, t(9)=0.351, p=0.734). In contrast, within the overnight group, there was a 13.29% increase in MEP size during Session 1 and a 29.65% increase in MEP size during Session 2; this difference between sessions in the facilitating effects of 20 Hz rTMS was significant (paired t-test, t(9)=3.788, p=0.004). More robust rTMS facilitation of MEPs in Session 2 was seen in 8/10 participants in the overnight group (Figure 2). When comparing the over-day versus overnight groups, there was no difference in the effect of rTMS within Session 1 (unpaired t-test with unequal variances, t(18)=0.258, p=0.799), indicating that the facilitating effect of one session of rTMS did not depend on the time-of-day of testing. However, the effect of the second rTMS session was significantly greater when the 12-hour interval between sessions spanned a night with sleep compared to the waking day (unpaired t-test with unequal variances, t(18)=2.89, p=0.012, Figure 3).
Figure 2. Individual responses to rTMS.
Data for the 10 individuals in each group are shown. As can be seen, all but two participants in the overnight group demonstrated more robust facilitation as a result of Session 2 compared to Session 1.
Figure 3. Group responses to rTMS.
The time-of-day of Session 1 did not influence the baseline MEPs or the average facilitation in MEPs following the 4-minute 20Hz rTMS protocol. However, when a night of sleep followed the initial session of rTMS, there was a 2-fold increase in the average facilitation of MEPs within Session 2. This data suggests that the physiological milieu during the 12-hour offline interval is critical for the engagement of processes of metaplasticity following rTMS priming during Session 1.
Discussion
We found that the cumulative effect of two sessions of rTMS spaced 12 hours apart was dependent on the timing of the sessions relative to the normal sleep-wake cycle: evening followed by morning stimulation to M1 led to a significantly greater overall facilitation in MEPs compared to morning followed by evening stimulation. The time-of-day of the first rTMS session, which is a function of both the number of consecutive hours awake and circadian phase, did not influence the baseline measure of corticospinal excitability. This finding is consistent with a recent TMS study demonstrating that baseline measures of corticospinal excitability are not influenced by the duration of prior waking activity or recent sleep (Doeltgen and Ridding, 2010). In addition, there was no significant difference in the resting MEPs from Session 1 to Session 2. This suggests that the first session of rTMS did not lead to a persistent change in intrinsic corticospinal excitability. Finally, the capacity of rTMS to facilitate MEPs within the first session did not differ between morning and evening stimulation. Therefore, the initial induction of plasticity was not dependent on the time-of-day. However, when the 12-hour interval between the sessions spanned a night with sleep, there was a two-fold increase in the facilitating effect of the second rTMS session. This pattern of results suggests that rTMS can prime neuronal circuits to enhance the capacity to undergo subsequent plastic changes, but the full expression of this type of metaplasticity is dependent upon the phase of the sleep-wake/circadian cycle.
In a recent study, the circadian pattern of cortisol secretion was linked to a time-of-day modulation of the ability to induce neuroplasticity in an initial session (Sale et al., 2008). That study used a paired associative stimulation (PAS) to induce an LTP-like increase in corticospinal excitability. PAS involves the pairing of TMS to the cortex with contralateral stimulation of a peripheral nerve. The discrepancy between our findings and that of Sale et al. (Sale et al., 2008) may be dependent upon differences in the nature of the induced plasticity. The metaplastic regulation of heterosynaptic plasticity, which occurs across converging pathways and is tested with PAS, may be distinct from the regulation of homosynaptic plasticity within a single pathway as studied here. In addition, participants in the Sale et al. study were tested after two hours awake as opposed to three hours in this study. Cortisol concentrations peak near the time of habitual waking and steadily decline to a nadir near habitual sleep (Van Cauter, 2005). Despite this small difference in timing between the studies relative to the 24-hour cortisol profile, it is possible that one hour could be sufficient for cortisol levels to decline below a critical threshold and minimize the effects on plasticity. In support of this possibility, the effects of PAS on plasticity were influenced from morning to afternoon by a time delay of as little as four hours (Sale et al., 2007).
