Abstract
Exposure to cigarette smoke is associated with airway epithelial mucus cell hyperplasia and a decrease in cilia and ciliated cells. Few models have addressed the long-term effects of chronic cigarette smoke exposure on ciliated epithelial cells. Our previous in vitro studies showed that cigarette smoke decreases ciliary beat frequency (CBF) via the activation of protein kinase C (PKC). We hypothesized that chronic cigarette smoke exposure in an in vivo model would decrease airway epithelial cell ciliary beating in a PKC-dependent manner. We exposed C57BL/6 mice to whole-body cigarette smoke 2 hours/day, 5 days/week for up to 1 year. Tracheal epithelial cell CBF and the number of motile cells were measured after necropsy in cut tracheal rings, using high-speed digital video microscopy. Tracheal epithelial PKC was assayed according to direct kinase activity. At 6 weeks and 3 months of smoke exposure, the baseline CBF was slightly elevated (∼ 1 Hz) versus control mice, with no change in β-agonist–stimulated CBF between control mice and cigarette smoke–exposed mice. By 6 months of smoke exposure, the baseline CBF was significantly decreased (2–3 Hz) versus control mice, and a β-agonist failed to stimulate increased CBF. The loss of β-agonist–increased CBF continued at 9 months and 12 months of smoke exposure, and the baseline CBF was significantly decreased to less than one third of the control rate. In addition to CBF, ciliated cell numbers significantly decreased in response to smoke over time, with a significant loss of tracheal ciliated cells occurring between 6 and 12 months. In parallel with the slowing of CBF, significant PKC activation from cytosol to the membrane of tracheal epithelial cells was detected in mice exposed to smoke for 6–12 months.
Keywords: chronic cigarette smoke, cilia, PKC
Cilia are fingerlike projections that sweep mucus and particles from the lungs, resulting in mucociliary clearance. Normal mucociliary clearance, which involves the production of mucus and the synchronized beating of ciliated airway epithelium, is a critical component in the clearance of pathogens within the upper and lower airways. Several environmental factors, including cigarette smoke, were shown to impede normal mucociliary clearance, resulting in increased pulmonary infections, accumulation of mucus, and airway obstruction.
Exposure to cigarette smoke was linked to the development of a variety of pulmonary diseases, including chronic obstructive pulmonary disease (COPD, including emphysema and chronic bronchitis) and lung cancer (1, 2). It is well established that exposure to cigarette smoke leads to airway epithelial mucus cell hyperplasia (3, 4), a loss of cilia (5–7), and reduced ciliary beating (8, 9).
Ciliary beating can be stimulated by a variety of mechanisms, one of which involves cyclic nucleotides. Several studies indicated that intracellular cyclic nucleotides act as important regulators of ciliary motility (10, 11). By increasing levels of cyclic adenosine 3′-5′ monophosphate (cAMP), ciliary motility is stimulated through the activation of the cAMP-dependent protein kinase A (PKA) (12). Conversely, agents that activate protein kinase C (PKC) are associated with decreased cilia motility (8, 13, 14).
One regulator of PKC is cigarette smoke, which was shown to induce and increase PKC activity in airway epithelial cells (8, 15, 16). We previously showed that protein kinase C epsilon (PKCɛ) localizes to ciliary axonemes in ciliated cells, but is primarily localized to the cytoplasm in basal cells. Upon activation, PKCɛ translocates to the plasma membrane, where it binds to and is stabilized by the receptor for activated C kinase 1 (RACK1) (17). Interactions of PKCɛ and RACK1 at the membrane were shown to be important for adhesion and migration in human glioma cells (18). Protein kinase C epsilon can regulate ciliary motility (14), and acts as an important mediator in ciliated cell attachment. Inhibiting PKCɛ in primary ciliated bovine brachial epithelial cells resulted in the detachment of ciliated cells from the basal cell monolayer, independent of apoptosis or cell death (19).
Although it is well-established that cigarette smoke predisposes individuals to pulmonary disease, mucus cell hyperplasia, decreased cilia, and decreased ciliary beat, animal models have not addressed the long-term effects of chronic cigarette smoke exposure on ciliated airway epithelium. Given that cigarette smoke was shown to activate PKC, we hypothesized that chronic exposure to cigarette smoke in an in vivo model would activate PKC and decrease airway epithelial ciliary beating. To test this hypothesis, we exposed C57BL/6 mice to whole-body cigarette smoke for up to 1 year. Our studies indicate that cigarette smoke decreased ciliary beat frequency (CBF), activated PKC, and reduced the number of ciliated cells in the airway epithelium.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice (Jackson Laboratories, Inc., Bar Harbor, ME), aged 7 weeks at time of arrival, were used in this study. The animals were group-housed (5/cage) in polycarbonate cages, with dry corncob bedding. The animal room was on a 12-hour light/dark cycle at a targeted temperature of 22°C ± 2°C and humidity of 50% ± 5%. Basal food in pellet form (Tekland 7012; Harlan, Madison, WI) and tap water were available ad libitum at all times during the study. Both control mice and cigarette smoke-exposed mice received the same diet. No significant weight loss was evident under any treatment conditions during the study period. All protocols conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. Treatment began after 1 wk of quarantine.
