Abstract
Vascular remodeling is an important pathological feature of pulmonary arterial hypertension (PAH), which leads to increased pulmonary vascular resistance, with marked proliferation of pulmonary artery smooth muscle cells (SMC) and/or endothelial cells (EC). Successful treatment of experimental PAH with a platelet-derived growth factor (PDGF) receptor tyrosine kinase inhibitor offers the perspective of “reverse remodeling” (i.e., the regression of established pulmonary vascular lesions). Here we ask the question: which forms of pulmonary vascular remodeling are reversible and can such remodeling caused by angiogenic proliferation of EC be reversed? It is important to emphasize that the report showing reduction of vascular remodeling by PDGF receptor tyrosine kinase inhibitor showed only a reduction of the pulmonary artery muscularization in chronic hypoxia and monocrotaline models, which lack the feature of clustered proliferated EC in the lumen of pulmonary arteries. The regression of vascular muscularization is an important manifestation, whereby proliferative adult SMC convert back to a nonproliferative state. In contrast, in vitro experiments assessing the contribution of EC to the development of PAH demonstrated that phenotypically altered EC generated as a consequence of a vascular endothelial growth factor receptor blockade did not reverse to normal EC. Whereas it is suggested that the proliferative state of SMC may be reversible, it remains unknown whether phenotypically altered EC can switch back to a normal monolayer-forming EC. This article reviews the pathogenetic concepts of severe PAH and explains the many forms in PAH with reversible or irreversible remodeling.
Keywords: remodeling, PAH, endothelial cell, smooth muscle cell
Pulmonary vascular remodeling is an important pathological feature of pulmonary arterial hypertension (PAH), which leads to increased pulmonary vascular resistance and reduced compliance, with marked proliferation of pulmonary artery smooth muscle cells (SMC) and/or endothelial cells (EC) resulting in the obstruction of blood flow in the resistance pulmonary arteries (1, 2). In a recent Perspective article, Rai and coworkers (3) characterized severe PAH as a quasi-neoplastic, angioproliferative disorder, and this concept provides a new framework for antiproliferative, antiangiogenic therapy in severe PAH.
Successful treatment with a platelet-derived growth factor (PDGF) receptor tyrosine kinase inhibitor of experimental pulmonary hypertension offers the prospect of “reverse remodeling” (i.e., the regression of established pulmonary vascular lesions) (4).
This review asks the question: which forms of pulmonary vascular remodeling are reversible and can the remodeling caused by the angiogenic proliferation of EC be reversed (5–8)? It is important to emphasize that the report showing a reduction of vascular remodeling by PDGF receptor tyrosine kinase inhibitor (4) showed only the reduction of pulmonary artery muscularization in chronic hypoxia and monocrotaline models, which lack the features of clustered proliferated EC in the lumen of pulmonary arteries (9, 10).
Further studies are necessary to characterize pulmonary vessel phenotypes to determine the reversibility of vascular remodeling and to stop angiogenesis in PAH.
SMC AND EC IN THE MANY FORMS OF PAH
Pulmonary vascular remodeling is characterized by the thickening of all three layers of the blood vessel wall (i.e., the adventitia, the media, and the intima). Such thickening is due to hypertrophy (cell growth) and/or hyperplasia (proliferation) of the predominant cell type within each of the layers, i.e., fibroblasts, SMC, and EC, as well as increased deposition of extracellular matrix components including collagen, elastin, and fibronectin (11). The thickening of the media occurs consistently in the arteries, at all levels of the pulmonary arterial tree, and less frequently in the veins (12). In addition, there is an extension of new smooth muscle into the partially muscular and nonmuscular peripheral arteries, and this is termed muscularization. This may be due to the differentiation of precursor cells (i.e., pericytes and “intermediate” cells, the latter being intermediate between pericytes and muscle cells in structure into SMC) (9). The other possibilities are the differentiation of fibroblasts and circulating mononuclear mesenchymal precursors into muscle cells (13) and the endothelial to mesenchymal transition (EMT) (14). The proliferation of pulmonary artery SMC in PAH is enhanced, whereas apoptosis is depressed (15–18). Many factors drive SMC proliferation, including bone morphogenetic protein receptor-2 (BMPR-2) mutations (19), the de novo expression of the anti-apoptotic protein survivin (15, 16), the increased expression/activity of the serotonin transporter (SERT) (20, 21) and the increased expression/activity of PDGF receptor. Dysfunctional voltage-sensitive potassium channels have been described in the pulmonary artery SMC from patients with idiopathic PAH (22), and impaired K+ channel performance has been linked to pulmonary vasoconstriction, whereas vasorelaxation due to nitric oxide and cyclic guanosine monophosphate has been linked to the activation of the Ca2+-dependent K+ channels (23). Moreover, it has been suggested that PAH has a cancer aspect because pulmonary artery SMC in PAH and cancer cells are both associated with mitochondrial disorders (24–26).