It is important to highlight a key distinction between the present study and other non-invasive studies of human plasticity. In this study, the observed behavioral effect of the first stimulation session on MEPs was no longer present 12 hours later at the time of the second session. However, following a night with sleep, the capacity of the network to undergo subsequent plastic changes was enhanced when the same stimulation was repeated. This example of metaplasticity should be distinguished from the modulation of plasticity induction (Abraham, 2008). For example, Nyffeler et al. (Nyffeler et al., 2006) used inhibitory theta-burst TMS to the frontal eye fields to prolong the latency of target-directed saccadic eye movements. The effect of a second theta-burst train spaced 15 minutes after the first disproportionately prolonged the duration of the behavioral effect and the magnitude of the response. Data from a control condition in that study demonstrated that the behavioral effect of the initial train lasts 30 minutes. Therefore, the second train occurred during the evolution of plastic changes induced from the first train. Their finding essentially demonstrates a dose-response effect of stimulation on the degree and persistence of induced plasticity. In a PAS study, Bergmann et al. (Bergmann et al., 2008) demonstrated that a night with sleep prolongs the behavioral effect of PAS on MEPs compared to the usual duration over the waking day. They demonstrated that MEP size decreased in all PAS conditions following a night with sleep, but the relative changes in MEP size induced from facilitatory and inhibitory PAS were preserved compared to control PAS. Their data is consistent with the synaptic homeostasis hypothesis (Tononi and Cirelli, 2003) and a general downscaling of synaptic connectivity during sleep but with preservation of the relative synaptic weights. This phenomenon is hypothesized to improve the signal-to-noise ratio of recent synaptic potentiation and to benefit memory. Therefore, sleep (or circadian phase) may modulate recently induced plastic changes. However, these studies showing modulation of conventional plasticity did not determine whether the capacity to induce subsequent plasticity from the identical stimulation conditions could increase long after the observable effects of the initial priming stimulus wore off, a key distinguishing feature between modulation of plasticity and metaplasticity (Abraham, 2008).
Processes of plasticity and metaplasticity are complex, protein synthesis-dependent, and they may share partially overlapping molecular mechanisms (Abraham, 2008). Gene expression of plasticity-related molecules and the ability to induce LTP can be influenced both by the sleep-wake history (Guzman-Marin et al., 2006, Gilestro et al., 2009, Tartar et al., 2006, Vyazovskiy et al., 2008) as well as the phase of the circadian cycle (Claridge-Chang et al., 2001, Dana and Martinez, 1984, Chaudhury et al., 2005). For example, sleep deprivation selectively increases the number of NR2A subunits of the glutamate NMDA receptors (Longordo et al., 2009), and a change in the ratio of NR2A/NR2B subunits shifts the threshold for subsequent LTP induction (Xu et al., 2009). In addition, high levels of endogenous adenosine from sleep loss impairs LTP through an adenosine A1 receptor mediated effect (Arrigoni et al., 2009). Alternatively, the effect of melatonin on calcium/calmodulin-dependent protein kinase (Fukunaga et al., 2002) or the effects of cortisol (Sale et al., 2008) may mediate circadian phase-dependent modulation of plasticity. Therefore, sleep-wake state and circadian phase may influence plastic or metaplastic processes through multiple, potentially interacting, mechanisms.
Repeated daily sessions of rTMS have become an effective therapeutic modality in human neuropsychiatric disorders such as depression (Padberg and George, 2009, Kim et al., 2009). Little is known about the optimal timing of repeated rTMS sessions to maximize plasticity and the cumulative effects of treatment. We chose to time study events relative to each participant’s habitual sleep-wake schedule so that participants within each group would be restricted to the comparable half of the sleep-wake/circadian cycle. While minimizing within-group variability improved the power to determine differences between the two groups, we were unable to determine whether there could be important variables within the overnight group that are critical for the observed effect. For example, it remains to be determined whether there is a critical window from the time of the first session before a night with sleep follows in order for the priming effect of rTMS to activate metaplastic processes: two stimulation sessions spaced 24-hours apart from morning to morning might show a smaller enhancement in the second session compared to sessions performed on two consecutive evenings. The duration of metaplasticity in experimental models can be as little as a few minutes to several days (Abraham, 2008), and it remains to be determined how long this effect lasts in humans. For example, would stimulation 48 hours later still be influenced by the first session, or would the system revert back to the initial capacity for plasticity?