Exposure to Cigarette Smoke
The C57BL/6 mice were studied in a single cohort. Animals were divided into groups at 2 to 3 months of age. One group was exposed to smoke, and one group was exposed to compressed air, as previously described (20). Briefly, cages containing C57BL/6 mice were placed in the exposure chamber of a Teague Small Animal Whole Body Smoke Exposure System (Model TE-10; Teague Enterprises, Davis, CA). Animals were exposed to whole-body mainstream and sidestream cigarette smoke via inhalation from 1R1 reference cigarettes (Tobacco and Health Research Institute, University of Kentucky, Lexington, KY) at 150 mg/m3 total smoking particles for 2 hours/day, 5 days/week, for up to 1 year. Control animals were sham-exposed to compressed air. Animals in groups of five were killed after 1 week, 1 month, 3 months, 6 months, 9 months, and 12 months of exposure.
Trachea Harvesting and Treatment
Tracheas were removed and maintained in a closed, sterile, 15-ml conical tube in serum-free M199 containing penicillin and streptomycin (100 units/100 μg per ml; Gibco, Carlsbad, CA) and fungizone (2 μg/ml; Gibco) at room temperature until processing (30–60 min). Tracheal rings were cut (0.5-mm width) from the distal end of the trachea, just above the carina. The rings were placed in Petri dishes containing serum-free M199 for determinations of CBF. Ciliary beat frequency was measured using Sisson Ammons video analysis (SAVA; Ammons Engineering, Mt. Morris, MI), and each whole-field analysis was averaged for the total number of motile points. The entire lumen of each trachea was used to measure the number of motile points and the CBF, to rule out selection bias. After the determination of baseline CBF, both the rings and the remaining tracheal tissue were stimulated with isoproterenol at a final concentration of 10 μM. The rings were incubated at 37°C and 5% CO2, and allowed to equilibrate at 25°C for 10 minutes. The final CBF reading was taken from the tracheal rings.
PKC Activity
The remaining tracheal tissue was opened with a longitudinal cut to expose the ciliated epithelium, and placed in a Petri dish containing serum-free M199. This tissue was removed from the medium, and the ciliated epithelium was gently scraped into cell lysis buffer, as described elsewhere (12). The epithelial lysate was then immediately flash-frozen in liquid nitrogen for kinase assay. Epithelial tracheal lysates were used to determine PKC activity, as previously described (21). Both the supernatant and particulate fractions were assayed for the presence of PKC activity. Total PKC activity was measured in cytosolic fractions, containing all soluble proteins and unactivated PKC, and in particulate fractions, containing nonsoluble material such as cell membranes (from ciliated cells and basal cells), nuclear particles, and cytoskeletal elements. The particulate fraction contains all translocated and activated PKC. The PKC assay was performed using 900 μM PKC substrate peptide (Peninsula, Belmont, CA), 12 mM calcium-acetate, 8 μM phosphatidyl-L-serine, 24 μg/ml phorbol 12-myristate 13-acetate, 30 mM dithiothreitol, 150 μM ATP, 45 mM magnesium acetate, and 10 μCi/ml [γ-32P] ATP (MP Biomedicals, Irvine, CA) in a Tris-HCl buffer (pH 7.5). Samples (20 μl) were added to 40 μl of the reaction mixture, and incubated for 15 minutes at 30°C. Spotting 50 μl of each sample onto P-81 phosphocellulose papers (Whatman, Clifton, NJ) halted the incubations. Papers were washed five times for 5 minutes in phosphoric acid (75 mM), washed in ethanol, dried, and counted in nonaqueous scintillant, as previously described (21). Kinase activity was expressed in relation to total cellular protein assayed, and was calculated as picomoles of phosphate incorporated per minute per milligram.
Tracheal Histology
Tracheae were fixed in 10% formalin and embedded in paraffin. Sections (4–5 μM) were cut and either stained with hematoxylin and eosin (H&E) or used later for an immunohistochemical staining procedure. The H&E-stained slides were examined according to phase microscopy, using a Primo Star microscope (Carl Zeiss, Thornwood, NY), histopathology was determined by a reviewer blinded to the treatment conditions, and images were selected at random. Photomicrographs were recorded digitally at 40× and 100× magnification, using a Canon EOS camera (Canon, Lake Success, NY).
Immunohistochemistry
Tissue sections were deparaffinized in xylene and rehydrated, and antigen retrieval was performed in PBS containing 0.1% trypsin for 1 hour in a humidified chamber. Control samples were blocked with 10% normal goat serum. Trachea sections were incubated in acetylated tubulin, a ciliary axoneme-specific protein (Sigma-Aldrich, St. Louis, MO), overnight at 4°C, rinsed in PBS, and incubated in Alexa 488 (Molecular Probes, Eugene, OR) for 1 hour at room temperature in a humidified chamber. Tissue sections were gently rinsed in PBS, mounted with Vectashield mounting media (Vector Labs, Burlingame, CA), and imaged using a 510 Meta confocal microscope (Carl Zeiss).
