Abstract
Prednisone was shown to induce hyperglycemia in dogs submitted to total pancreatectomy and pancreatic islet autotransplantation. The hyperglycemia caused by a 10-day course of prednisone, 1 mg/kg/day, starting on the day of operation was reversible within 1 week after steroid discontinuance. Three weeks after prednisone was stopped, there was no detectable adverse effect on glucose homeostasis as judged by fasting blood sugar levels and intravenous glucose tolerance test results. Four months after transplantation, glucose disappearance was delayed in animals previously treated with the prednisone compared with those previously treated with prednisone plus insulin or control animals. This was accompanied by lower insulin values on intravenous glucose tolerance testing and suggests a long-term subtle effect on islet function. The mechanism of the steroid effect is not known. However, this model could be used to test the diabetogenicity of other immunosuppressive agents including cyclosporine, FK 506, and azathioprine.
Since steroids were introduced as immunosuppressive agents for clinical renal transplantation,1 prednisone has been widely used in many different kinds of organ transplants. A steroid diabetogenic effect was recognized at the outset.2 The complication was not surprising because it was already known from small animal experiments that these agents could induce “steroid diabetes.”3 Although many subsequent reports have emphasized the etiologic role of steroids in post-transplant diabetes, 4–7 Leonard et al. 8 were unable to show that prednisone was toxic to human pancreatic islets in vitro or after their transplantation into nude mice. The aim of this study was to reexamine this controversial question in an animal model in which the factor of rejection was excluded. Delineation of the presence or absence of a steroid effect on islet graft function is of immediate clinical relevance in view of the resurgence of interest in human islet transplantation for diabetes mellitus.9–12
MATERIAL AND METHODS
Fourteen adult mongrel dogs weighing 19 to 25 kg were randomly divided into three groups. Each dog was made diabetic by total pancreatectomy, and the excised pancreas was used as the source of islet autografts. Dogs in group I (control group; n = 6) were not given prednisone or insulin after islet autotransplantation. Dogs in group II (prednisone group; n = 4) were treated with prednisone, 1 mg/kg body weight/day (The Upjohn Co., Kalamazoo, Mich.), orally for 10 days after islet autotransplantation. Dogs in group III (prednisone plus insulin; n = 4) were treated with prednisone, 1 mg/kg/day, plus 25 to 30 units/day regular human insulin given subcutaneously (Eli Lilly & Co., Indianapolis, Ind.) for 10 days after islet autotransplantation.
The method used for islet isolation was a modification of those published previously.13, 14 The dog’s pancreatic ducts were cannulated during the pancreatectomy, and 250 ml Hanks’ solution containing 1 mg/ml collagenase (type p, lot 09; Boehringer Mannheim Biochemicals, Indianapolis, Ind.) was injected. The pancreas was then placed in a digestion chamber and subjected to 20 to 25 minutes of enzymatic digestion.14 The separated islets were purified with Euro-Collins Ficoll gradients9 (densities were 1.108, 1.096, and 1.037) in a cell separator (COBE 2991; Cobe Laboratories, Lake-wood, Colo.).15, 16 The purified islets were cultured overnight in CMRL 1066 plus 10% fetal calf serum plus penicillin (100 units/ml) plus streptomycin (100 μm/ml) at 37° C, 95% air, 5% CO2. The islets were injected into the portal vein 1 day after pancreatectomy. The islet yield expressed as 150 unit size equivalents/kg recipient weight17 was not significantly different in the three groups.
Fasting plasma glucose levels were determined in all animals with a Beckman glucose analyzer (Beckman Instruments, Inc., Fullerton, Calif.) before and daily for 3 weeks after islet autotransplantation and weekly thereafter. An intravenous glucose tolerance test (IVGTT) was performed in all animals 3 weeks and 4 months after transplantation, with 0.5 gm glucose/kg body weight. Samples were obtained before and 5, 10, 30, 60, 90, and 120 minutes after glucose injection. The K value was calculated from the straight line describing the change in glucose over time according to the standard formula.18 Statistics are presented as mean ± SEM, and significance of differences was assessed by Student’s t test.