An additional feature that is seen in some forms of PAH in humans is a complex vascular lesion known as a plexiform lesion (27). These lesions contain a disorganized monoclonal EC proliferation in a stroma of myofibroblasts (28, 29). These so-called plexiform or complex vascular lesions are characterized by apoptosis-resistant (30–35), phenotypically altered EC (36–41). Recently, conclusive evidence that idiopathic PAH pulmonary artery EC have a hyperproliferative apoptosis-resistant phenotype compared with cells from control lungs has been shown using cell proliferation, DNA synthesis, and the evaluation of cell death pathways (42). Likewise, human herpes virus 8 (HHV-8) infection of pulmonary microvascular EC results in an apoptotic-resistant phenotype characteristic of severe PAH (43), although it is unclear whether HHV-8 has a pathogenetic role in idiopathic PAH (44–46). Moreover, dysfunctional endothelial progenitor cells, which are hyperproliferative with impaired ability to form vascular networks, are involved in the vascular remodeling associated with PAH (47).
An impairment of the EC function in patients with PAH leads to local thrombosis (48, 49), the interruption of which may improve the prognosis of patients with PAH. Moreover, the expression of tissue factor (TF), the membrane glycoprotein that initiates coagulation (50), facilitates angiogenesis (50), mediates arterial injury in the systemic circulation (51), and increases in the pulmonary arterioles and plexiform lesions of the rats, thus indicating that the induction of TF may contribute to the progression of PAH (52).
Xu and colleagues used an in vitro experiment with pulmonary artery EC from idiopathic PAH (IPAH EC) and control lungs (control EC) to show that glucose metabolism plays the primary role for energy requirements of IPAH EC based on the 3-fold greater glycolytic rate of IPAH EC compared with control EC, thus indicating that there is mitochondrial disorder in EC in idiopathic PAH, like SMC in PAH and cancer cells (53). A share of a mitochondrial disorder in SMC and EC in PAH may support the EMT hypothesis.
REVERSIBLE AND IRREVERSIBLE MODELS OF PULMONARY HYPERTENSION
In contrast to the human disease, both classical rodent models of mild to moderate pulmonary hypertension—the chronic hypoxia and monocrotaline models—lack clustered proliferated EC in the lumen of pulmonary arteries (9, 10). Pulmonary EC constitute a stable cell population with a very low turnover rate and, apparently, neither severe chronic hypoxia/hypoxemia nor monocrotaline pyrrole causes the emergence of a proliferative, dysfunctional EC phenotype (54). The defining pulmonary vascular alteration in both of these models, medial muscular thickening of proliferating SMC, is potentially reversible upon reexposure to normoxia or with the passage of time after monocrotaline injection (9, 11, 55).
Neointimal pulmonary vascular occlusive lesions that consist of proliferating SMC are found in rats after the combination of pneumonectomy with monocrotaline injection (56–60). A combination of compensatory lung growth after a pneumonectomy, hemodynamic factors, and endothelial injury by monocrotaline pyrrole may combine to produce this neointimal pulmonary vascular disease (60). Neointimal vascular occlusion is reversible with simvastatin in this rat model, through its antiproliferative and proapoptotic effects on vascular SMC (61).
A vascular endothelial growth factor (VEGF) receptor blockade with the VEGF-RI/VEGF-R II antagonist SU5416 combined with chronic hypoxia results in severe angioproliferative PAH in adult rats (39). The defining pulmonary vascular alteration in this model, arterial occlusion by proliferating EC, is not reversible upon reexposure to normoxia and with passage of time after SU5416 injection (39). Together, these rat models offer the perspective that medial muscular thickening due to proliferating SMC may be reversible and arterial occlusion by proliferating EC may conversely be irreversible.