Statistics
Data are presented as the mean ± SEM. Statistics were performed using a two-tailed, nonpaired t test for CBF, and one-way ANOVA for PKC activity, to determine significant changes among treatment groups, using GraphPad Prism software (GraphPad, La Jolla, CA).
RESULTS
Effect of Smoke Exposure on CBF
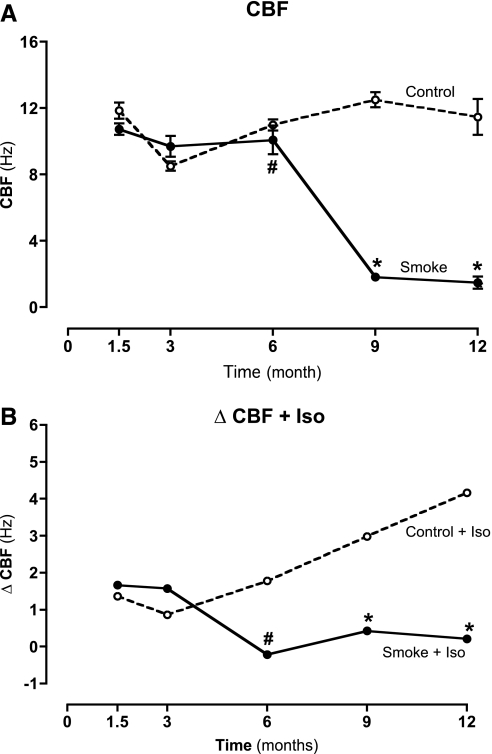
We previously showed that exposure to cigarette smoke in mice initially increases the baseline CBF, but subsequently causes cilia slowing after stimulation with β-agonists (9, 15). To determine the effects of cigarette smoke on CBF for a period of months instead of weeks, cut tracheal rings were measured after necropsy, using high-speed digital video microscopy (22). Mice exposed to cigarette smoke for 1.5 to 3 months demonstrated a slight, but not significant, increase in baseline CBF (∼1 Hz), compared with control mice (Figure 1A). The exposure of tracheal rings, excised from mice aged 1.5 to 3 months, to 10 μM isoproterenol for 30 minutes significantly increased CBF in both smoke-exposed and non–smoke-exposed mice (Figure 1B). After 6 months of smoke exposure, however, a significant decrease in baseline CBF (∼2–3 Hz; P < 0.05) was evident (Figure 1B). In addition, isoproterenol failed to stimulate CBF in mice exposed to smoke for 6 months (Figure 1B). The loss of β-agonist responsiveness continued through 12 months of smoke exposure, and at 1 year, CBF responsiveness was significantly decreased (P < 0.01) to less than one third of the baseline CBF in control mice (Figure 1B). Our data demonstrate that a decrease in CBF β-agonist stimulation in smoke-exposed C57BL/6 mice occurs over time. After only 6 months of smoke exposure, baseline cilia beating was significantly reduced and unresponsive to β-agonist stimulation. Maintaining tracheal sections in M199 culture media exerted no significant impact on CBF over the time course of the measurements.
Figure 1.
(A) Ciliary beat frequency (CBF) in tracheal epithelial cells of mice exposed to cigarette smoke. No change in baseline CBF was evident in mice exposed to cigarette smoke for 1.5 months and 3 months. Smoke exposure for 6 mo caused a small but significant (#P < 0.05) decrease in baseline CBF of media-only cells. From 9 months onward, baseline CBF slowed significantly (*P < 0.01). Vertical axis represents the mean (±SEM) of CBF (n = 10). (B) Ciliary beat frequency (CBF) in tracheal epithelial cells stimulated with a β-agonist, isoproterenol (Iso; 10 μM), from mice exposed to cigarette smoke. Isoproterenol was able to stimulate an increase in CBF in mice exposed to smoke for 1.5 to 3 months. However, isoproterenol failed to stimulate an increase in CBF of mice exposed to smoke for 6 months or longer. Vertical axis represents the mean (±SEM) ΔCBF (n = 10).
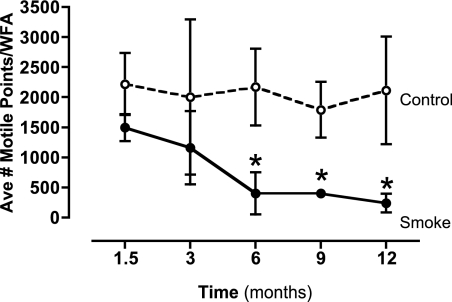
Because smoke exposure is also associated with a loss of cilia, we examined the effect of prolonged smoke exposure on ciliary number. To quantify the number of motile cilia in cigarette smoke–exposed mice contributing to changes in CBF, mouse tracheal rings were mapped using SAVA, and each whole-field analysis was averaged for the total number of motile points. Although a trend toward a reduced number of motile cilia was evident early after smoke exposure (1.5–3 mo), cilia motile points were significantly reduced from 6 to 12 months of exposure onward (P < 0.01) (Figure 2). These results suggest that the numbers of motile cilia are reduced over a period of time, with a significant decrease in motile cilia occurring after only 6 months of exposure to cigarette smoke.