RESULTS
The mean (± SEM) numbers of islets transplanted in the three groups were as follows: group I, 12,689 ± 1647 islets/kg weight; group II, 15,866 ± 945 islets/kg weight; and group II, 14,320 ± 1245 islets/kg weight (Table I). The daily postoperative fasting blood sugar levels for the first 3 postoperative weeks are shown in Fig. 1. After islet transplantation, in the untreated control group, all dogs remained normoglycemic for the duration of the study (mean plasma glucose level, 102 ± 2.5 mg/dl). The dogs treated with prednisonefor 10 days after islet transplantation were hyperglycemic during therapy (mean plasma glucose level, 198 ± 10 mg/dl). However, the fasting blood sugar levels returned to normal within the first week after the prednisone was stopped (Fig. 1). The dogs treated with prednisone plus insulin for 10 days were normoglycemic during this time and remained so after discontinuation of treatment (mean plasma glucose level, 106 ± 9.8 mg/dl). Four months after transplantation, fasting plasma glucose levels were 119 ± 11 mg/dl in group I, 123 ± 13 mg/dl in group II, and 123 ± 11 mg/dl in group III (data not shown).
Table I.
Description of dog islet grafts
| Group | Dog No. | Body weight (kg) | Sex | Pancreatic weight (gm) | Islet no. (× 1000)* | Islet/kg dog weight |
|---|---|---|---|---|---|---|
| Nonprednisone | 1 | 19 | F | 44.5 | 157.5 | 8,289.5 |
| 2 | 20 | F | 41 | 284.3 | 14,215 | |
| 3 | 18 | M | 39 | 247.9 | 13,772.2 | |
| 4 | 20 | F | 40 | 387.7 | 19,385 | |
| 5 | 21 | F | 38 | 198.9 | 9,473 | |
| 6 | 23 | M | 40 | 250.2 | 10,878 | |
| Mean | 20.17 ± 0.70 | 40.5 ± 0.92 | 254.42 ± 32.2 | 12,688.78 ± 1646.3 | ||
| Prednisone | 1 | 21 | F | 38 | 385.3 | 18,345.2 |
| 2 | 23 | F | 43 | 358.9 | 15,605.4 | |
| 3 | 22 | M | 40.2 | 302.4 | 13,745.5 | |
| 4 | 19 | M | 37 | 299.6 | 15,768.4 | |
| Mean | 21.25 ± 0.85 | 39.5 ± 1.33 | 336.55 ± 21.2 | 15,866.13 ± 945.2 | ||
| Prednisone + insulin | 1 | 24 | M | 39.9 | 357.8 | 14,908.5 |
| 2 | 22 | M | 44.4 | 368.2 | 16,736.4 | |
| 3 | 25 | F | 52 | 370.2 | 14,808 | |
| 4 | 24 | M | 35.2 | 380.2 | 10,829.5 | |
| Mean | 23.75 ± 0.63 | 42.88 ± 3.57 | 369.1 ± 4.59 | 14,320.6 ± 1245.2 |
Number of islets of an average size equivalence of 150 units.
Fig. 1.
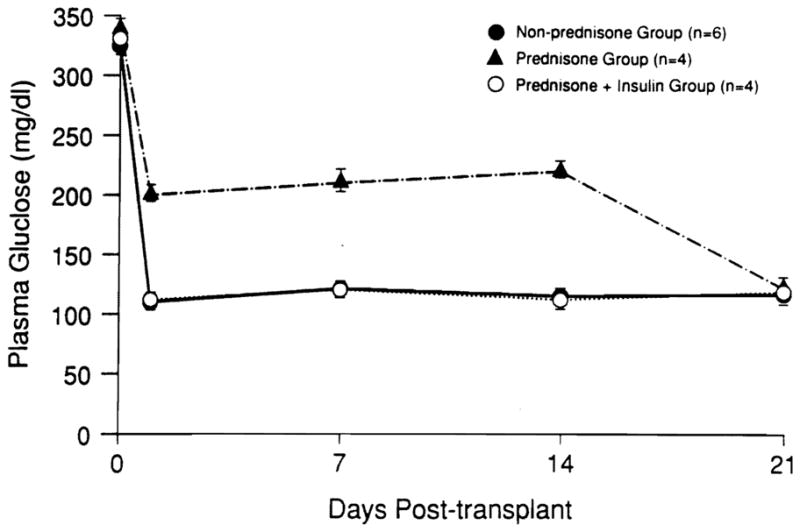
Weekly fasting plasma glucose levels of (non-prednisone-treated group) control group (n = 6), prednisone-treated group (n = 4), and prednisone plus insulin group (n = 4) up to 3 weeks after islet autotransplantation.