REVERSAL OF MUSCULARIZATION
Although less well studied, the shift of vascular SMC, whereby proliferative adult vascular SMC convert back to a nonproliferative state, is an important observation. This particular phenotype switch is essential for limiting SMC accumulation and for the termination of vascular remodeling. The regulatory factors that drive proliferative SMC into a nonproliferative state, and hold them in that state, are critical for effective vascular remodeling and for limiting vascular disease (62). Li and coworkers generated unique lines of nonimmortalized human vascular SMC that are capable of a conversion from proliferative to nonproliferative states (63). In the presence of serum, these SMC proliferate, migrate, and elaborate extracellular matrix similar to primary SMC. Upon withdrawal of the serum, however, they undergo a reproducible program of cellular maturation whereby they exit the cell cycle, migrate into multilayered aggregates, and then acquire the ability to rapidly contract. Li and colleagues (63) concluded that this shift to a nonproliferative and mature phenotype may be attributed to cellular plasticity, rather than the selective expansion (or loss) of distinct cell subpopulations, because the SMC are clonal. Although this plasticity of SMC has been shown in systemic arteries, the same plasticity may therefore be essential for reversing the vascular remodeling caused by proliferating SMC in PAH.
In vitro experiments conducted to assess the contribution of EC to the development of PAH have demonstrated that a VEGF receptor blockade with the VEGF-RI/VEGF-R II antagonist SU5416, under conditions of increased fluid shear stress, causes initial apoptosis followed by exuberant proliferation of the surviving EC (40). These EC are hyperproliferative and apoptosis-resistant and express the anti-apoptotic protein survivin (40). Moreover, human pulmonary microvascular EC transdifferentiated with SU5416 in vitro demonstrate that the shift to a transdifferentiated phenotype could be attributed to selection of distinct cell subpopulations (i.e., stem-like cells), and suggest that endothelial–mesenchymal transdifferentiation might be an important contributor to pathophysiological vascular remodeling in complex vascular lesion of PAH (41), because, although bone marrow–derived cells could participate in arterial neointimal formation after mechanical injury, they do not contribute substantially to pulmonary arterial remodeling in an experimental PAH model (64). Moreover, those EC are not capable of returning to normal EC states over 10 passages from the withdrawal of SU5416 (41). Likewise, Arciniegas and coworkers suggested that EMT was an important contributor to vascular remodeling in PAH, but might be reversible (65).
DRUGS AND “REVERSIBLE OR IRREVERSIBLE REMODELING”
The drugs that are currently used for the treatment of PAH act not only by opposing any abnormal vasoconstriction, but also by inhibiting the growth of normal SMC: endothelin-receptor antagonists, phosphodiesterase type 5 inhibitors, and prostacyclin derivatives (66). Some patients with IPAH have been treated successfully with vasodilators with normal or nearly normal hemodynamics. It is unclear whether they have been diagnosed very early before remodeling occurred or they are a different phenotype. However, since vascular remodeling likely becomes progressively as the disease advances in patients who are resistant to these agents (67), new drugs may be developed to specifically target pulmonary vascular remodeling for these patients.
A few cases of clinical and hemodynamic improvements with the PDGF receptor tyrosine kinase inhibitors have also been reported in human PAH (68–70). Therefore, agents like these drugs may perhaps achieve reversal of remodeling, that is, the regression of established muscularized pulmonary arteries (4). A recently completed phase II clinical trial evaluating the safety and efficacy of imatinib mesylate, a tyrosine kinase inhibitor with antineoplastic activity, in PAH failed to meet the primary efficacy end point of improvement in exercise capacity; however, many secondary end points, including pulmonary hemodynamics, were significantly improved (71). However, the effects of these drugs in human PAH are currently missing and there is no data on reverse remodeling with this class of drugs in humans. It is probable that most patients with severe PAH at the time of their diagnosis have irreversible structural alterations of their microscopically small pulmonary arterioles, that is, irreversible pulmonary vascular remodeling believed to be caused by angiogenic proliferation of phenotypically altered and transdifferentiated EC (5–8, 40, 41).
