Abstract
Sphingosine 1–phosphate (S1P) is a key endogenous regulator of the response to lung injury, maintaining endothelial barrier integrity through interaction with one of its receptors, S1P1. The short-term administration of S1P or S1P1 receptor agonists enhances endothelial monolayer barrier function in vitro, and attenuates injury-induced vascular leak in the lung and other organ systems in vivo. Although S1P1 agonists bind to and activate S1P1, several of these agents also induce receptor internalization and degradation, and may therefore act as functional antagonists of S1P1 after extended exposure. Here we report on the effects of prolonged exposure to these agents in bleomycin-induced lung injury. We demonstrate that repeated administration of S1P1 agonists dramatically worsened lung injury after bleomycin challenge, as manifested by increased vascular leak and mortality. Consistent with these results, prolonged exposure to S1P1 agonists in vitro eliminated the ability of endothelial cell monolayers to respond appropriately to the barrier-protective effects of S1P, indicating a loss of normal S1P–S1P1 signaling. As bleomycin-induced lung injury progressed, continued exposure to S1P1 agonists also resulted in increased pulmonary fibrosis. These data indicate that S1P1 agonists can act as functional antagonists of S1P1 on endothelial cells in vivo, which should be considered in developing these agents as therapies for vascular leak syndromes. Our findings also support the hypothesis that vascular leak is an important component of the fibrogenic response to lung injury, and suggest that targeting the S1P–S1P1 pathway may also be an effective therapeutic strategy for fibrotic lung diseases.
Keywords: lung injury, vascular leak, fibrosis, sphingosine 1–phosphate, S1P1
CLINICAL RELEVANCE.
Although the short-term administration of sphingosine 1–phosphate receptor-1 (S1P1) agonists prevents vascular leak in models of acute lung injury, we demonstrate that with prolonged exposure, these agents act as functional antagonists of S1P1 and worsen pulmonary vascular leak after injury. The ability of these agents to act as functional antagonists should be considered in efforts to target the S1P–S1P1 pathway therapeutically in vascular leak syndromes. Moreover, prolonged exposure to S1P1 agonists also exacerbates the fibroproliferative response to lung injury, providing evidence for a pathogenetic link between increased vascular permeability and the development of pulmonary fibrosis.
Sphingosine 1–phosphate (S1P) is a bioactive lysophospholipid that mediates diverse cellular processes through interactions with at least five distinct G protein–coupled receptors, termed S1P1–5 (1, 2). S1P signaling was shown to play important roles in cell migration, survival, contraction, proliferation, and cell–cell interactions (3).
Extensive data indicate that S1P, signaling via S1P1, plays a critical role in maintaining endothelial barrier integrity. S1P is normally present in human and mouse plasma at concentrations of approximately 0.5–1 μM (4–6). The activation of S1P1 on endothelial cells by S1P induces cytoskeletal rearrangements that promote the formation of intercellular adherens junctions and tight junctions, resulting in an enhancement of endothelial monolayer barrier function (7–9). S1P analogues that bind to and activate S1P1 were similarly shown to promote endothelial barrier integrity in vitro (10–12).
Several lines of evidence support the importance of the S1P–S1P1 pathway for regulating vascular permeability in vivo. Mice that are genetically deficient for the S1P-producing enzyme sphingosine kinase 1 (Sphk1) develop worse vascular leak after lung injury (13, 14). The genetic deletion of both sphingosine kinases (Sphk1 and Sphk2) results in a complete lack of circulating S1P and increased vascular leak in multiple organ systems, even in the absence of tissue injury (15). Similarly, interrupting S1P–S1P1 signaling with S1P1 receptor antagonists also increases vascular leak (16). Conversely, short-term treatment with S1P or S1P1 receptor agonists was shown to prevent vascular leak in numerous animal models of acute lung injury, and in models of renal and cutaneous injury (10, 12, 16–22). Because increased vascular permeability is a hallmark of tissue injury, there is a great deal of interest in developing S1P1 agonists as potential therapeutic agents for conditions in which vascular leak is thought to play a central role (22, 23).
The most well-known S1P analog is FTY720, a derivative of the fungal compound myriocin. FTY720 was initially identified as a potent immunosuppressant, because it causes a profound peripheral lymphopenia by inducing lymphocyte sequestration in secondary lymphoid organs (24–26). It has been extensively studied in humans as a potential therapy for solid organ transplant rejection and multiple sclerosis (27–33). FTY720 is rapidly phosphorylated in vivo to FTY720-P, which binds with high affinity to and activates four of the five S1P receptors, S1P1,3–5 (34, 35). Its effects on the S1P1 receptor have been most well-studied. Although FTY720-P activates S1P1 in vitro, it also causes a loss of S1P1 cell-surface expression through receptor internalization and degradation, and can therefore act as a “functional antagonist” of the receptor (36–40). Such functional antagonism of S1P1 on lymphocytes is thought to explain the ability of FTY720 to induce lymphocyte sequestration (41, 42). Numerous S1P1-selective agonists have been developed which, like FTY720, were also shown to induce peripheral lymphopenia (43–45), presumably through functional antagonism of S1P1 on lymphocytes.
As noted already, the short-term administration of FTY720 or S1P1-selective agonists prevents injury-induced vascular leak in vivo. However, studies demonstrating the barrier-protective effects of these agents focused on early time points (≤ 24 h) after tissue injury, with the administration of a single dose of agonist (10, 12, 16–22). In vitro studies show that S1P1 agonists, similar to their effects on lymphocytes, can also induce the time-dependent internalization and degradation of S1P1 on endothelial cells (38–40). Therefore, S1P1 agonists may initially enhance endothelial barrier function and prevent vascular leak by augmenting S1P1 signaling, but prolonged exposure to these agents may exert the opposite effect, disrupting endothelial barrier integrity and worsening vascular leak through functional antagonism of S1P1 on endothelial cells. To determine whether this functional antagonism of endothelial S1P1 occurs in vivo, we studied the effects of sustained administration of two such agents, FTY720 and the S1P1-selective agonist, AUY954, in the bleomycin mouse model of lung injury. We found that prolonged exposure to FTY720 dramatically worsened mortality, histologic evidence of lung injury, and vascular leak after bleomycin challenge. Prolonged exposure to AUY954 similarly exacerbated bleomycin-induced vascular leak, consistent with the functional antagonism of endothelial S1P1. Uninjured mice treated with FTY720 and AUY954 also demonstrated changes consistent with increased pulmonary vascular permeability, although these findings were milder and not associated with mortality or major histologic sequelae. As the response to bleomycin injury progressed, sustained treatment with S1P1 agonists also led to increased lung fibrosis, suggesting that S1P–S1P1 signaling may represent an important pathway for limiting the fibrotic response to injury.
MATERIALS AND METHODS
Cells and Reagents
All mice were purchased from the National Cancer Institute–Frederick Animal Production Program (Frederick, MD). Human umbilical-vein endothelial cells (HUVECs) were purchased from Lonza (Walkersville, MD) and maintained in EGM-2 medium (Lonza), except where indicated. S1P was purchased from Avanti Polar Lipids (Alabaster, AL). FTY720 and FTY720-P were purchased from Cayman Chemicals (Ann Arbor, MI). SEW2871 was purchased from Fisher Scientific (Pittsburgh, PA). AUY954 was synthesized according to the protocol described by Pan and colleagues (44). Stock solutions were prepared in methanol (S1P), water (FTY720), or DMSO (FTY720-P, SEW2871, and AUY954). FITC-labeled albumin and FITC-labeled phalloidin were purchased from Sigma (St. Louis, MO). For in vivo administration, FTY720 and AUY954 stock solutions were diluted to the appropriate concentrations in sterile water. For in vitro experiments, S1P, FTY720-P, and SEW2871 stock solutions were diluted in serum-free media with 0.1% fatty acid–free BSA, and sonicated before use.