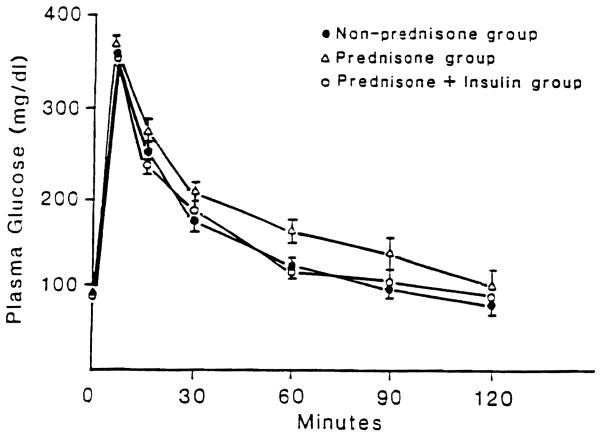
IVGTTs were performed 3 weeks and 4 months after transplantation. There was no significant difference among the groups, although there was a trend for a lower k value in group II. Four months after transplantation, fasting plasma glucose levels among the three groups were indistinguishable. However, the disappearance rate of intravenously injected glucose was slower in group II than in groups I and III (Table II; Fig. 2). Insulin values obtained during the 4-month IVGTT are shown in Fig. 3. Insulin secretion in all groups was similar at the late points on the IVGTT; however, at the points before 30 minutes, insulin was lower in the prednisone-treated group compared with the control and prednisone plus insulin groups.
Table II.
Plasma glucose levels and k values after islet autotransplantation
| Control |
Prednisone |
Prednisone + insulin |
||||
|---|---|---|---|---|---|---|
| 3 wk | 4 mo | 3 wk | 4 mo | 3 wk | 4 mo | |
| FPG (mg/dl) | 115 ± 14 | 119 ± 11 | 120 ± 24 | 123 ± 13 | 99 ± 19 | 123 ± 11 |
| k Values | 2.14 | 2.41 | 1.57 | 1.21* | 2.37 | 2.42 |
| %/min | ± 0.42 | ± 0.42 | ± 0.25 | ± 0.09 | ± 0.40 | ± 0.35 |
p < 0.05 compared with control and prednisone plus insulin groups.
FPG, Fasting plasma glucose.
Fig. 2.
IVGTT 4 months after islet autotransplantation.
Fig. 3.
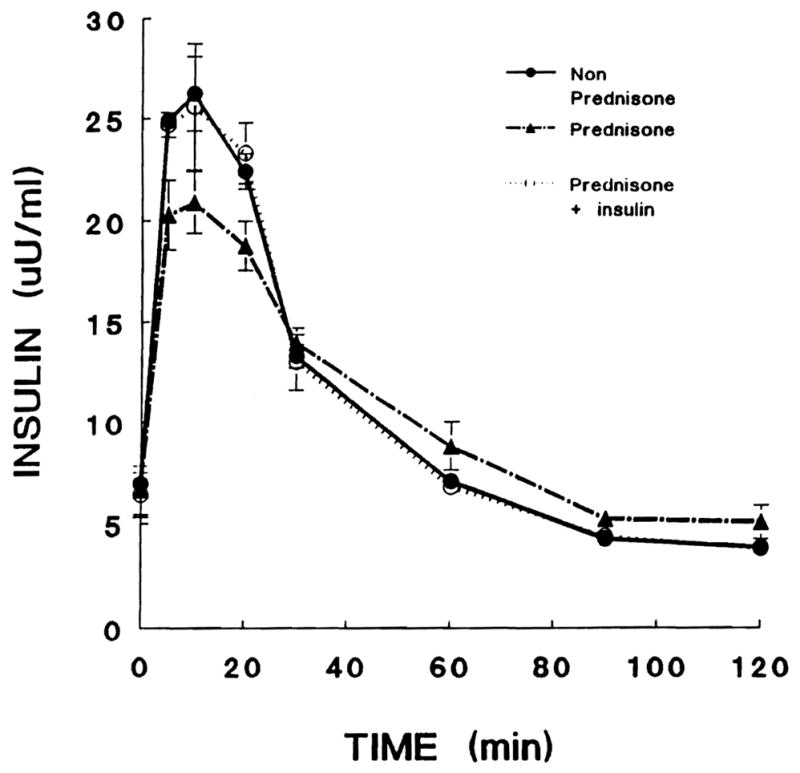
Insulin response to intravenously injected glucose 4 months after islet autotransplantation.
DISCUSSION
In this study we examined the effect of 1 mg/kg prednisone on islet autografts in which variables related to rejection could be excluded. The amount of prednisone in this study was chosen because of observations by Leonard et al.8 that 1 mg/kg/ day prednisone did not inhibit human fetal pancreatic growth in nude mice and this dose range is commonly used in whole organ transplantation in humans.