Rapamycin, an antiproliferative immunosuppressor that arrests the cells in the G1 phase of the cell cycle (72), was examined in randomized, vehicle-controlled trials using a rodent model of severe PAH (57). Because of this approval for clinical practice, the potential of rapamycin for rapid translation to human PAH therapies is offered. Rapamycin is used clinically in cardiovascular medicine, as an antiproliferative agent applied to coronary stents to reduce local restenosis (73). Rapamycin inhibits hypoxia-induced activation of S6 kinase in PA adventitial fibroblasts (74), suggesting a possibility for therapeutic benefit in PAH. Rapamycin has also been shown to attenuate experimental PAH and suppress neointimal SMC proliferation in a pneumonectomy with monocrotaline-PAH model (57). In that study, however, rapamycin failed to reverse established PAH, thus suggesting that antiproliferative (rapamycin) agents were not sufficient to induce apoptosis of neointimal SMC and that additional proapoptotic agents might be needed.
THE ROLE OF IMMUNITY
There is strong circumstantial evidence for an immune pathogenesis of PAH, i.e., PAH is associated with rheumatoid arthritis, systemic lupus erythematosus, collagen diseases (e.g., scleroderma and mixed connective tissue disease), hypothyroidism, hypersensitivity pneumonitis, and infection with HIV (7, 75, 76). Barst and colleagues (77) and Morse and coworkers (78, 79) detected an association of MHC class II alleles with PAH.
Recently, Daley and colleagues demonstrated that activation of the immune system can induce impressive pulmonary arterial remodeling, that is, pulmonary arterial muscularization without intimal EC proliferation. This pulmonary arterial muscularization in the mouse did not cause pulmonary hypertension (80). Novel treatment strategies for PAH should target the apoptosis-resistant phenotypically altered vascular cells and the disruption of the extracellular matrix between the obliterating cells. Drugs that turn the in situ local vascular immune response off may be essential to the treatment strategies for PAH because immune responses are likely to regulate signals that control cell proliferation, or the differentiation of smooth muscle actin–positive cells, and the rearrangement of the cellular organization which thus results in a severely remodeled arterial wall (80).
The use of the immunosuppressants methotrexate and prednisone to treat pulmonary hypertension with nonspecific inflammatory signs has shown promising beneficial effects in some patients (81). Ogawa and coworkers reported an immunosuppressant, prednisolone, to have an antiproliferative effect on cultured SMC of pulmonary arteries from patients with idiopathic PAH, thus suggesting that prednisolone may be potentially useful therapeutically in patients with idiopathic PAH (82). Moreover, they have shown that PDGF-induced nuclear translocation of NF-κB may play an important role in stimulating pulmonary arterial SMC proliferation (and/or enhancing pulmonary arterial SMC survival), whereas prednisolone may exert both anti-inflammatory and antiproliferative effects on PASMC by inhibiting NF-κB nuclear translocation (83).
However, it should be acknowledged that, to date, the treatment of PAH with immunosuppressive drugs has been disappointing with the exception of less severe PAH associated with connective tissue disease (84).
CONCLUSIONS
The treatment strategies for PAH should consider that there are at least two types of remodeling: pulmonary vascular disease that develops predominantly because of increased muscularization of vessel walls, and pulmonary vascular disease that develops because of EC proliferation (85). The SMC shift between a proliferative and nonproliferative phenotype may be attributed to cellular plasticity, rather than selective expansion of distinct cell subpopulations (63), thus suggesting that this form of vascular remodeling may be reversible. However, phenotypically altered and hyperproliferative SMC in PAH cannot easily convert back to a normal SMC. A recent study has demonstrated irreversible PAH in congenital heart disease (CHD) to be strongly associated with impaired apoptotic regulation of EC (35), with endothelial damage and with increased circulating EC counts (86), thus suggesting that vascular remodeling that develops because of phenotypically altered EC may be irreversible.