Animals and Administration of Bleomycin
Adult male C57Bl/6 mice, 7–9 weeks old (∼ 25 g), were used in all experiments. Mice were administered a single intratracheal dose of bleomycin at 1.2 U/kg (standard dose) or 0.1 U/kg (low dose), in a total volume of 50 μl. Control mice were injected intratracheally with 50 μl sterile saline. For FTY720 experiments, mice were injected intraperitoneally with vehicle or FTY720 at 5 mg/kg or 0.5 mg/kg, three times per week. For AUY954 experiments, mice were injected intraperitoneally with vehicle or AUY954 at 5 mg/kg once daily. All administrations of FTY720, AUY954, and vehicle were initiated 1 hour before bleomycin challenge, and continued throughout the duration of the experiments. Bronchoalveolar lavage (BAL) was performed, and lungs were harvested at the time points indicated. All protocols were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care, and all mice were maintained in a specific pathogen–free environment certified by the American Association for Accreditation of Laboratory Animal Care.
Administration of LPS
Mice were administered a single intratracheal dose of 50 μg of lipopolysaccharide (LPS) or sterile saline in a total volume of 50 μl. BAL was performed 24 hours after LPS challenge. FTY720 at 0.5 mg/kg or vehicle was administered intraperitoneally once daily, beginning 24 hours before LPS challenge (48 h before BAL).
Lung Histology
Lungs were inflated with 10% buffered formalin at 25 cm H2O for 15 minutes, excised, fixed in 10% formalin for 72 hours, and embedded in paraffin. Multiple paraffin-embedded 5-μm sections were stained with hematoxylin–eosin or Masson trichrome stain. Images were obtained with a Nikon Eclipse ME600 microscope with a Nikon DXM 1200C digital camera and NIS Elements AR 2.30 software (Nikon Instruments Inc., Melville, NY).
Mouse BAL
Mouse lungs were lavaged with six successive 0.5-ml aliquots of PBS with 0.6 mM EDTA (3 ml in total). These BAL samples were centrifuged at 540 × g, at 4°C, for 5 minutes. The BAL supernatants were transferred into siliconized, low-binding microcentrifuge tubes (Fisher Scientific) for subsequent analysis. The BAL cell pellets were resuspended in PBS for determinations of cell count, cytospin analysis, and cytofluorimetry.
BAL Total Protein, D-Dimer, and Thrombin–Antithrombin Determination
Concentrations of BAL supernatant total protein were determined using the commercially available BCA Protein Assay Kit (Pierce/Thermo Scientific, Rockford, IL), according to the manufacturer's instructions. D-dimer activity and thrombin–antithrombin (TAT) concentrations in BAL supernatants were determined with commercially available ELISA kits obtained from Fisher Scientific and USCN Life (Wuhan, P.R. China), respectively.
BAL Leukocyte Counts, Cell Differentials, and Cytofluorimetry
BAL total cell counts were determined using a Cellometer Auto T4 (Nexcelom, Lawrence, MA) cell counter. Total leukocytes (obtained by excluding the percentages of red blood cells) and percentages of mononuclear cells and neutrophils were determined in preparations of cells centrifuged with a Cytospin 3 (Shandon/Thermo Scientific, Rockford, IL) and stained with Hema 3 stain (Millipore, Billerica, MA). Cells recovered from BAL were incubated at 4°C for 20 minutes with 2.4G2 anti-FcγIII/II receptor (BD Pharmingen, Franklin Lakes, NJ), and stained with monoclonal antibodies to mouse CD3, CD4, and CD8 directly conjugated to allophycocyanin (APC), FITC, and phycoerythrin (PE), respectively (all from BD Pharmingen) at 4°C for 30 minutes. Percentages of CD3+, CD4+, and CD8+ cells were determined by cytofluorimetry, using a FACSCalibur Cytometer (Becton–Dickinson, Franklin Lakes, NJ), and analyzed using CellQuest software (BD Biosciences, San Jose, CA).
Evans Blue Dye Extravasation Assay
Evans blue dye extravasation was assessed in saline-challenged and bleomycin-challenged mice, as described previously (46). Briefly, Evans blue dye (Sigma) was injected intravenously at 20 mg/kg into mice 2 hours before they were killed. When the mice were killed, blood was recovered into a heparinized syringe by cardiac puncture. The right ventricle was then perfused with PBS to remove intravascular dye from the lungs, which were then removed and homogenized. Evans blue dye was extracted from the homogenates by the addition of 1.5 ml of formamide, followed by incubation overnight at 60°C, and centrifugation at 5,000 × g for 30 minutes. The absorption (A) of Evans blue dye in the lung supernatants and plasma was measured at 620 nm and corrected for the presence of heme pigments as follows: A620 (corrected) = A620 − (1.426 × A740 + 0.030). The concentrations of Evans blue dye in the lungs and plasma were then determined against a standard curve, and the degree of vascular leak in each lung set was presented as an Evans blue dye index, calculated as the ratio of the total amount of Evans blue dye in those lungs to the concentration of Evans blue dye in the plasma of that mouse.
Measurement of Lung Hydroxyproline
Total lung hydroxyproline was determined as described previously (47). Briefly, lungs were homogenized in PBS and hydrolyzed overnight in 6 N HCl at 120°C. A 25-μL aliquot was then desiccated, resuspended in 25 μL H2O, and added to 0.5 ml of 1.4% chloramine T (Sigma), 10% n-propanol, and 0.5 M sodium acetate, pH 6.0. After 20 minutes of incubation at room temperature, 0.5 ml of Erlich's solution (1 M p-dimethylaminobenzaldehyde from Sigma, in 70% n-propanol and 20% perchloric acid) were added, and a 15-minute incubation at 65°C followed. Absorbance was measured at 550 nm, and the amount of hydroxyproline was determined against a standard curve.
Endothelial Permeability Assay
HUVECs were grown to confluence on semipermeable, fibronectin-coated transwell inserts in 24-well plates, with media above (luminal chamber) and below (abluminal chamber) the monolayers. Intact monolayers were serum-starved for 1 hour, and then stimulated with S1P (1 μM), FTY720-P (1 μM), or SEW2871 (10 μM), with FITC–albumin (1 mg/ml) added to the luminal chamber. After 1 hour of incubation at 37°C, transwell inserts were removed from the wells, and fluorescence in the abluminal chamber was measured using a Thermo Fluoroskan Ascent FC (Thermo Scientific) (excitation/emission, 485/518 nm). The concentration of FITC–albumin in the abluminal chamber was determined against a standard curve. Permeability was reported as an albumin flux index, and the ratio of abluminal FITC–albumin concentration was compared with baseline conditions. Where indicated, intact HUVEC monolayers were pretreated with FTY720-P, SEW2871, or medium alone for 24 hours before performing the permeability assay.
Visualization of Endothelial Cytoskeletal Rearrangement
HUVECs were grown to confluence on fibronectin-coated, four-well Lab-Tek chamber slides (Nunc). Where indicated, FTY720-P (1 μM) or SEW2871 (10 μM) was added to media 24 hours before stimulation. Cells were serum-deprived for 1 hour, and then stimulated with S1P (1 μM), thrombin (1 U/ml), or both for 30 minutes. Cells were then washed with PBS, fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, and incubated with FITC–phalloidin for 15 minutes before image acquisition with the microscope equipment already described, using a ×10 optical lens and ×40 objective lens (total magnification, ×400).
Statistical Analyses
Except where indicated in figure legends, differences between treatment and control groups were analyzed for statistical significance with two-tailed Student t tests, using Microsoft Excel software (Microsoft, Redmond, WA). For comparisons of survival after bleomycin challenge, we performed log-rank tests using GraphPad Prism software (GraphPad Software, La Jolla, CA). P < 0.05 was considered statistically significant.