As expected, 1 mg/kg/day prednisone markedly elevated the fasting glucose levels of the islet recipients during acute treatment. The effect was reversible on discontinuation of prednisone. The IVGTT results 3 weeks after discontinuation of steroid therapy were not significantly different among the groups. Surprisingly, 4 months after islet autotransplantation, glucose disappearance rate was delayed in the prednisone group compared with the control group and prednisone plus insulin, group, although fasting glucose levels were normal in all groups. These results indicated that there was a subtle long-term diabetogenic effect of an acute course of steroids. Studies performed in animals with reduced B cell mass, or in animals infused chronically with glucose, indicate that hyperglycemia per se can lead to the development of B cell unresponsiveness to glucose.19–21 This could explain why in our study insulin treatment given acutely prevented steroid-induced hyperglycemia, resulting in a long-term beneficial effect on the transplanted islets. Although in vitro the effects of high concentrations of glucose on islets are rapidly reversible, 22 there is experimental evidence that hyperglycemia might irreversibly damage B cells.23 In fact, cats rendered hyperglycemic for 1 month remained diabetic despite discontinuing intraperitoneal glucose administration, and their islets showed hydropic degeneration.24 This was also observed in partially pancreatectomized dogs intravenously infused with glucose.23 It has been shown that chronic hyperglycemia induces metabolic adaption rather than degenerative lesions.25 However, short-term exposure to high glucose concentrations leads normal human islets to a selective insensitivity to glucose, which is reversible with restoration of normoglycemia.22 Another possibility is that steroids alter other islet cell functions, such as glucose transporters, as has been shown in peripheral targets for insulin action.26 This would not explain why insulin treatment had a beneficial effect in our experiments. Steroid-induced hyperglycemia is not necessarily caused by a direct effect on islet cell function. It could be explained by alterations in hepatic glucose production, peripheral glucose disposal, or a combination of these alterations. 5 Our data indicate some residual effect on islet function. Hyperglycemia caused by high-dose steroid therapy for a protracted period could adversely affect the islets as with Lukens’ diabetes.24
More recently, Kaufman et al.27 showed that prednisone at a dose of 1 or 2 mg/kg/day had permanent toxic effects on canine islet autograft function, as evidenced by loss of the islet grafts. One of the possible reasons for the disparity between their results and ours could be the difference in the mean number of transplanted islets. In our study, dogs received 15,867 ± 945 islets/kg recipient body weight in the prednisone group and 14,320 ± 1245 islets/kg in the prednisone plus insulin group. In contrast, in their study, only 7600 ± 1200 islets/kg were transplanted in prednisone-treated animals. This represents a significant (p < 0.05) decrease in transplanted B cell mass. Another possible difference is the duration of steroid treatment. In our study we examined the potential consequences of short-term prednisone treatment, whereas the duration of steroid treatment was longer in the study of Kaufman et al. Nevertheless, we observed impaired insulin secretion and higher glucose levels during IVGTT 4 months after islet transplantation in the prednisone-treated animals. This could indicate a potential long-term toxic effect of prednisone on islet engraftment or function even when a short-course treatment is used. This toxic effect appears to be reversed by acute insulin treatment. Further long-term studies will be necessary to address the issue of mechanisms and reversibility of the steroid effect on islet function. This is an important issue in the field of islet allotransplantation because steroids represent the dose-maneuverable component of multiple drug regimens that are used to prevent or reverse graft rejection in the early postoperative period.
Acknowledgments
Supported by research grants from the Juvenile Diabetes Foundation International grant 1911421, the Veterans Administration, and Project grant DK 29961 from the National Institutes of Health, Bethesda, Md.