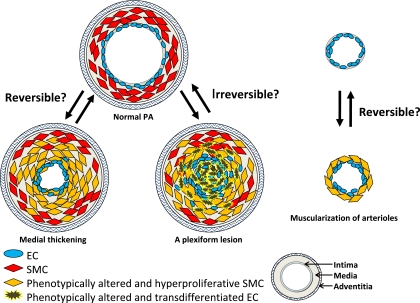
Moreover, after inducing apoptosis of phenotypically altered vascular cells, it may be necessary to allow normal EC to spread out and again form a monolayer. Whether phenotypically altered EC can switch back to a normal monolayer-forming EC therefore remains to be elucidated (Figure 1).
Figure 1.
Reversible or irreversible remodeling in pulmonary arterial hypertension (PAH): a hypothetical mechanism. This is a hypothetical figure of a cross-section of pulmonary arteries. Pulmonary vascular remodeling in PAH is characterized by the thickening of the media, a plexiform lesion, and muscularization. Such thickening is due to hyperplasia (proliferation) of phenotypically altered smooth muscle cells (SMC). A plexiform lesion is composed of apoptosis-resistant phenotypically altered endothelial cells (EC). Muscularization is the extension of new smooth muscle into the partially muscular and nonmuscular peripheral arteries. The SMC shift between a proliferative and nonproliferative phenotype may be attributed to cellular plasticity, rather than selective expansion of distinct cell subpopulations, suggesting that this form of vascular remodeling (i.e., likely medial thickening and muscularization) may be reversible. However, irreversible PAH in congenital heart disease is strongly associated with the impaired apoptotic regulation of EC and with endothelial damage, thus suggesting that vascular remodeling, which develops because of phenotypically altered EC (i.e., likely a plexiform lesion) may therefore be irreversible.
In conclusion, the disobliteration or reopening of occluded small pulmonary vessels is increasingly recognized as a treatment goal in PAH. The reopening of even a fraction of the obliterated arterioles would reduce pulmonary vascular resistance and thus unload the stressed right ventricle. Further studies are necessary to characterize the pulmonary vessel phenotypes to determine the reversibility of vascular remodeling in PAH.
This study was supported by National Institutes of Health (NIH) Grant 5P01 HL66254–03 PI; an NIH Program Project Grant (to N.F.V.); Research Grants for the Respiratory Failure Research Group from the Ministry of Health, Labor and Welfare, Japan; and Chiba foundation for health promotion and disease prevention.
This article represents a selective perusal of the pertinent literature together with a discussion of our own findings, and not a comprehensive or formal “balanced” review as outlined by a referee.
S.S. conceived of the report, contributed to its design and conception and drafted the manuscript. K.T. drafted the manuscript and contributed to its design and conception. N.F.V. contributed to its design and drafted the manuscript. All authors read and approved the final manuscript.
This work is dedicated to the memory of Dr. J. T. Reeves.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0389TR on December 11, 2009
Author Disclosure: S.S. received a sponsored grant from Chiba foundation for health promotion and disease prevention for $1,001–$5,000. K.T. has a dependent who received a sponsored grant from Japan Society of the Promotion of Science for $5,001–$10,000. N.F.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:13S–24S. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;351:1425–1436. [DOI] [PubMed] [Google Scholar]
- 3.Rai PR, Cool CD, King JAC, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2008;178:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 2005;115:2811–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 6.Hirose S, Hosoda Y, Furuya S, Otsuki T, Ikeda E. Expression of vascular endothelial growth factor and its receptors correlates closely with formation of the plexiform lesion in human pulmonary hypertension. Pathol Int 2000;50:472–479. [DOI] [PubMed] [Google Scholar]
- 7.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 2005;26:1110–1118. [DOI] [PubMed] [Google Scholar]
- 8.Tuder RM, Cool CD, Yeager ME, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension. Clin Chest Med 2001;22:405–418. [DOI] [PubMed] [Google Scholar]
- 9.Meyrick B, Reid L. Hypoxia-induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am J Pathol 1980;100:151–178. [PMC free article] [PubMed] [Google Scholar]