Figure 2.
Quantification of numbers of motile cilia in mice exposed to cigarette smoke. Tracheal rings of mice were mapped using SAVA, and each whole-field analysis (WFA) was averaged for the total number of motile points. Ave #, average number. Smoke exposure for 6 to 12 months caused a significant (*P < 0.01) decrease in the numbers of motile cilia detected.
Effect of Smoke Exposure on Cilia Loss
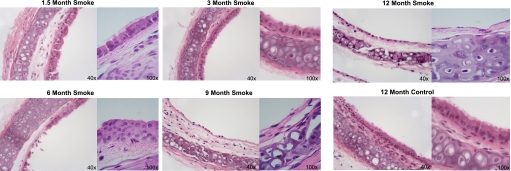
It was previously established that smoking results in a loss of cilia within the airway epithelium (7), and the loss of motile points over time is consistent with this observation. To confirm a loss of cilia after prolonged smoke exposure, histologic sections of control and smoke-exposed mouse tracheae were sectioned and stained with a cilia axoneme-specific antibody directed against acetylated tubulin antibody or H&E to observe the ciliated respiratory epithelia. Mice exposed to smoke for 1.5 to 3 months retained a normal pseudostratified columnar ciliated epithelial cell layer, which was similar to that of 12-month control mice (Figure 3). By 6 months, smoke-exposed mice revealed some areas of sparse or detached ciliated cells (Figure 3). Ciliated cell loss was most evident in the mice exposed to smoke for 6 to 12 months, resulting in an almost complete loss of ciliated cells by 12 months (Figure 3). Only a thin layer of basal epithelial cells was detected lining the airway in the trachea of mice exposed to smoke for 9 to 12 months.
Figure 3.
Histologic sections represent mouse tracheae after exposure to cigarette smoke (magnifications, ×40 and ×100) for 1.5 to 12 months. Mice exposed to cigarette smoke for 1.5 to 3 months retained a pseudostratified columnar ciliated epithelial layer of cells similar to that in control mice (sham-exposed to room air) and harvested at 1 year. Mice exposed to smoke for 6 months reveal some areas of sparse or detaching ciliated cells. Mice exposed to smoke for 9 to 12 months have lost most of their ciliated cells. Control mice retain cilia throughout the 12-month study.
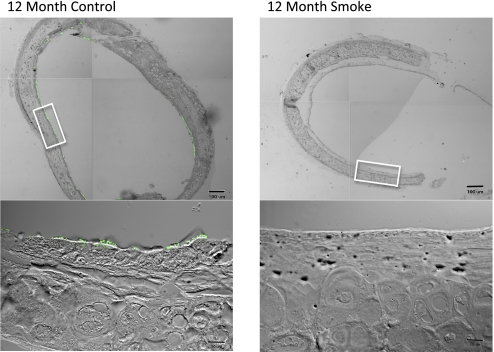
To confirm the cilia loss observed in H&E-stained tracheal sections, we examined the immunohistochemical staining of acetylated tubulin, a cilia axoneme-specific protein, in mouse tracheal sections. Confocal microscopy images revealed the presence of acetylated tubulin-positive cilia in 1.5- to 12-month non–smoke-exposed mice and 1.5- to 6-month smoke-exposed mice. However, after 6 months of smoke exposure, there was a decrease in cilia within the tracheal epithelium. Although a few cilia were evident in mice exposed to smoke for 9 months, the cilia were sparse, and large areas of nonciliated airway epithelium were evident. By 12 months, the trachea appeared to be mostly denuded of cilia in smoke-exposed mice (Figure 4).
Figure 4.
Immunohistochemical staining for acetylated tubulin in tracheae of mice exposed or not exposed to smoke for 12 months. Cilia (green) are present at 12 months in mice not exposed to smoke (left column). After 12 months of smoke exposure, immunofluorescence images reveal a loss of cilia (right column). Lower images depict enlargements of boxed sections indicated in corresponding top images.
Effect of Smoke Exposure on PKC Activity
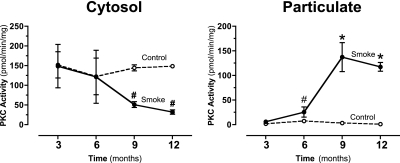
We previously showed that PKCɛ constituted a possible novel signaling pathway through which ciliated cells are attached to basal cells and maintained (19). To determine the effects of cigarette smoke exposure on PKC activity, mouse tracheal epithelium was isolated and PKC activity was measured, as described in Materials and Methods. Because of the limited amount of mouse tracheal epithelium available for this study, multiple individual PKC isoform activities could not be measured. Instead, total PKC activity was measured in both the control and smoke-exposed mice. Total cytosolic PKC activity was significantly reduced in mice exposed to smoke for 9 to 12 months. In contrast, a significant increase in PKC activity was evident in the particulate fractions of mice exposed to smoke for 9 to 12 months. These results indicate that the activated PKC translocates (suggesting that novel and/or classic PKCs are involved) to the particulate fraction, where it is predominately localized in the mice exposed for 9 to 12 months to cigarette smoke (Figure 5).