RESULTS
The Nonselective S1P Receptor Agonist FTY720 Worsens Lung Injury and Mortality after Bleomycin Challenge
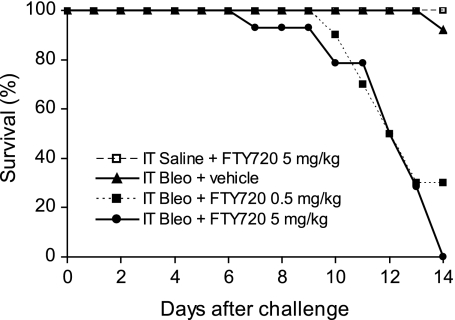
We first examined the effects of repeated administrations of the nonselective S1P receptor agonist FTY720 on mortality after intratracheal instillations of bleomycin. Although bleomycin challenge alone at a dose of 1.2 U/kg induced minimal mortality at 2 weeks, the administration of FTY720 at 5 mg/kg or 0.5 mg/kg three times per week to bleomycin-challenged mice had a striking effect, inducing 100% and 70% mortality, respectively (Figure 1). In the absence of bleomycin challenge, treatment with FTY720 had no overt toxicity and no effect on survival.
Figure 1.
Effects of nonselective S1P receptor agonist FTY720 on survival after bleomycin (Bleo) challenge. Mice received a single intratracheal (IT) dose of bleomycin (1.2 U/kg) or saline, and were treated with intraperitoneal vehicle or FTY720 at 5 or 0.5 mg/kg, three times per week, starting on Day 0. FTY720 treatment at both doses led to an increase in mortality within 2 weeks after bleomycin challenge. Data are combined from three independent experiments; n = 10–25 mice/group in total. For both FTY720 doses tested, P < 0.0001, comparing bleomycin + vehicle with bleomycin + FTY720 by log-rank test.
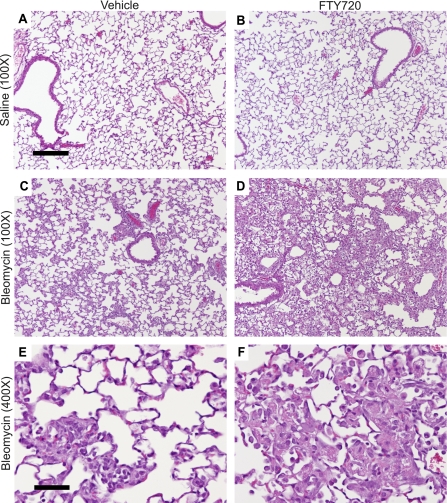
The early response to bleomycin-induced lung injury in mice mimics the exudative phase of acute respiratory distress syndrome (ARDS) in humans, characterized by vascular leak, intra-alveolar coagulation, hyaline membrane deposition, and inflammatory-cell recruitment (48). The time course of mortality we observed in bleomycin-challenged mice treated with FTY720 (during Days 7–14 after challenge; Figure 1) is therefore more consistent with death from worsened exudative responses, rather than from progressive lung fibrosis, which typically does not become established until Day 14 after bleomycin challenge (48). To assess whether the effects of prolonged FTY720 treatment on mortality after bleomycin challenge were attributable to worsened lung injury, we performed histologic analyses of mouse lungs on Day 7 after bleomycin challenge, just before the onset of mortality in FTY720-treated mice. Mice injected intratracheally with saline and treated with vehicle or FTY720 showed no histologic evidence of lung injury (Figures 2A and 2B). Lungs from bleomycin-challenged mice treated with vehicle showed the characteristic pattern of injury seen in this model, with patchy areas of alveolar damage adjacent to areas of normal lung (Figures 2C and 2E). Concurrent treatment with FTY720, however, resulted in more extensive lung injury, with a more diffuse pattern of alveolar damage and very little normal lung (Figure 2D). On high-power magnification, the most striking finding in FTY720-treated mice was the extensive intra-alveolar accumulation of amorphous eosinophilic material, representing the hyaline membranes characteristic of lung injury (Figure 2F).
Figure 2.
Effects of FTY720 on lung injury after bleomycin challenge. Representative hematoxylin–eosin staining of mouse lung sections on Day 7 after intratracheal saline or bleomycin and treatment with vehicle or FTY720 at 5 mg/kg intraperitoneally, three times per week. (A, B) No histologic evidence of lung injury was seen after intratracheal saline with vehicle or FTY720 treatment. (C–F) Bleomycin challenge induced histologic evidence of lung injury, characterized by intra-alveolar hyaline membrane deposition, which was more severe in FTY720-treated mice. Representative images are shown from each treatment group; n = 3 mice/group. Scale bar = 200 μm (A–D) or 50 μm (E, F).
FTY720 and the S1P1-Selective Agonist AUY954 Worsen Lung Vascular Leak and Intra-Alveolar Coagulation after Lung Injury
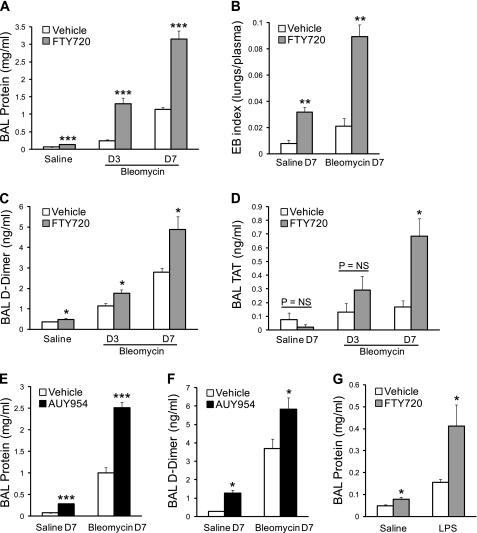
We assessed lung vascular leak in saline-challenged and bleomycin-challenged mice by measuring BAL fluid total protein concentrations and performing Evans blue dye extravasation assays. The concentration of BAL total protein rose on Days 3 and 7 after bleomycin challenge, and this increase in vascular leak was greatly exaggerated with repeated administrations of FTY720 (Figure 3A). Evans blue dye extravasation assays performed on Day 7 confirmed this increase in vascular leak with FTY720 treatment (Figure 3B). Interestingly, control mice challenged with intratracheal saline also showed an increase in BAL total protein and Evans blue dye extravasation after FTY720 treatment (Figures 3A and 3B), indicating that FTY720 can disrupt the capillary–alveolar barrier, even in the absence of direct lung injury. Although FTY720 treatment resulted in an increase in vascular leak even in the absence of bleomycin challenge, this finding was not associated with mortality (Figure 1) or obvious histologic abnormalities (Figure 2B), likely because of the capacity of the uninjured lung to clear increased extravascular water and proteins. As expected, bleomycin lung injury also induced the intra-alveolar activation of the coagulation cascade, which was determined by measuring BAL D-dimer and TAT levels (Figures 3C and 3D). Both of these indices of intra-alveolar coagulation were increased in FTY720-treated mice compared with vehicle-treated controls after bleomycin challenge (Figures 3C and 3D).
Figure 3.
Effects of FTY720 and the S1P1-selective agonist AUY954 on lung vascular leak and intra-alveolar coagulation after lung injury. (A) Bronchoalveolar lavage (BAL) total protein concentration was increased in FTY720-treated mice compared with vehicle-treated mice on Day 3 (D3) and Day 7 (D7) after bleomycin challenge. (B) Evans blue (EB) dye extravasation assay confirmed the presence of increased lung vascular leak at D7 after bleomycin. Seven days of exposure to FTY720 increased lung vascular leak, even in the absence of bleomycin-induced lung injury (A and B, Saline D7). (C, D) Intra-alveolar coagulation after bleomycin injury was also increased in FTY720-treated mice compared with vehicle-treated control mice, as determined by BAL D-dimer and thrombin–antithrombin concentrations. (E, F) Treatment with the S1P1-selective agonist AUY954 at 5 mg/kg/day similarly increased lung vascular leak, both in the presence (Bleomycin D7) and absence (Saline D7) of lung injury, and it also increased intra-alveolar coagulation. (G) BAL total protein concentration was increased in FTY720-pretreated mice compared with vehicle-pretreated mice, 24 hours after intratracheal LPS challenge, as well as in saline-challenged control mice. Data are presented as means ± SEM, with n = 4–5 mice/group. *P < 0.05, **P < 0.005, and ***P < 0.0005, comparing FTY720-treated or AUY954-treated versus vehicle-treated groups at each time point.