In this study, the 12-hour over-day and overnight intervals of the normally circadian entrained (i.e. non-jet-lagged) sleep-wake cycle differed by both the prevailing sleep-wake state as well as the range of circadian phases across the intervals. Future studies can extend our findings by using protocols to disentangle whether the changes in metaplasticity across the sleep-wake/circadian cycle are dependent upon sleep or the circadian night during the offline interval between the sessions. For example, participants can be scheduled to live on a non-24-hour day to uncouple the normal phase relationship between sleep-wake and circadian cycles (Czeisler et al., 1999, Dijk et al., 1992, Wyatt et al., 2004, Cohen et al., 2010). Alternatively, nap protocols that have been used in memory consolidation paradigms (Mednick et al., 2003) can be used to determine whether a short interval of sleep during the circadian day is sufficient to enhance the effect of repeated sessions of rTMS. We should note that a limitation of this study is the lack of objective sleep measures. However, we caution interpretation of studies that rely on correlation of behavioral responses (such as memory consolidation) to features of the sleep EEG, since sleep timing, duration, and architecture (for example REM sleep and electrophysiological features of NREM sleep including the incidence, frequency, amplitude, and duration of sleep spindles) are strongly influenced by circadian phase (Czeisler et al., 1980, Dijk et al., 1997). Therefore, a correlation of behavior with overnight sleep variables does not remove the circadian confound (Dijk and Von Schantz, 2005), and the experimental dissociation of sleep-wake and circadian cycles is necessary to demonstrate which physiological process regulates metaplasticity.
Studies of different stimulation sites and parameters, which may engage distinct mechanisms of metaplasticity, may uncover unique benefits of wakefulness or the circadian day. Future experiments to extend these findings may lead to refined rTMS treatment protocols by: 1) determining the optimal timing between repeated sessions; 2) assessing the benefit of naps between rTMS sessions within a day; or 3) advancing hypothesis-driven pharmacological targets that may enhance metaplastic processes such as manipulating melatonin, cortisol, or adenosine systems. Ultimately, this study suggests that rTMS may be a useful model to study basic mechanisms of metaplasticity in humans.
Acknowledgements
Work on this study was supported by a grant from the National Center for Research Resources: Harvard Clinical and Translational Science Center (UL1 RR025758); and NIH grant K24 RR018875 to A.P.-L; L.O. was supported by NIH fellowship F32MH080493; C.F. was supported by the Foundation for Science and Technology, Portugal (SFRH / BPD / 44126 / 2008); M.E. is supported by the Mind, Brain, and Behavior Interfaculty Initiative at Harvard University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
DAC – received honoraria for presenting research findings at the Providence Sleep Research Interest Group.
APL – has served on scientific advisory boards for Novavision, Northstar Neuroscience, Nexstim; has received grant support from Nexstim
Other authors report no financial disclosures.