Figure 5.
Protein kinase C (PKC) activity in tracheal epithelial cells of mice exposed to cigarette smoke. The vertical axis represents the mean (±SEM) of PKA activity in pmol/min/mg of ciliated tracheal epithelial tissue within each exposure group (n = 10). Smoke exposure for 6 to 12 months caused a significant (#P < 0.05 and *P < 0.01, vs. time-matched controls.) increase in the particulate fraction levels of PKC activity. Smoke exposure for 9 to 12 months caused a significant (#P < 0.05) decrease in the cytosolic fraction levels of PKC activity. This result indicates the activated translocation of the enzyme to the particulate fraction.
DISCUSSION
It is well-established that cigarette smoke causes pulmonary diseases, decreased CBF, cilia shedding, and impeded mucociliary clearance (7). For decades, researchers have exposed mice to cigarette smoke in hopes of understanding the processes of such diseases and the impaired control mechanisms involved in mucociliary clearance. Short-term studies of smoke exposure in rats showed a significant increase in mucociliary clearance (23, 24). Such results agree with our own findings in short-term (6 wk) studies of mice exposed to cigarette smoke, in which we saw a significant increase in baseline CBF (9).
Although there is an abundance of short-term, smoke-exposed mouse models, few studies of mice have addressed the effects of long-term smoke exposure. Our goal was to determine the effects of long-term smoke exposure on cilia loss and the potential role of PKC in this process. We hypothesized that in a model of long-term smoke exposure, ciliary beating would decrease in a PKC-dependent manner, and would be unresponsive to β-agonist stimulation. Initially, no significant difference in baseline CBF was evident between mice exposed to smoke for 1 to 3 months and sham air-exposed mice. As we anticipated, β-agonist stimulation resulted in a significant increase in CBF. However, by 6 months of smoke exposure, we observed a significant decrease in CBF baselines between smoke-exposed and sham-exposed mice. Ciliary beat frequency continued to decrease even further in our mice exposed to smoke for 9 to 12 months. Furthermore, a β-agonist failed to stimulate CBF in any mice after 6 or more months of smoke exposure.
Studying the long-term effects of cigarette smoke on ciliated airway epithelium in a mouse model presents limitations because of the duration of the study and the small amounts of tissue retrieved. Because of limitations in the size of mouse tracheae and the limited amount of epithelium recovered from those tracheae, we were only able to measure total PKC levels, and not the panoply of specific PKC isoforms. The size of the animals also limited our ability to measure CBF in vivo. Although the ability to measure total airway clearance in mice exists, and would indicate some functional levels of CBF, these experiments were not performed. Instead, we relied on tracheal ring slices to measure CBF. This model for CBF has been well-established by us and others (16, 25, 26).
The consequences of exposure to cigarette smoke include lung inflammation and the loss of normal pseudostratified columnar ciliated epithelium. Some reports suggested that cilia length is also decreased in smokers, a phenomenon that was not investigated in our studies (27–29). Rather than assaying small changes in cilia length, we measured quantitative differences in beat frequency, numbers of motile cilia (points), and the qualitative loss of ciliated cells. Histologic preparations from smoke-exposed mice produced results consistent with the observations of others (30). It is also important to point out that ciliated cells attach to basal cells, and not to the basement membrane (31). We did not observe a histologic difference between 12-month sham-exposed mice and 1- to 3-month smoke-exposed mice that retained a pseudostratified columnar ciliated epithelial layer. However, after 6 mo of smoke exposure, some areas of sparse or detached ciliated cells were evident. Epithelial shedding continued and increased in mice exposed to smoke for 9 to 12 months. By 12 months, an almost complete loss of cilia appeared to occur within the trachea, leaving only a thin layer of basal epithelial cells lining the interior of the trachea.
In addition, we closely examined cilia in smaller airways, using whole-lung H&E sections. Ciliated cells within these smaller airways were detected and did not appear to be significantly denuded, as observed in the trachea (data not shown). We also observed similar changes in the alveolar airspaces consistent with emphysema, as reported in other models of long-term exposure to cigarette smoke (32, 33). Morphometric analyses of alveolar spaces showed an increase in the mean linear intercept of mice exposed to smoke, compared with control mice (data not shown).
This study also replicated the established concept of epithelial fragility, as shown to occur in diseases such as COPD and asthma (34–36). Enhanced epithelial denudation is often observed in histologic preparations from diseased lungs, because of increased epithelial fragility. Although lung epithelial cells were demonstrated to detach in vitro in response to cigarette smoke (37, 38), to our knowledge, this study is the first of epithelial cell shedding in an in vivo cigarette smoke exposure model. In our study, only those tissues from mice exposed to cigarette smoke demonstrated epithelial shedding. The CBF of tracheal sections was also examined immediately ex vivo in our study, independent of histologic processing. Beating cilia were evident even in tracheal sections of mice exposed to smoke for 12 months. However, the number of motile cilia (measured in motile points) was significantly reduced when compared with control mice. Our data suggest that PKC is involved in cilia loss, and may also contribute to the epithelial fragility observed in histologic sections of tissues exposed to cigarette smoke.