Phosphorylated FTY720 is an agonist at four of the five S1P receptors (S1P1,3–5), and activation of S1P3 was reported to disrupt epithelial barrier function (49). Therefore, to determine whether our observations with FTY720 were attributable to its effects on S1P1, we treated bleomycin-challenged and saline-challenged mice with the S1P1-selective agonist AUY954. AUY954 has high affinity for S1P1, with negligible effects on other S1P receptors (44). As with other S1P1 agonists, it was shown to induce profound peripheral lymphopenia (44). Similar to our findings in the FTY720 experiments, prolonged treatment with AUY954 also resulted in a dramatic increase in lung vascular leak, as determined by measurement of BAL total protein, with and without bleomycin-induced lung injury (Figure 3E). AUY954 also caused an increase in coagulation within the airspaces, as determined by BAL D-dimer concentrations (Figure 3F).
Our findings that prolonged exposure to S1P1 agonists exacerbates vascular leak after bleomycin lung injury differ from previous observations that short-term exposure to these agents protects against vascular leak at early time points after injury. To investigate whether these contrasting results reflect differences in duration of exposure to S1P1 agonists, rather than differences between the bleomycin model and other models of lung injury, we examined the effects of sustained administration of FTY720 on LPS-induced lung injury in mice. When administered 1 hour after LPS challenge, FTY720 was shown to protect against pulmonary vascular leak in this model, when evaluated ≤ 24 hours after injury (12, 17). We treated mice with FTY720 at 0.5 mg/kg daily, starting 24 hours before intratracheal LPS (or sterile saline) challenge, and we assessed the degree of protein extravasation in BAL at 24 hours after lung injury (48 hours after initiating FTY720 treatment). This more prolonged (48-h) exposure to FTY720 resulted in a significant increase in BAL total protein concentration, with and without LPS-induced lung injury (Figure 3G), suggesting an increase in pulmonary vascular leak, consistent with our findings in the bleomycin model.
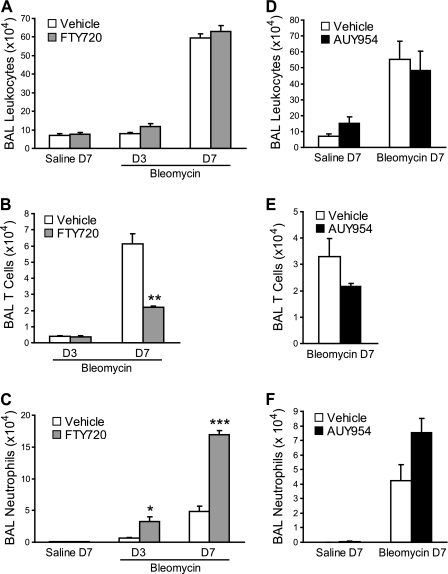
Effects of FTY720 and AUY954 on Inflammation after Bleomycin Challenge
We observed the characteristic accumulation of leukocytes in the airspaces of mice after bleomycin lung injury (Figure 4). Despite the dramatic increase in bleomycin-induced vascular leak with FTY720 treatment, no differences were evident in the total numbers of BAL leukocytes between FTY720-treated and vehicle-treated mice (Figure 4A). However, differences were evident in the subsets of leukocytes. FTY720 treatment resulted in a decrease in BAL lymphocytes (Figure 4B), consistent with the expected induction of peripheral lymphopenia by S1P1 agonists. Moreover, an increase in BAL neutrophils occurred with FTY720 treatment (Figure 4C). A similar pattern of BAL leukocyte accumulation was seen in AUY954-treated mice after bleomycin challenge (Figures 4D–4F), although the decrease in lymphocytes and increase in neutrophils did not reach statistical significance in these experiments.
Figure 4.
Effects of FTY720 and AUY954 on BAL leukocytes after bleomycin challenge. (A–C) FTY720 treatment had no effect on total leukocyte accumulation in the airspaces after bleomycin lung injury, although a decrease in BAL T cells occurred, as well as an increase in BAL neutrophils, in FTY720-treated compared with vehicle-treated mice. (D–F) Similar effects on BAL leukocyte accumulation were evident with AUY954 treatment, with no difference in total leukocytes and trends toward decreased T cells and increased neutrophils. Data are presented as means ±SEM, with n = 5 mice/group. *P < 0.05, **P < 0.005, and ***P < 0.0005, comparing FTY720-treated or AUY954-treated versus vehicle-treated groups at each time point.
Prolonged Exposure to S1P1 Agonists Results in a Loss of S1P–S1P1 Signaling in Endothelial Monolayers
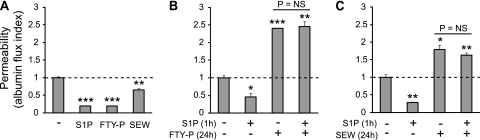
The initial response of endothelial cell monolayers to S1P1 agonist treatment in vitro is the stabilization and enhancement of intercellular junctions, which increases monolayer barrier function. However, S1P1 agonists were also shown to cause a time-dependent loss of S1P1 surface expression in many cell types, including endothelial cells, because of agonist-induced receptor internalization and degradation (36–40). Therefore, we hypothesized that with sustained exposure to S1P1 agonists, the down-regulation and functional antagonism of endothelial cell surface S1P1 would impair the ability of these cells to respond appropriately to the barrier-enhancing properties of S1P. To test this hypothesis, we assessed the effects of S1P on HUVEC monolayer barrier function in the presence or absence of FTY720-P or the S1P1-selective agonist SEW2871. SEW2871 is a weak S1P1 agonist, and although it appears to cause minimal receptor degradation at concentrations ≤ 500 nM (37, 43), the loss of endothelial S1P1 expression was seen at higher concentrations (≥ 1 μM) (39). As expected, short-term (1-hour) stimulation with S1P (1 μM), FTY720-P (1 μM), or SEW2871 (10 μM) decreased monolayer permeability to FITC-labeled albumin (Figure 5A), consistent with S1P1-mediated barrier enhancement. In contrast, prolonged (24-hour) exposure to FTY720-P or SEW2871 had the opposite effect, increasing monolayer permeability (Figures 5B and 5C). Furthermore, prolonged exposure to these S1P1 agonists eliminated the ability of S1P to enhance barrier function (Figures 5B and 5C), indicating a loss of normal S1P-S1P1 signaling.
Figure 5.
Effects of prolonged exposure to FTY720-P and the S1P1-selective agonist SEW2871 on endothelial monolayer permeability. Human umbilical vein endothelial cells (HUVECs) were grown to confluence on semipermeable, fibronectin-coated membranes. HUVEC monolayer permeability to FITC-labeled albumin over 1 hour (1h) was then determined. (A) S1P (1 μM), FTY720-P (FTY-P; 1 μM), and SEW2871 (SEW; 10 μM) all decreased HUVEC monolayer permeability when cells were stimulated for 1 hour. In contrast, when cells were pretreated with (B) FTY720-P or (C) SEW2871 for 24 hours (24h), monolayer permeability was increased at baseline, and failed to decrease in response to S1P. Data are presented as means ± SEM (n = 3), and are representative of two or three independent experiments. *P < 0.05, **P < 0.005, and ***P < 0.00005, versus untreated cells.
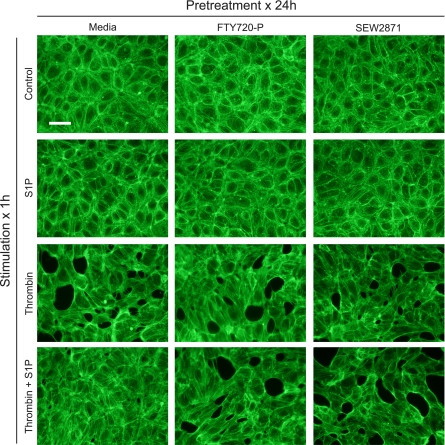
We also examined the effects of prolonged S1P1 agonist exposure on endothelial monolayers by visualizing HUVEC actin cytoskeletal rearrangements in response to thrombin (Figure 6). In the resting state, confluent HUVECs demonstrated a cortical ring arrangement of the actin cytoskeleton, which promotes tight intercellular adhesions. Treatment with thrombin for 1 hour resulted in actin stress fiber formation, with a disruption of cell–cell interactions and the creation of paracellular gaps. Thrombin-mediated stress fiber formation was effectively blocked by the co-administration of S1P. However, pretreating HUVEC monolayers with FTY720-P or SEW2871 for 24 hours eliminated the ability of S1P to prevent thrombin-mediated stress fiber formation (Figure 6).