References
- 1.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–95. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE. Experience in renal transplantation. Vol. 117. Philadelphia: WB Saunders; 1964. p. 161.p. 221. [Google Scholar]
- 3.Ingle DJ. Experimental steroid diabetes. Diabetes. 1956;5:187. doi: 10.2337/diab.5.3.187. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz DS, Simmons RL, Callender CO, et al. Steroid diabetes in renal transplant recipients: pathogenic factors and prognosis. Surgery. 1973;5:759–65. [PubMed] [Google Scholar]
- 5.Fennell RS, Van Deusen J, Riley WJ. Steroid-induced diabetes in pediatric renal transplant recipients. Int J Pediatr Nephrol. 1983;4:103. [PubMed] [Google Scholar]
- 6.Gunnarsson R, Lundgren G, Magnusson G, Ost L, Groth C. Steroid diabetes: a sign of overtreatment with steroids in renal graft recipients? Scand J Urol Nephrol. 1980;54:135. [PubMed] [Google Scholar]
- 7.Ruiz JO, Simmons RL, Callender CO, Kjellstrand CM, Buselmeier TJ, Najarian JS. Steroid diabetes in renal transplant recipients: pathogenetic factors and prognosis. Surgery. 1973;73:759–65. [PubMed] [Google Scholar]
- 8.Leonard DK, Landry AS, Sollinger HW, et al. Prednisone, azathioprine, and cyclosporine A toxicity on human fetal pancreas. J Surg Res. 1989;46:625–32. doi: 10.1016/0022-4804(89)90033-4. [DOI] [PubMed] [Google Scholar]
- 9.Tzakis AG, Ricordi C, Alejandro R, et al. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet. 1990;336:402–6. doi: 10.1016/0140-6736(90)91946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricordi C, Tzakis AG, Carroll P, et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplantation. 1992;53:407–14. doi: 10.1097/00007890-199202010-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnock GL, Kneteman NM, Ryan E, et al. Normoglycaemia after transplantation of freshly isolated and cryopreserved pancreatic islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:55–8. doi: 10.1007/BF00404026. [DOI] [PubMed] [Google Scholar]
- 12.Scharp DW, Lacy PE, Santiago JV, et al. Results of our first nine intraportal islet allografts in type 1, insulin-dependent diabetic patients. Transplantation. 1991;51:76–85. doi: 10.1097/00007890-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 14.Ricordi C, Socci C, Davalli A, et al. Isolation of elusive pig islet. Surgery. 1990;107:688–94. [PubMed] [Google Scholar]
- 15.Lake SP, Bassert D, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes. 1989;38(suppl):143–5. doi: 10.2337/diab.38.1.s143. [DOI] [PubMed] [Google Scholar]
- 16.Alejandro R, Strasser S, Zucker PZ, et al. Isolation of pancreatic islets from dogs. Transplantation. 1990;50:207–10. doi: 10.1097/00007890-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ricordi C, Gray DWR, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–95. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 18.Alsever RN, Gotlin RW. Handbook of endocrine tests in adults and children. 2. Chicago: Year Book; 1980. p. 95. [Google Scholar]
- 19.Leahy JL, Bonner-Weir S, Weir GC. Abnormal glucose regulation of insulin secretion in models of reduced B-cell mass. Diabetes. 1984;33:667–73. doi: 10.2337/diab.33.7.667. [DOI] [PubMed] [Google Scholar]
- 20.Leahy JL, Cooper HE, Deal DA, Weir GC. Chronic hvperglycemia is associated with impaired glucose influence on insulin secretion. J Clin Invest. 1986;77:908–15. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leahy JL, Bonner-Weir S, Weir GC. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest. 1988;81 :1407–14. doi: 10.1172/JCI113470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davalli AM, Ricordi C, Socci C, et al. Abnormal sensitivity to glucose of human islets cultured in a high glucose medium: reversibility after an additional culture in a normal glucose medium. J Clin Endocrinol Metab. 1991;72:202–3. doi: 10.1210/jcem-72-1-202. [DOI] [PubMed] [Google Scholar]
- 23.Imamura T, Koffler M, Helderman JH, et al. Severe diabetes induced in subtotally depancreatectomized dogs by sustained hyperglycemia. Diabetes. 1988;37:600–9. doi: 10.2337/diab.37.5.600. [DOI] [PubMed] [Google Scholar]
- 24.Dohan FC, Lukens FDW. Lesions of pancreatic islets produced in cats by administration of glucose. Science. 1947;105:183–4. doi: 10.1126/science.105.2720.183. [DOI] [PubMed] [Google Scholar]
- 25.Andersson A. Long-term effects of glucose on insulin release and glucose oxidation by mouse pancreatic islets maintained in tissue culture. Biochem J. 1973;140:377–82. doi: 10.1042/bj1400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olefsky JM. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975;56:1499–508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman DB, Morel P, Condie R, et al. Beneficial and detrimental effects of RBC-adsorbed antilymphocyte globulin and prednisone on purified canine islet autograft and allograft function. Transplantation. 1991;51:37–42. doi: 10.1097/00007890-199101000-00005. [DOI] [PubMed] [Google Scholar]