- 10.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 1997;150:1349–1360. [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther 2001;92:1–20. [DOI] [PubMed] [Google Scholar]
- 12.Dingemanns KP, Wagenvoort CA. Pulmonary arteries and veins in experimental hypoxia. Am J Pathol 1978;93:353–368. [PMC free article] [PubMed] [Google Scholar]
- 13.Jones R, Jacobson M, Steudel W. Alpha-smooth-muscle actin and microvascular precursor smooth-muscle cells in pulmonary hypertension. Am J Respir Cell Mol Biol 1999;20:582–594. [DOI] [PubMed] [Google Scholar]
- 14.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 2009;10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 2005;115:1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 2004;95:830–840. [DOI] [PubMed] [Google Scholar]
- 17.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation 2005;112:423–431. [DOI] [PubMed] [Google Scholar]
- 18.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 2002;105:244–250. [DOI] [PubMed] [Google Scholar]
- 19.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 2001;104:790–795. [DOI] [PubMed] [Google Scholar]
- 20.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res 2004;94:1263–1270. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. The 5-HT transporter transactivates the PDGF beta receptor in pulmonary artery smooth muscle cells. FASEB J 2007;21:2725–2734. [DOI] [PubMed] [Google Scholar]
- 22.Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX, Insel PA. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol 2007;292:L294–L303. [DOI] [PubMed] [Google Scholar]
- 23.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxinsensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA 1994;91:7583–7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, et al. A mitochondria-K1 channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2003;11:37–51. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, et al. An abnormal mitochondrial-HIF-1-Kv channel pathway disrupts oxygen-sensing and triggers pulmonary arterial hypertension (PAH) in fawn-hooded rats: similarities to human PAH. Circulation 2006;113:2630–2641. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA 2007;104:11418–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voelkel NF, Tuder RM, Weir EK. Pathophysiology of primary pulmonary hypertension: from physiology to molecular mechanisms. In: Rubin LJ, Rich S, editors. Primary pulmonary hypertension. New York: Marcel Dekker, Inc.; 1997. pp. 83–129.
- 28.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 1999;155:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voelkel NF, Tuder RM. Severe pulmonary hypertensive diseases: a perspective. Eur Respir J 1999;14:1246–1250. [DOI] [PubMed] [Google Scholar]
- 30.Rabinovitch M. Elastase and the pathobiology of unexplained pulmonary hypertension. Chest 1998;114:213–224. [DOI] [PubMed] [Google Scholar]
- 31.Rubin LJ. Cellular and molecular mechanisms responsible for the pathogenesis of primary pulmonary hypertension. Pediatr Pulmonol Suppl 1999;18:194–197. [PubMed] [Google Scholar]
- 32.Wagenvoort CA, Wagenvoort N. Primary pulmonary hypertension: a pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation 1970;42:1163–1171. [Google Scholar]
- 33.Wohrley JD, Frid MG, Moiseeva EP, Orton EC, Belknap JK, Stenmark KR. Hypoxia selectively induces proliferation in a specific subpopulation of smooth muscle cells in the bovine neonatal pulmonary arterial media. J Clin Invest 1995;96:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation 2005;111:534–538. [DOI] [PubMed] [Google Scholar]
- 35.Lévy M, Maurey C, Celermajer DS, Vouhé PR, Danel C, Bonnet D, Israël-Biet D. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol 2007;49:803–810. [DOI] [PubMed] [Google Scholar]
- 36.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992;327:70–75. [DOI] [PubMed] [Google Scholar]
- 37.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993;328:1732–1739. [DOI] [PubMed] [Google Scholar]
- 38.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 1991;114:464–469. [DOI] [PubMed] [Google Scholar]
- 39.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001;15:427–438. [DOI] [PubMed] [Google Scholar]
- 40.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 2005;19:1178–1180. [DOI] [PubMed] [Google Scholar]