Although no significant cilia loss was observed until 6 to 12 months of smoke exposure, significant cilia loss and decreased CBF appear to correlate with the translocation of PKC activity from the cytosol to the particulate faction.
We previously reported on the importance of protein kinases in the regulation of ciliary beat. Specifically, PKA and PKG activation were shown to increase CBF, resulting in increased mucociliary clearance (12, 31). Protein kinase C activation was also demonstrated to reduce CBF, thus impeding mucociliary clearance (13, 15). Cigarette smoke was shown to induce and increase in vivo PKC activity in airway epithelium (8, 15, 16). Two PKC isoforms that are prevalent within the airway epithelium, and that translocate to lipid membranes upon activation, are PKCα and PKCɛ. Measuring translocation activity is indicative of this. In isoenzymes that do not translocate when activated (atypical PKCs), no change in particulate fraction activity would occur (39). Therefore, the PKC isoenzyme activated is one that would be translocated to the membrane. Using an in vitro bovine bronchial epithelial cell model, we previously demonstrated a loss of ciliated cells from the basal epithelium through the regulation of PKCɛ.
We demonstrated that PKCɛ localizes to the cytoplasm of basal cells and within the ciliary axoneme of ciliated cells (19). A variety of second messengers may activate PKCɛ, including diacylglyercerol (DAG), fatty acids, and phosphatidylinositol 3,4,5,-triphosphate (PIP3). Activated PKCɛ translocates from the cytoplasm to the membrane or cytoskeleton in response to DAG or PIP3, whereas the binding of certain fatty acids causes a translocation to the Golgi network (40). The activation of PKCɛ in bovine bronchial epithelial basal cells results in the translocation of PKCɛ to the plasma membrane, where it localizes with RACK1. Interestingly, ciliated cells do not contain RACK1 (17). Previous research showed that the interaction of PKCɛ and RACK1 is important in functions such as cell adhesion and migration (18). Based on the localization of PKCɛ/RACK1 interactions, we suspect that the basal epithelium may play an active role in the shedding of ciliated cells.
In our study, we observed a shift of PKC activity from the cytosol to the membrane (particulate fractions) at 9 to 12 months in smoke-exposed mice. The activity of PKC significantly decreased in the cytosol (P < 0.05) at 9 to 12 months in smoke-exposed mice (Figure 5), whereas PKC assays revealed a significant increase in PKC concentrations within the particulate fractions of mice exposed to smoke for 6 to 12 months (#P < 0.05 and *P < 0.01, vs. time-matched controls) (Figure 5). Based on evidence from previous research and the apparent translocation of PKC activity in this study, we suspect that PKCɛ may play a role in decreasing cilia motility and ciliated cell detachment in our model of smoke exposure in mice.
Our results also indicate that significant changes in CBF, the loss of motile cilia, PKC translocation, and cilia shedding do not occur until after 6 months of exposure to cigarette smoke. At this juncture, it remains unclear why this type of injury only begins to appear after 6 months of smoke exposure. We speculate that changes in key signaling and target proteins occur at that time, and regulate the attachment of ciliated cells to the basal epithelium. As with other markers of cigarette smoke–induced lung remodeling, such as alveolar emphysema, injury often does not appear in the mouse until extended periods of time. Clearly, changes in oxidative stress and inflammatory mediators would be expected over the course of such a chronic lung exposure model. Further studies are needed to define a potential role for such mediators in ciliated cell detachment.
We conclude that long-term exposure to cigarette smoke has the potential to alter normal mucociliary protection through the loss of ciliated cells and the desensitization of cilia to stimulated increases in beating through PKC.