References
- Abraham WC. Metaplasticity: Tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Arrigoni E, Lu J, Vetrivelan R, Saper CB. Long-term synaptic plasticity is impaired in rats with lesions of the ventrolateral preoptic nucleus. Eur J Neurosci. 2009;30:2112–2120. doi: 10.1111/j.1460-9568.2009.07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann TO, Molle M, Marshall L, Kaya-Yildiz L, Born J, Roman Siebner H. A local signature of ltp- and ltd-like plasticity in human nrem sleep. Eur J Neurosci. 2008;27:2241–2249. doi: 10.1111/j.1460-9568.2008.06178.x. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the drosophila head. Neuron. 2001;32:657–671. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Off-line learning of motor skill memory: A double dissociation of goal and movement. Proc Natl Acad Sci USA. 2005;102:18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Robertson EM. Motor sequence consolidation: Constrained by critical time windows or competing components. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Wang W, Wyatt JK, Kronauer RE, Dijk D-J, Czeisler CA, Klerman EB. Uncovering residual effects of chronic sleep loss on human performance. Sci. Transl. Med. 2010;2:14ral3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: Its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- Dana RC, Martinez JL., Jr Effect of adrenalectomy on the circadian rhythm of ltp. Brain Res. 1984;308:392–395. doi: 10.1016/0006-8993(84)91086-2. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Fratello F, Marzano C, Moroni F, Curcio G, Tempesta D, Pellicciari MC, Pirulli C, Ferrara M, Rossini PM. Cortical plasticity induced by transcranial magnetic stimulation during wakefulness affects electroencephalogram activity during sleep. PLoS One. 2008;3:e2483. doi: 10.1371/journal.pone.0002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. l997;505(Pt 3):851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005;20:279–290. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- Doeltgen SH, Ridding MC. Behavioural exposure and sleep do not modify corticospinal and intracortical excitability in the human motor system. Clin Neurophysiol. 2010;121:448–452. doi: 10.1016/j.clinph.2009.11.085. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interferin with theories of sleep and memory: Sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Ferrara M, Iaria G, Tempesta D, Curcio G, Moroni F, Marzano C, De Gennaro L, Pacitti C. Sleep to find your way: The role of sleep in the consolidation of memory for navigation in humans. Hippocampus. 2008;18:844–851. doi: 10.1002/hipo.20444. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Horikawa K, Shibata S, Takeuchi Y, Miyamoto E. Ca2+/calmodulin-dependent protein kinase ii-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J Neurosci Res. 2002;70:799–807. doi: 10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006;575:807–819. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on rem sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Rothwell JC, Ahmed MA, Shawky OA, Farouk M. Modulation of motor cortical excitability following rapid-rate transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:140–145. doi: 10.1016/j.clinph.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kim DR, Pesiridou A, O'Reardon JP. Transcranial magnetic stimulation in the treatment of psychiatric disorders. Curr Psychiatry Rep. 2009;11:447–452. doi: 10.1007/s11920-009-0068-z. [DOI] [PubMed] [Google Scholar]
- Longordo F, Kopp C, Mishina M, Lujan R, Luthi A. Nr2a at ca1 synapses is obligatory for the susceptibility of hippocampal plasticity to sleep loss. J Neurosci. 2009;29:9026–9041. doi: 10.1523/JNEUROSCI.1215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000a;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000b;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Morris RG. New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: A nap is as good as a night. Nat Neurosci. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Hulme SR. Metaplasticity: New insights through electrophysiological investigations. J Integr Neurosci. 2008;7:315–336. doi: 10.1142/s0219635208001782. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Wurtz P, Luscher HR, Hess CW, Senn W, Pflugshaupt T, von Wartburg R, Luthi M, Muri RM. Extending lifetime of plastic changes in the human brain. Eur J Neurosci. 2006;24:2961–2966. doi: 10.1111/j.1460-9568.2006.05154.x. [DOI] [PubMed] [Google Scholar]
- Ogiue-Ikeda M, Kawato S, Ueno S. The effect of repetitive transcranial magnetic stimulation on long-term potentiation in rat hippocampus depends on stimulus intensity. Brain Res. 2003;993:222–226. doi: 10.1016/j.brainres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, Luxen A, Maquet P. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J. Cogn. Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res. 2007;181:615–626. doi: 10.1007/s00221-007-0960-x. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 2008;28:8285–8293. doi: 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- Tartar J, Ward C, McKenna J, Thakkar M, Arrigoni E, McCarley R, Brown R, Strecker R. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokay T, Holl N, Kirschstein T, Zschorlich V, Kohling R. High-frequency magnetic stimulation induces long-term potentiation in rat hippocampal slices. Neurosci Lett. 2009;461:150–154. doi: 10.1016/j.neulet.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Van Cauter E. Endocrine physiology. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. Fourth ed. Philadelphia: Elsevier, Saunders; 2005. [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- Xu Z, Chen RQ, Gu QH, Yan JZ, Wang SH, Liu SY, Lu W. Metaplastic regulation of long-term potentiation/long-term depression threshold by activity-dependent changes of nr2a/nr2b ratio. J Neurosci. 2009;29:8764–8773. doi: 10.1523/JNEUROSCI.1014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]