- 41.Sakao S, Taraseviciene-Stewart L, Cool CD, Tada Y, Kasahara Y, Kurosu K, Tanabe N, Takiguchi Y, Tatsumi K, Kuriyama T, et al. VEGF-R blockade causes endothelial cell apoptosis, expansion of surviving CD34+ precursor cells and transdifferentiation to smooth muscle-like and neuronal-like cells. FASEB J 2007;21:3640–3652. [DOI] [PubMed] [Google Scholar]
- 42.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L548–L554. [DOI] [PubMed] [Google Scholar]
- 43.Bull TM, Meadows CA, Coldren CD, Moore M, Sotto-Santiago SM, Nana-Sinkam SP, Campbell TB, Geraci MW. Human herpesvirus-8 infection of primary pulmonary microvascular endothelial cells. Am J Respir Cell Mol Biol 2008;39:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cool CD, Rai PR, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, Geraci MW, Brown KK, Routes JM, Tuder RM, et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med 2003;349:1113–1122. [DOI] [PubMed] [Google Scholar]
- 45.Galambos C, Montgomery J, Jenkins FJ. No role for kaposi sarcoma-associated herpesvirus in pediatric idiopathic pulmonary hypertension. Pediatr Pulmonol 2006;41:122–125. [DOI] [PubMed] [Google Scholar]
- 46.Bendayan D, Sarid R, Cohen A, Shitrit D, Shechtman I, Kramer MR. Absence of human herpesvirus 8 DNA sequences in lung biopsies from Israeli patients with pulmonary arterial hypertension. Respiration 2008;75:155–157. [DOI] [PubMed] [Google Scholar]
- 47.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 2009;180:780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisenberg PR, Lucore C, Kaufman L, Sobel BE, Jaffe AS, Rich S. Fibrinopeptide A levels indicative of pulmonary vascular thrombosis in patients with primary pulmonary hypertension. Circulation 1990;82:841–847. [DOI] [PubMed] [Google Scholar]
- 49.Welsh CH, Hassell KL, Badesch DB, Kressin DC, Marlar RA. Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. Chest 1996;110:710–717. [DOI] [PubMed] [Google Scholar]
- 50.Mackman N. Regulation of the tissue factor gene. Thromb Haemost 1997;78:747–754. [PubMed] [Google Scholar]
- 51.Pyo R, Jensen KK, Wiekowski MT, Manfra D, Alcami A, Taubman MB, Lira SA. Inhibition of intimal hyperplasia in transgenic mice conditionally expressing the chemokine-binding protein M3. Am J Pathol 2004;164:2289–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, et al. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L583–L590. [DOI] [PubMed] [Google Scholar]
- 53.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA 2007;104:1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reid LM, Davies P. Control of cell proliferation in pulmonary hypertension. In: Pulmonary physiology and pathophysiology. Weir EK, Reeves JT, editors. New York: Marcel Dekker, Inc.; 1989. pp.541–611.
- 55.Kakusaka I, Kaneko N, Kiyatake K, Fujita A, Suzuki A, Nakano K, Okada O, Sugita T, Watanabe S, Kuriyama T. Effects of various doses of monocrotaline administration on the development of pulmonary hypertension and its regression in rats. Nihon Kyobu Shikkan Gakkai Zasshi 1989;27:51–56. [PubMed] [Google Scholar]
- 56.Faul JL, Nishimura T, Berry GJ, Benson GV, Pearl RG, Kao PN. Triptolide attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 2000;162:2252–2258. [DOI] [PubMed] [Google Scholar]
- 57.Nishimura T, Faul JL, Berry GJ, Veve I, Pearl RG, Kao PN. 40-O-(2-Hydroxyethyl)-rapamycin attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 2001;163:498–502. [DOI] [PubMed] [Google Scholar]
- 58.Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qiu D, Pearl RG, Kao PN. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med 2002;166:1403140–1403148. [DOI] [PubMed] [Google Scholar]
- 59.Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury: a neointimal model of pulmonary hypertension. Am J Pathol 1997;151:1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 60.Nishimura T, Faul JL, Berry GJ, Kao PN, Pearl RG. Effect of a surgical aortocaval fistula on monocrotaline-induced pulmonary hypertension. Crit Care Med 2003;31:1213–1218. [DOI] [PubMed] [Google Scholar]
- 61.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 2003;108:1640–1645. [DOI] [PubMed] [Google Scholar]
- 62.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004;84:767–801. [DOI] [PubMed] [Google Scholar]