Figure 6.
Effects of prolonged exposure to FTY720-P and SEW2871 on endothelial cell cytoskeletal rearrangements induced by thrombin. HUVEC actin filaments were visualized with FITC-labeled phalloidin staining. Baseline cortical arrangement of the actin cytoskeleton was unaffected by 24 hours of exposure to FTY720 or SEW2871 (top row). Stimulation with thrombin (1 U/ml) for 1 hour induced stress fiber formation with cellular distortion and paracellular gap formation, a process inhibited by costimulation with S1P. Pretreating HUVEC monolayers with FTY720 or SEW2871 for 24 hours eliminated the ability of S1P to attenuate thrombin-induced cytoskeletal changes. Images are representative of two independent experiments. Scale bar = 50 μm.
FTY720 and AUY954 Worsen Lung Fibrosis after Low-Dose Bleomycin Challenge
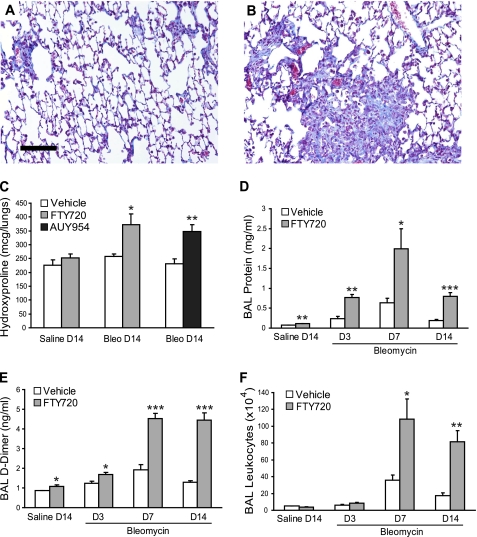
In the bleomycin model, fibrosis develops during the second week after lung injury, and can be reliably assessed by Day 14 (48). As demonstrated in Figure 1, however, challenging mice with our standard dose of bleomycin (1.2 U/kg) and treating them with FTY720 resulted in very high mortality, with most of the animals dying during the second week. Therefore, to ensure survival for at least 2 weeks and allow us to compare the development of lung fibrosis between vehicle-treated and FTY720-treated mice, we lowered the bleomycin dose 12-fold (to 0.1 U/kg), and used our lower dose of FTY720 (0.5 mg/kg, three times per week). With this treatment strategy, all mice survived for 2 weeks. By itself, this low-dose bleomycin challenge resulted in minimal lung fibrosis on Day 14, as assessed by Masson trichrome staining (Figure 7A). However, when mice challenged with low-dose bleomycin were treated with FTY720, an increase in collagen deposition was evident (Figure 7B). Quantification of total lung collagen by hydroxyproline measurement confirmed that low-dose bleomycin challenge alone did not induce significant fibrosis, whereas low-dose bleomycin with concurrent FTY720 treatment significantly increased lung collagen (Figure 7C). Furthermore, the S1P1-selective agonist AUY954 induced a similar degree of fibrosis after low-dose bleomycin challenge, indicating that the profibrotic effects of FTY720 were mediated by S1P1 (Figure 7C).
Figure 7.
Effects of S1P1 agonists on lung injury and fibrosis after low-dose bleomycin challenge. (A) Trichrome staining of mouse lung sections (scale bar = 100 μm) on Day 14 after low-dose intratracheal bleomycin challenge (0.1 U/kg) demonstrated minimal collagen accumulation. (B) Treatment with FTY720 at 0.5 mg/kg, three times per week, resulted in increased collagen deposition in the lungs. (C) Quantification of fibrosis with total lung hydroxyproline measurement confirmed the increase in lung fibrosis in FTY720-treated mice. Treatment with the S1P1-selective agonist AUY954 also increased lung fibrosis on Day 14 after low-dose bleomycin challenge. (D–F) FTY720 at 0.5 mg/kg, three times per week, also resulted in increased (D) BAL total protein concentration, (E) D-dimer activity, and (F) leukocyte accumulation after low-dose bleomycin challenge. Data are presented as means ± SEM, with n = 5 mice/group. *P < 0.05, **P < 0.005, and ***P < 0.0005, comparing FTY720-treated or AUY954-treated versus vehicle-treated groups at each time point.
Even at this lower dose of 0.5 mg/kg three times per week, FTY720 treatment increased lung vascular leak (Figure 7D) and intra-alveolar coagulation (Figure 7E) at all time points after bleomycin challenge, and in saline-treated control mice. In contrast to findings with our standard dose of bleomycin, FTY720 treatment induced a significant increase in total BAL leukocytes after low-dose bleomycin challenge (Figure 7F), although with a similar decrease in T cells and an increase in neutrophils (data not shown).
DISCUSSION
In this study, we examined the effects of prolonged exposure to S1P1 agonists on lung injury, using the bleomycin model. In contrast to the protective effects of short-term exposure to S1P1 agonists, prolonged exposure to two such agents, the nonselective S1P receptor agonist FTY720 and the S1P1-selective agonist AUY954, exacerbated the lung injury induced by bleomycin. Sustained administration of FTY720 resulted in a dramatic worsening of mortality and histologic evidence of lung injury, and both FTY720 and AUY954 treatment increased vascular leak, intra-alveolar coagulation, and fibrosis after bleomycin challenge. Furthermore, prolonged exposure to S1P1 agonists resulted in increased pulmonary vascular leak in the absence of direct injury, similar to the phenotype of mice that lack circulating S1P or are treated with S1P1 antagonists (15, 16). These observed increases in vascular leak with sustained administrations of FTY720 and AUY954 are consistent with our hypothesis that prolonged exposure to S1P1 agonists can result in functional antagonism of S1P1 on endothelial cells in vivo, leading to a loss of the barrier-protective effects of endogenous S1P–S1P1 signaling.
The results of our study are consistent with the large body of evidence that S1P is an important endogenous regulator of endothelial permeability, and that its ability to restore and enhance endothelial barrier function is mediated by the S1P1 receptor. S1P–S1P1 signaling induces endothelial cytoskeletal rearrangements that tighten intercellular junctions, thereby decreasing the permeability of endothelial cell monolayers in vitro (7–12). The importance of endogenous S1P–S1P1 signaling for maintaining vascular barrier integrity in vivo is highlighted by recent studies showing how a genetic deficiency in the enzymes that produce circulating S1P, or a pharmacologic inhibition of S1P1, results in increased vascular permeability under basal conditions and in response to tissue injury (13–16). Conversely, augmenting endogenous S1P–S1P1 signaling through the short-term administration of S1P or S1P1 agonists was shown to reduce vascular leak in many different animal models of acute tissue injury (10, 12, 16–22). Accordingly, there is great interest in developing S1P1 agonists as potential therapeutic agents for pathologic conditions in which vascular leak is believed to play a central role (22, 23).
The results of our study initially seem to contradict published reports in which S1P1 agonists protected animals against injury-induced vascular leak. However, those studies focused on early time points (≤ 24 hours) after tissue injury, with the administration of a single dose of agonist (10, 12, 16–22). Although these agents all bind to and activate S1P1 (hence their designation as agonists), they can also induce receptor internalization and degradation, and may therefore act as functional antagonists of S1P1 upon prolonged exposure (36–38). In fact, such functional antagonism of S1P1 on lymphocytes is thought to explain the ability of these agents to induce peripheral lymphopenia (41, 42). S1P1 agonists were also shown to induce receptor degradation and functional antagonism of S1P1 on endothelial cells in vitro (38–40). It is reasonable to hypothesize, therefore, that prolonged exposure to S1P1 agonists also results in functional antagonism of S1P1 on endothelial cells in vivo, with the resulting loss of endogenous S1P–S1P1 signaling leading to the increased vascular leak that we observed in our experiments.