This work was supported by Department of Veterans Affairs Merit Review grant and by National Institutes of Health grants AA017993 (T.A.W.), AA08769 (J.H.S.), and ES013856 (S.I.R.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0297OC on December 30, 2009
Author Disclosure: T.A.W. and J.H.S. received sponsored grants from the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism ($100,000 and more) for research. S.I.R. has had and currently has a number of relationships with companies that provide products and/or services relevant to the outpatient management of COPD. These relationships include serving as a consultant, advice regarding clinical trials, speaking at continuing medical education programs, and performing funded research at basic and clinical levels. S.I.R. does not own any stock in any pharmaceutical companies. S.I.R. received compensation for consultancies from Abbott ($1,001–$5,000), KOL Connection (up to $1,000), Able Associates (up to $1,000), Leerink Swan (up to $1,000), Almirall ($5,001–$10,000), MedaCorp (up to $1,000), Almirall/Forest (up to $1,000), Mpex (up to $1,000), Altana ($1,001–$5,000), Novartis ($10,001–$50,000), Anthera (up to $1,000), Otuska ($1,001–$5,000), APT Pharma/Britnall (up to $1,000), Pfizer ($1,001–$5,000), Aradigm ($1,001–$5,000), Propagate (up to $1,000), Astra-Zeneca ($1,001–$5,000), Pulmatrix (up to $1,000), Boehringer Ingelheim ($1,001–$5,000), Quintiles (up to $1,000), Britnall and Nicolini (up to $1,000), Roche ($1,001–$5,000), Defined Health (up to $1,000), Scimed (up to $1,000), Dunn Group (up to $1,000), TargeGen ($1,001–$5,000), Eaton Associates (up to $1,000), Theravance ($1,001–$5,000), Gerson (up to $1,000), UBS ($1,001–$5,000), GlaxoSmithKline ($10,001–$50,000), VantagePoint (up to $1,000), Infomed (up to $1,000), VantagePoint Mgmt ($1,001–$5,000), and Johnson & Johnson ($1,001–$5,000). S.I.R. served on advisory boards for Abbott ($1,001–$5,000), Nycomed ($10,001–$50,000), Almirall ($10,001–$50,000), Nycomed/Strategicare ($1,001–$5,000), Boehringer Ingelheim ($1,001–$5,000), Pfizer ($1,001–$5,000), COPDForum ($1,001–$5,000), Pharmaxis ($1,001–$5,000), Dey ($1,001–$5,000), Schering-Plough ($1,001–$5,000), GlaxoSmithKline ($10,001–$50,000), TargeGen ($5001–$10,000), and Novartis ($10,001–$50,000). S.I.R. received compensation for lectures from ACCP (up to $1,000), Network for Continuing Ed (up to $1,000), Astra-Zeneca ($10,001–$50,000), Novartis ($10,001–$50,000), Creative Educational Concepts ($1,001–$5,000), Pfizer ($10,001–$50,000), the France Foundation ($1,001–$5,000), and SOMA ($1,001–$5,000). S.I.R. received compensation in the form of industry-sponsored grants from Almirall ($10,001–$50,000), Lorillard ($10,001–$50,000), Astra-Zeneca ($50,001–$100,000), Novartis ($100,001 or more), Biomarck ($100,001 or more), Pfizer ($100,001 or more), Centocor ($50,001–$100,000), Philip Morris ($100,001 or more), GlaxoSmithKline ($100,001 or more), Roche ($100,001 or more), and IFSH ($10,001–$50,000). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Trofor A, Frasila EI. The habit of smoking—a sure step towards COPD. Pneumologia 2007;56:85–90. [PubMed] [Google Scholar]
- 2.Proctor RN. The global smoking epidemic: a history and status report. Clin Lung Cancer 2004;5:371–376. [DOI] [PubMed] [Google Scholar]
- 3.Vastag E, Matthys H, Kohler D, Gronbeck L, Daikeler G. The connections between smoking, mucociliary clearance and airway obstruction. Fortschr Med 1984;102:629–634. [PubMed] [Google Scholar]
- 4.Cohen D, Arai SF, Brain JD. Smoking impairs long-term dust clearance from the lung. Science 1979;204:514–517. [DOI] [PubMed] [Google Scholar]
- 5.Sisson S, Tripp J, Paris W, Cooper DK, Zuhdi N. Medication noncompliance and its relationship to financial factors after heart transplantation. J Heart Lung Transplant 1994;13:930. [PubMed] [Google Scholar]
- 6.Abdi S, Evans MJ, Cox RA, Lubbesmeyer H, Herndon DN, Traber DL. Inhalation injury to tracheal epithelium in an ovine model of cotton smoke exposure: early phase (30 minutes). Am Rev Respir Dis 1990;142:1436–1439. [DOI] [PubMed] [Google Scholar]
- 7.Sisson JH, Papi A, Beckmann JD, Leise KL, Wisecarver J, Brodersen BW, Kelling CL, Spurzem JR, Rennard SI. Smoke and viral infection cause cilia loss detectable by bronchoalveolar lavage cytology and dynein ELISA. Am J Respir Crit Care Med 1994;149:205–213. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt TA, Schmidt SC, Rennard SI, Tuma DJ, Sisson JH. Acetaldehyde-stimulated PKC activity in airway epithelial cells treated with smoke extract from normal and smokeless cigarettes. Proc Soc Exp Biol Med 2000;225:91–97. [DOI] [PubMed] [Google Scholar]
- 9.Elliott MK, Sisson JH, West WW, Wyatt TA. Differential in vivo effects of whole cigarette smoke exposure versus cigarette smoke extract on mouse ciliated tracheal epithelium. Exp Lung Res 2006;32:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lansley AB, Sanderson MJ, Dirksen ER. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am J Physiol 1992;263:L232–L242. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt TA, Forget MA, Adams JM, Sisson JH. Both cAMP and cGMP are required for maximal ciliary beat stimulation in a cell-free model of bovine ciliary axonemes. Am J Physiol Lung Cell Mol Physiol 2005;288:L546–L551. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol 1998;275:L827–L835. [DOI] [PubMed] [Google Scholar]