- 63.Li S, Fan YS, Chow LH, Van Den Diepstraten C, van Der Veer E, Sims SM, Pickering JG. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circ Res 2001;89:517–525. [DOI] [PubMed] [Google Scholar]
- 64.Sahara M, Sata M, Morita T, Nakamura K, Hirata Y, Nagai R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation 2007;115:509–517. [DOI] [PubMed] [Google Scholar]
- 65.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L1–L8. [DOI] [PubMed] [Google Scholar]
- 66.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2005;352:308–309. [DOI] [PubMed] [Google Scholar]
- 67.Reeves JT, Groves BM, Turkevich D. The case for treatment of selected patients with primary pulmonary hypertension. Am Rev Respir Dis 1986;134:342–346. [DOI] [PubMed] [Google Scholar]
- 68.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 2005;353:1412–1413. [DOI] [PubMed] [Google Scholar]
- 69.Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med 2006;145:152–153. [DOI] [PubMed] [Google Scholar]
- 70.Souza R, Sitbon O, Parent F, Simonneau G, Humbert M. Long term imatinib treatment in pulmonary arterial hypertension. Thorax 2006;61:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghofrani HA, Barst RJ, Benza RL, Champion HC, Fagan KA, Grimminger F, Humbert M, Simonneau G, Stewart DJ, Ventura C, et al. Future perspectives for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S108–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycinreceptor complex. Nature 1994;369:756–758. [DOI] [PubMed] [Google Scholar]
- 73.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1773–1780. [DOI] [PubMed] [Google Scholar]
- 74.Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol 2005;98:722–731. [DOI] [PubMed] [Google Scholar]
- 75.Rabinovitch M. Autoimmune disease and unexplained pulmonary hypertension. Circulation 1992;85:380–381. [DOI] [PubMed] [Google Scholar]
- 76.Mouthon L, Guillevin L, Humbert M. Pulmonary arterial hypertension: an autoimmune disease? Eur Respir J 2005;26:986–988. [DOI] [PubMed] [Google Scholar]
- 77.Barst RJ, Flaster ER, Menon A, Fotino M, Morse JH. Evidence for the association of unexplained pulmonary hypertension in children with the major histocompatibility complex. Circulation 1992;85:249–258. [DOI] [PubMed] [Google Scholar]
- 78.Morse JH, Barst RJ, Fotino M, Zhang Y, Flaster E, Gharavi AE, Fritzler MJ, Dominguez M, Angles-Cano E. Primary pulmonary hypertension, tissue plasminogen activator antibodies, and HLA-DQ7. Am J Respir Crit Care Med 1997;155:274–278. [DOI] [PubMed] [Google Scholar]
- 79.Morse JH, Barst RJ, Fotino M, Zhang Y, Flaster E, Fritzler MJ. Primary pulmonary hypertension: immunogenetic response to high-mobility group (HMG) proteins and histone. Clin Exp Immunol 1996;106:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 2008;205:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bellotto F, Chiavacci P, Laveder F, Angelini A, Thiene G, Marcolongo R. Effective immunosuppressive therapy in a patient with primary pulmonary hypertension. Thorax 1999;54:372–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ogawa A, Nakamura K, Matsubara H, Fujio H, Ikeda T, Kobayashi K, Miyazaki I, Asanuma M, Miyaji K, Miura D, et al. Prednisolone inhibits proliferation of cultured pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertension. Circulation 2005;112:1806–1812. [DOI] [PubMed] [Google Scholar]
- 83.Ogawa A, Firth AL, Yao W, Rubin LJ, Yuan JX. Prednisolone inhibits PDGF-induced nuclear translocation of NF-_B in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2008;295:L648–L657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jais X, Launay D, Yaici A, Le Pavec J, Tchérakian C, Sitbon O, Simonneau G, Humbert M. Immunosuppressive therapy in lupus- and mixed connective tissue disease-associated pulmonary arterial hypertension: a retrospective analysis of twenty-three cases. Arthritis Rheum 2008;58:521–531. [DOI] [PubMed] [Google Scholar]
- 85.Proceedings of the 3rd World Symposium on Pulmonary Arterial Hypertension. Venice, Italy, June 23–25, 2003. J Am Coll Cardiol 2004;43:1S–90S. [DOI] [PubMed] [Google Scholar]
- 86.Smadja DM, Gaussem P, Mauge L, Israël-Biet D, Dignat-George F, Peyrard S, Agnoletti G, Vouhé PR, Bonnet D, Lévy M. Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation 2009;119:374–381. [DOI] [PubMed] [Google Scholar]