Other potential explanations for the discrepancy between our findings and previous reports about S1P1 agonists include differences in dosing or a specific interaction between these agonists and bleomycin itself. However, we observed increases in vascular leak and mortality with both FTY720 doses tested (0.5 and 5 mg/kg), which overlap with the range of doses shown to exert barrier-protective effects in short-term models of tissue injury (0.1–2 mg/kg). Furthermore, we observed increases in vascular leak with S1P1 agonist treatment in the absence of bleomycin challenge, suggesting that these findings are not specific to the lung injury induced by bleomycin. Our finding that prolonged FTY720 treatment can also exacerbate the lung injury induced by intratracheal LPS further supports our hypothesis that this agent can act as a functional antagonist of endothelial S1P1 in vivo. The contrast between our findings with sustained FTY720 treatment in the LPS model and previous findings with short-term administrations of FTY720 in this model highlight the time-dependent nature of this agent's ability to act as either an agonist or antagonist of S1P1.
To support our hypothesis that the effects of S1P1 agonists on endothelial cells depend on the duration of exposure, we used an in vitro assay of endothelial monolayer barrier function, taking advantage of the known barrier-enhancing effect of S1P–S1P1 signaling in HUVECs. As previously shown, short-term (1-h) exposure to S1P or S1P1 agonists decreases endothelial monolayer permeability and protects against thrombin-mediated barrier disruption. However, prolonged (24-hour) exposure to S1P1 agonists exerts the opposite effect, increasing monolayer permeability and eliminating the ability of S1P to enhance barrier function. This elimination of S1P-mediated barrier protection suggests an inhibition of normal S1P–S1P1 signaling, consistent with the hypothesis that in the setting of prolonged exposure, S1P1 agonists behave as functional antagonists of endothelial S1P1.
The increase in baseline endothelial monolayer permeability with prolonged exposure to S1P1 agonists in vitro is also noteworthy. This finding may be attributable to an inhibition of basal S1P–S1P1 signaling (because of S1P present in the growth medium), or it may represent unopposed S1P signaling through S1P2 or S1P3, which were reported to have barrier-disruptive properties (49–51). Interestingly, prolonged exposure to FTY720-P appears to have a greater effect on increasing baseline monolayer permeability than does SEW2871. Although this finding may be attributed to the higher affinity of FTY720-P for the S1P1 receptor or its more pronounced effects on receptor degradation, it may also be attributed to the ability FTY720-P (but not SEW2871) to activate other S1P receptors, and specifically S1P3.
Our data support previous work indicating that S1P1 agonists can act as functional antagonists of S1P1 expressed in endothelial cells in vitro, and also suggest that this phenomenon is important in vivo. In the lung, this functional antagonism of S1P1 caused increased vascular leak, both at baseline and in response to direct lung injury. Therefore, caution should be exercised in developing S1P1 agonists as potential therapies for vascular leak syndromes, because prolonged exposure to these agents may paradoxically worsen leak.
Our findings may have direct clinical implications. In a Phase II clinical trial of FTY720 for relapsing multiple sclerosis (MS), pulmonary toxicity, manifested by dyspnea and mild decreases in pulmonary function, was observed in a significant number of patients who received FTY720 at the highest dose (5 mg/day) (30). The lower dose (1.25 mg/day) was equally efficacious at treating MS, but had minimal pulmonary toxicity. Recent Phase II and Phase III clinical trials of FTY720 for the prevention of renal transplant rejection also reported mild, statistically nonsignificant decreases in pulmonary function at doses of 5 and 2.5 mg/day (31, 32). Although these pulmonary effects are nonspecific, such findings could result from minor perturbations in lung endothelial barrier function. However, the FTY720 doses in our animal experiments are significantly higher (in mg/kg/d) than the doses used in those clinical trials. For example, our lower dose of 0.5 mg/kg three times per week would be equivalent to approximately 12 mg/day in a 60-kg human. Whether or not FTY720 (or other S1P1 agonists) can exacerbate pulmonary vascular leak at clinically relevant doses remains to be determined. Nevertheless, our data suggest that monitoring for the development or exacerbation of lung injury merits consideration for patients treated with S1P1 agonists.
In addition to studying the effects of S1P1 agonists on the initial phases of lung injury, we examined how these agents affect the later fibrotic phase. Given the excessive mortality when FYT720 treatment was combined with our standard dose of intratracheal bleomycin, we used a low-dose bleomycin challenge to ensure adequate survival at 2 weeks, when fibrosis could be reliably assessed and compared between control and treatment groups. We also examined the effects of S1P1 agonists on lung vascular leak, intra-alveolar coagulation, and inflammatory cell accumulation after low-dose bleomycin challenge.
Although this low-dose bleomycin challenge induced a negligible amount of lung fibrosis in vehicle-treated mice, we found that concurrent treatment with FTY720 or AUY954 greatly exacerbated the fibrotic response. As was the case in our standard-dose bleomycin experiments, significant increases were evident in vascular leak and intra-alveolar coagulation with S1P1 agonist treatment under these conditions. A large body of evidence indicates that intra-alveolar coagulation is an important component of the fibrotic response to lung injury, either through the establishment of a provisional fibrin matrix within the airspaces, or through thrombin-mediated activation of proteinase-activated receptors (52–59). Increased extravasation of plasma proteins, including clotting factors, into the alveolar space as a result of increased vascular leak can lead to increased intra-alveolar activation of the coagulation cascade. Therefore, it is reasonable to hypothesize that the increased fibrosis we observed in FTY720-treated and AUY954-treated mice resulted from the observed increases in vascular leak and coagulation.
Other differences in the response to bleomycin injury elicited by S1P1 agonist treatment preclude us from definitively attributing the increased fibrosis we observed to functional antagonism of endothelial S1P1 and increased vascular leak. Most notable of these differences were alterations in leukocyte accumulation in the airspaces, although the importance of inflammatory cells in the development of lung fibrosis after injury is a matter of controversy. According to numerous publications, the fibrotic and inflammatory responses to bleomycin appear to be independent of each other (46, 60–66). Specifically, the systemic depletion of neutrophils or a genetic deficiency in T cells does not prevent the development of fibrosis in the bleomycin model (62, 64, 65). Moreover, a genetic deletion of lysophosphatidic acid receptor-1 protects against bleomycin-induced lung fibrosis without affecting inflammatory cell accumulation in the airspaces (46), and the inhibition of transforming growth factor (TGF)-β activation prevents fibrosis while increasing inflammation after bleomycin challenge (66). Nevertheless, we cannot exclude the possibility that the increased overall inflammatory response or reduction in BAL T cells we observed with prolonged S1P1 agonist treatment may have contributed to the exaggerated fibrotic response to low-dose bleomycin. S1P can also mediate other processes that may contribute to the development of lung fibrosis, including cellular apoptosis, fibroblast chemotaxis, angiogenesis, and TGF-β signaling (7, 67–78). Therefore, further elucidation of the effects of S1P1 agonists on these biological processes will be of interest for a better understanding of how S1P–S1P1 signaling modifies the fibrotic response to injury.
The response to lung injury involves a complex series of biological processes, including epithelial cell death, inflammatory cell recruitment and activation, increased vascular permeability, activation of the coagulation cascade, deposition of extracellular matrix by fibroblasts, and subsequent re-epithelialization. If their timing, magnitude, and balance are appropriate, these responses lead to a resolution of injury and restoration of normal lung structure and function. However, if any or all of these processes are exaggerated, progressive lung dysfunction can ensue. Dysregulation of the wound-healing response to injury may also lead to progressive scarring, such as occurs in idiopathic pulmonary fibrosis and the fibroproliferative phase of ARDS (79, 80). Endogenous S1P–S1P1 signaling appears to be a critical regulatory component of the response to injury, limiting endothelial barrier disruption and vascular leak. Our data suggest that S1P–S1P1 signaling may be important for regulating not only the early exudative phase of lung injury, but also the later fibroproliferative phase. If our hypothesis that vascular leak promotes fibrosis is correct, then the development of agents to induce sustained increases in S1P–S1P1 signaling without functional antagonism of the receptor could potentially benefit patients with vascular leak syndromes, and with other diseases characterized by excessive fibrosis.