- 13.Salathe M, Pratt MM, Wanner A. Protein kinase C-dependent phosphorylation of a ciliary membrane protein and inhibition of ciliary beating. J Cell Sci 1993;106:1211–1220. [DOI] [PubMed] [Google Scholar]
- 14.Wong LB, Park CL, Yeates DB. Neuropeptide Y inhibits ciliary beat frequency in human ciliated cells via nPKC, independently of PKA. Am J Physiol 1998;275:C440–C448. [DOI] [PubMed] [Google Scholar]
- 15.Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol 2007;36:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vander Top EA, Wyatt TA, Gentry-Nielsen MJ. Smoke exposure exacerbates an ethanol-induced defect in mucociliary clearance of Streptococcus pneumoniae. Alcohol Clin Exp Res 2005;29:882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slager RE, Devasure JM, Pavlik JA, Sisson JH, Wyatt TA. RACK1, a PKC targeting protein, is exclusively localized to basal airway epithelial cells. J Histochem Cytochem 2008;56:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besson A, Wilson TL, Yong VW. The anchoring protein RACK1 links protein kinase C epsilon to integrin beta chains. Requirements for adhesion and motility. J Biol Chem 2002;277:22073–22084. [DOI] [PubMed] [Google Scholar]
- 19.Slager RE, Sisson JH, Pavlik JA, Johnson JK, Nicolarsen JR, Jerrells TR, Wyatt TA. Inhibition of protein kinase C epsilon causes ciliated bovine bronchial cell detachment. Exp Lung Res 2006;32:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohnishi T, Arnold LL, He J, Clark NM, Kawasaki S, Rennard SI, Boyer CW, Cohen SM. Inhalation of tobacco smoke induces increased proliferation of urinary bladder epithelium and endothelium in female C57BL/6 mice. Toxicology 2007;241:58–65. [DOI] [PubMed] [Google Scholar]
- 21.Wyatt TA, Kharbanda KK, Tuma DJ, Sisson JH. Malondialdehyde-acetaldehyde-adducted bovine serum albumin activates protein kinase C and stimulates interleukin-8 release in bovine bronchial epithelial cells. Alcohol 2001;25:159–166. [DOI] [PubMed] [Google Scholar]
- 22.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 2003;211:103–111. [DOI] [PubMed] [Google Scholar]
- 23.Coote K, Nicholls A, Atherton HC, Sugar R, Danahay H. Mucociliary clearance is enhanced in rat models of cigarette smoke and lipopolysaccharide-induced lung disease. Exp Lung Res 2004;30:59–71. [DOI] [PubMed] [Google Scholar]
- 24.Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med 1996;154:1868–1902. [DOI] [PubMed] [Google Scholar]
- 25.Winters SL, Davis CW, Boucher RC. Mechanosensitivity of mouse tracheal ciliary beat frequency: roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am J Physiol Lung Cell Mol Physiol 2007;292:L614–L624. [DOI] [PubMed] [Google Scholar]
- 26.Delmotte P, Sanderson MJ. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am J Respir Cell Mol Biol 2006;35:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang SC. Microscopic properties of whole mounts and sections of human bronchial epithelium of smokers and nonsmokers. Cancer 1957;10:1246–1262. [DOI] [PubMed] [Google Scholar]
- 28.Nagai A, Thurlbeck WM. Scanning electron microscopic observations of emphysema in humans: a descriptive study. Am Rev Respir Dis 1991;144:901–908. [DOI] [PubMed] [Google Scholar]
- 29.Serafini SM, Michaelson ED. Length and distribution of cilia in human and canine airways. Bull Eur Physiopathol Respir 1977;13:551–559. [PubMed] [Google Scholar]
- 30.Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol 2008;59:19–34. [PubMed] [Google Scholar]
- 31.Stout SL, Wyatt TA, Adams JJ, Sisson JH. Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem 2007;55:433–442. [DOI] [PubMed] [Google Scholar]
- 32.Nikula KJ, March TH, Seagrave J, Finch G, Barr E, Menache M, Hahn F, Hobbs C. A mouse model of cigarette smoke-induced emphysema. Chest 2000;117:246S–247S. [PubMed] [Google Scholar]
- 33.Hodge-Bell KC, Lee KM, Renne RA, Gideon KM, Harbo SJ, McKinney WJ. Pulmonary inflammation in mice exposed to mainstream cigarette smoke. Inhal Toxicol 2007;19:361–376. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery PK. Morphology of the airway wall in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;143:1152–1161. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery PK. Pathology of asthma. Br Med Bull 1992;48:23–39. [DOI] [PubMed] [Google Scholar]
- 36.Jeffery PK. Histological features of the airways in asthma and COPD. Respiration 1992;59:13–16. [DOI] [PubMed] [Google Scholar]
- 37.Lannan S, Donaldson K, Brown D, MacNee W. Effect of cigarette smoke and its condensates on alveolar epithelial cell injury in vitro. Am J Physiol 1994;266:L92–L100. [DOI] [PubMed] [Google Scholar]
- 38.Rickard KA, Taylor J, Rennard SI. Observations of development of resistance to detachment of cultured bovine bronchial epithelial cells in response to protease treatment. Am J Respir Cell Mol Biol 1992;6:414–420. [DOI] [PubMed] [Google Scholar]
- 39.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J 1998;332:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akita Y. Protein kinase C-epsilon (PKC-epsilon): its unique structure and function. J Biochem 2002;132:847–852. [DOI] [PubMed] [Google Scholar]