Acknowledgments
The authors thank C.P. Leary and H.S. Shin for their assistance with this work.
This work was supported by a Pulmonary Fibrosis Foundation grant, a Coalition for Pulmonary Fibrosis/American Thoracic Society grant, a Nirenberg Center for Advanced Lung Disease grant, and National Institutes of Health grant R01-HL095732 (A.M.T.), an American Thoracic Society Fellows Career Development Award and National Institutes of Health grant T32-HL007874 (B.S.S.), and National Institutes of Health grants R01-DA019674, NIH R01-NS048478, and NIH R01-DC009505 (J.C.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0345OC on January 15, 2010
Author Disclosure: J.C. serves on the advisory board for Amira Pharmaceuticals and has received $5,001–$10,000. B.S.S. received sponsored grants from the American Thoracic Society for $10,001–$50,000 and the National Institutes of Health for more than $100,001. A.M.T. serves on an advisory board for Amira Pharmaceuticals and has received $10,001–$50,000, and received lecture fees for less than $1,000. He also received expert witness fees from Kasowitz, Benson, Torres and Friedman, LLP, for $1,001–$5,000, and an industry-sponsored grant from DebioPharm, S.A., for $50,001–$100,000, and has a patent pending for “Lysophosphatidic Acid Receptor Targeting for Lung Disease.” He has received sponsored research grants from the LAM Treatment Alliance for $5,001–$10,000, the Coalition for Pulmonary Fibrosis/American Thoracic Society and the Pulmonary Fibrosis Foundation, both for $50,001–$100,000, and the National Heart, Lung and Blood Institute/National Institutes of Health and the Nirenberg Center for Advanced Lung Disease, both for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1–phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 1998;279:1552–1555. [DOI] [PubMed] [Google Scholar]
- 2.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 2004;73:321–354. [DOI] [PubMed] [Google Scholar]
- 3.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol 2008;160:25–46. [DOI] [PubMed] [Google Scholar]
- 4.Berdyshev EV, Gorshkova IA, Garcia JG, Natarajan V, Hubbard WC. Quantitative analysis of sphingoid base-1–phosphates as bisacetylated derivatives by liquid chromatography–tandem mass spectrometry. Anal Biochem 2005;339:129–136. [DOI] [PubMed] [Google Scholar]
- 5.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, et al. Extracellular export of sphingosine kinase–1a contributes to the vascular S1P gradient. Biochem J 2006;397:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1–phosphate in blood. FASEB J 2007;21:1202–1209. [DOI] [PubMed] [Google Scholar]
- 7.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1–phosphate. Cell 1999;99:301–312. [DOI] [PubMed] [Google Scholar]
- 8.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1–phosphate promotes endothelial cell barrier integrity by EDG-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM, Argraves WS. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem 2008;283:25074–25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor–induced vascular permeability. J Biol Chem 2003;278:47281–47290. [DOI] [PubMed] [Google Scholar]
- 11.Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 2007;19:1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camp SM, Bittman R, Chiang ET, Moreno-Vinasco L, Mirzapoiazova T, Sammani S, Lu X, Sun C, Harbeck M, Roe M, Natarajan V, Garcia JG, Dudek SM. Synthetic analogues of FTY720 differentially regulate pulmonary vascular permeability in vivo and in vitro. J Pharmacol Exp Ther 2009;331:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase–1 reverses the increase in lung vascular permeability through sphingosine-1–phosphate receptor signaling in endothelial cells. Circ Res 2008;103:1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadgaonkar R, Patel V, Grinkina N, Romano C, Liu J, Zhao Y, Sammani S, Garcia JG, Natarajan V. Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am J Physiol Lung Cell Mol Physiol 2009;296:L603–L613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1–phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 2009;119:1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2006;2:434–441. [DOI] [PubMed] [Google Scholar]
- 17.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1–phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004;169:1245–1251. [DOI] [PubMed] [Google Scholar]
- 18.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1–phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 2004;170:987–993. [DOI] [PubMed] [Google Scholar]
- 19.Awad AS, Ye H, Huang L, Li L, Foss FW Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1–phosphate 1 receptor activation reduces ischemia–reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 2006;290:F1516–F1524. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki M, Kreisel F, Richardson SB, Kreisel D, Krupnick AS, Patterson GA, Gelman AE. Sphingosine 1–phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am J Transplant 2007;7:751–758. [DOI] [PubMed] [Google Scholar]
- 21.Szczepaniak WS, Zhang Y, Hagerty S, Crow MT, Kesari P, Garcia JG, Choi AM, Simon BA, McVerry BJ. Sphingosine 1–phosphate rescues canine LPS-induced acute lung injury and alters systemic inflammatory cytokine production in vivo. Transl Res 2008;152:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu HB, Cui NQ, Wang Q, Li DH, Xue XP. Sphingosine-1–phosphate and its analogue FTY720 diminish acute pulmonary injury in rats with acute necrotizing pancreatitis. Pancreas 2008;36:e10–e15. [DOI] [PubMed] [Google Scholar]
- 23.Uhlig S, Gulbins E. Sphingolipids in the lungs. Am J Respir Crit Care Med 2008;178:1100–1114. [DOI] [PubMed] [Google Scholar]
- 24.Adachi K, Kohara T, Nakao N, Arita M, Chiba K, Mishina T, Sasaki S, Fujita T. Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1,3-propanediols: discovery of a novel immunosuppressant, FTY720. Bioorg Med Chem Lett 1995;5:853–856. [Google Scholar]
- 25.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats: I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol 1998;160:5037–5044. [PubMed] [Google Scholar]
- 26.Yanagawa Y, Sugahara K, Kataoka H, Kawaguchi T, Masubuchi Y, Chiba K. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats: II. FTY720 prolongs skin allograft survival by decreasing t cell infiltration into grafts but not cytokine production in vivo. J Immunol 1998;160:5493–5499. [PubMed] [Google Scholar]
- 27.Kahan BD, Karlix JL, Ferguson RM, Leichtman AB, Mulgaonkar S, Gonwa TA, Skerjanec A, Schmouder RL, Chodoff L. Pharmacodynamics, pharmacokinetics, and safety of multiple doses of FTY720 in stable renal transplant patients: a multicenter, randomized, placebo-controlled, Phase I study. Transplantation 2003;76:1079–1084. [DOI] [PubMed] [Google Scholar]
- 28.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B, Pellet P, et al. FTY720, a novel immunomodulator: efficacy and safety results from the first Phase 2A study in de novo renal transplantation. Transplantation 2005;79:1553–1560. [PubMed] [Google Scholar]
- 29.Salvadori M, Budde K, Charpentier B, Klempnauer J, Nashan B, Pallardo LM, Eris J, Schena FP, Eisenberger U, Rostaing L, et al. FTY720 versus MMF with cyclosporine in de novo renal transplantation: a 1-year, randomized controlled trial in Europe and Australasia. Am J Transplant 2006;6:2912–2921. [DOI] [PubMed] [Google Scholar]
- 30.Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006;355:1124–1140. [DOI] [PubMed] [Google Scholar]
- 31.Tedesco-Silva H, Pescovitz MD, Cibrik D, Rees MA, Mulgaonkar S, Kahan BD, Gugliuzza KK, Rajagopalan PR, Esmeraldo RM, Lord H, et al. Randomized controlled trial of FTY720 versus MMF in de novo renal transplantation. Transplantation 2006;82:1689–1697. [DOI] [PubMed] [Google Scholar]
- 32.Tedesco-Silva H, Szakaly P, Shoker A, Sommerer C, Yoshimura N, Schena FP, Cremer M, Hmissi A, Mayer H, Lang P. FTY720 versus mycophenolate mofetil in de novo renal transplantation: six-month results of a double-blind study. Transplantation 2007;84:885–892. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a Phase II extension study. Neurology 2009;72:73–79. [DOI] [PubMed] [Google Scholar]
- 34.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. Alteration of lymphocyte trafficking by sphingosine-1–phosphate receptor agonists. Science 2002;296:346–349. [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, et al. The immune modulator FTY720 targets sphingosine 1–phosphate receptors. J Biol Chem 2002;277:21453–21457. [DOI] [PubMed] [Google Scholar]
- 36.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1–phosphate G-protein–coupled receptors. FASEB J 2004;18:551–553. [DOI] [PubMed] [Google Scholar]
- 37.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo–active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol 2005;12:703–715. [DOI] [PubMed] [Google Scholar]
- 38.LaMontagne K, Littlewood-Evans A, Schnell C, O'Reilly T, Wyder L, Sanchez T, Probst B, Butler J, Wood A, Liau G, et al. Antagonism of sphingosine-1–phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res 2006;66:221–231. [DOI] [PubMed] [Google Scholar]
- 39.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1–phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem 2007;282:9082–9089. [DOI] [PubMed] [Google Scholar]
- 40.Krump-Konvalinkova V, Chwalla I, Siess W. FTY720 inhibits S1P-mediated endothelial healing: relationship to S1P1-receptor surface expression. Biochem Biophys Res Commun 2008;370:603–608. [DOI] [PubMed] [Google Scholar]
- 41.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355–360. [DOI] [PubMed] [Google Scholar]
- 42.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1–phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 2004;279:15396–15401. [DOI] [PubMed] [Google Scholar]
- 43.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, et al. Sphingosine 1–phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 2004;279:13839–13848. [DOI] [PubMed] [Google Scholar]
- 44.Pan S, Mi Y, Pally C, Beerli C, Chen A, Guerini D, Hinterding K, Nuesslein-Hildesheim B, Tuntland T, Lefebvre S, et al. A monoselective sphingosine-1–phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem Biol 2006;13:1227–1234. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, Schaeffer MT, Chapman J, Cameron M, Guerrero M, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol 2008;74:1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tager AM, Lacamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA(1) links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 2008;14:45–54. [DOI] [PubMed] [Google Scholar]
- 47.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol 2004;31:395–404. [DOI] [PubMed] [Google Scholar]
- 48.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L152–L160. [DOI] [PubMed] [Google Scholar]
- 49.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H. S1P3 receptor–induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA 2005;102:9270–9275. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1–phosphate receptor-2 (S1P2r) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 2007;27:1312–1318. [DOI] [PubMed] [Google Scholar]
- 51.Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D, Lee MJ. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol 2009;296:H33–H42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olman MA, Mackman N, Gladson CL, Moser KM, Loskutoff DJ. Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse. J Clin Invest 1995;96:1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor–1 gene. J Clin Invest 1996;97:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olman MA, Simmons WL, Pollman DJ, Loftis AY, Bini A, Miller EJ, Fuller GM, Rivera KE. Polymerization of fibrinogen in murine bleomycin-induced lung injury. Am J Physiol 1996;271:L519–L526. [DOI] [PubMed] [Google Scholar]
- 55.Imokawa S, Sato A, Hayakawa H, Kotani M, Urano T, Takada A. Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med 1997;156:631–636. [DOI] [PubMed] [Google Scholar]
- 56.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 2000;106:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gunther A, Lubke N, Ermert M, Schermuly RT, Weissmann N, Breithecker A, Markart P, Ruppert C, Quanz K, Ermert L, et al. Prevention of bleomycin-induced lung fibrosis by aerosolization of heparin or urokinase in rabbits. Am J Respir Crit Care Med 2003;168:1358–1365. [DOI] [PubMed] [Google Scholar]
- 58.Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Chambers RC. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol 2005;166:1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG,. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest 2009;119:2550–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keane MP, Belperio JA, Moore TA, Moore BB, Arenberg DA, Smith RE, Burdick MD, Kunkel SL, Strieter RM. Neutralization of the CXC chemokine, macrophage inflammatory protein–2, attenuates bleomycin-induced pulmonary fibrosis. J Immunol 1999;162:5511–5518. [PubMed] [Google Scholar]
- 61.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–646. [DOI] [PubMed] [Google Scholar]
- 62.Manoury B, Nenan S, Guenon I, Lagente V, Boichot E. Influence of early neutrophil depletion on MMPS/TIMP-1 balance in bleomycin-induced lung fibrosis. Int Immunopharmacol 2007;7:900–911. [DOI] [PubMed] [Google Scholar]
- 63.Russo RC, Guabiraba R, Garcia CC, Barcelos LS, Roffe E, Souza AL, Amaral FA, Cisalpino D, Cassali GD, Doni A, et al. Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol 2009;40:410–421. [DOI] [PubMed] [Google Scholar]
- 64.Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis 1979;120:893–899. [DOI] [PubMed] [Google Scholar]
- 65.Helene M, Lake-Bullock V, Zhu J, Hao H, Cohen DA, Kaplan AM. T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol 1999;65:187–195. [DOI] [PubMed] [Google Scholar]
- 66.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha V beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez T, Thangada S, Wu MT, Kontos CD, Wu D, Wu H, Hla T. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc Natl Acad Sci USA 2005;102:4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, et al. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol 2005;25:4237–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Long JS, Natarajan V, Tigyi G, Pyne S, Pyne NJ. The functional PDGFbeta receptor–S1P1 receptor signaling complex is involved in regulating migration of mouse embryonic fibroblasts in response to platelet derived growth factor. Prostaglandins Other Lipid Mediat 2006;80:74–80. [DOI] [PubMed] [Google Scholar]
- 70.Donati C, Cencetti F, Nincheri P, Bernacchioni C, Brunelli S, Clementi E, Cossu G, Bruni P. Sphingosine 1–phosphate mediates proliferation and survival of mesoangioblasts. Stem Cells 2007;25:1713–1719. [DOI] [PubMed] [Google Scholar]
- 71.Keller CD, Rivera Gil P, Tolle M, van der Giet M, Chun J, Radeke HH, Schafer-Korting M, Kleuser B. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and SMAD3 signaling. Am J Pathol 2007;170:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, Yokoyama M. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol 2007;37:395–404. [DOI] [PubMed] [Google Scholar]
- 73.Swaney JS, Moreno KM, Gentile AM, Sabbadini RA, Stoller GL. Sphingosine-1–phosphate (S1P) is a novel fibrotic mediator in the eye. Exp Eye Res 2008;87:367–375. [DOI] [PubMed] [Google Scholar]
- 74.Hashimoto M, Wang X, Mao L, Kobayashi T, Kawasaki S, Mori N, Toews ML, Kim HJ, Cerutis DR, Liu X, et al. Sphingosine 1–phosphate potentiates human lung fibroblast chemotaxis through the S1P2 receptor. Am J Respir Cell Mol Biol 2008;39:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hagen N, Van Veldhoven PP, Proia RL, Park H, Merrill AH Jr, van Echten-Deckert G. Subcellular origin of sphingosine 1–phosphate is essential for its toxic effect in lyase-deficient neurons. J Biol Chem 2009;284:11346–11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caballero S, Swaney J, Moreno K, Afzal A, Kielczewski J, Stoller G, Cavalli A, Garland W, Hansen G, Sabbadini R, et al. Anti–sphingosine-1–phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Exp Eye Res 2009;88:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schnitzer SE, Weigert A, Zhou J, Brune B. Hypoxia enhances sphingosine kinase 2 activity and provokes sphingosine-1–phosphate–mediated chemoresistance in A549 lung cancer cells. Mol Cancer Res 2009;7:393–401. [DOI] [PubMed] [Google Scholar]
- 78.Gellings Lowe N, Swaney JS, Moreno KM, Sabbadini RA. Sphingosine-1–phosphate and sphingosine kinase are critical for transforming growth factor–beta–stimulated collagen production by cardiac fibroblasts. Cardiovasc Res 2009;82:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 80.Meduri GU, Belenchia JM, Estes RJ, Wunderink RG, el Torky M, Leeper KV Jr. Fibroproliferative phase of ARDS: clinical findings and effects of corticosteroids. Chest 1991;100:943–952. [DOI] [PubMed] [Google Scholar]