Abstract
Reactive airway disease is mediated by smooth muscle contraction initiated through several agonist-dependent pathways. Activation of type 1 N-methyl-D-aspartate receptors (NMDA-R1s) by plasminogen activators (PAs) has been linked to control of vascular tone, but their effect on airway smooth muscle contractility has not previously been studied to our knowledge. We observed that NMDA-R1s are expressed by human airway smooth muscle cells and constitutively inhibit the contraction of isolated rat tracheal rings in response to acetylcholine (Ach). Both tissue-type PA (tPA) and urokinase-type PA (uPA) bind to NMDA-R1 and reverse this effect, thereby enhancing Ach-induced tracheal contractility. Tracheal contractility initiated by Ach is reduced in rings isolated from tPA−/− and uPA−/− mice compared with their wild-type counterparts. The procontractile effect of uPA or tPA was mimicked and augmented by the nitric oxide synthase inhibitor, l-NAME. uPA and tPA further enhanced the contractility of rings denuded of epithelium, an effect that was inhibited by the NMDA-R antagonist, MK-801. Binding of PAs to NMDA-R1 and the subsequent activation of the receptor were inhibited by PA inhibitor type 1, by a PA inhibitor type 1–derived hexapeptide that recognizes the tPA and uPA docking domains, as well as by specific mutations within the docking site of tPA. These studies identify involvement of PAs and NMDA-R1 in airway contractility, and define new loci that could lead to the development of novel interventions for reactive airway disease.
Keywords: tissue plasminogen activator, urokinase NMDA receptor, lungs
In the second half of the 20th century, asthma became a worldwide health concern, and its prevalence continues to rise (1). The pathogenesis of asthma is complex and includes airway smooth muscle cell (SMC) contraction, inflammation, edema, altered mucus production, and airway remodeling, among other processes. Contractility of airway SMC is central to the pathogenesis of asthma (2, 3) and its treatment (4). Although the cellular mechanisms that lead to excessive SMC contraction are of importance, these are only partially understood at the present time (3).
N-methyl-D-aspartate receptors (NMDA-Rs) have been identified in the large airways (5–7), where they have been implicated in the regulation of vascular tone and airway contractility in experimental systems (5, 8). However, the involvement of NMDA-Rs in the physiological regulation of airway contractility and the endogenous mediators that might engage these receptors in patients with asthma have not been fully elucidated. Clues to the function of NMDA-Rs within the lung may come from their activity in other tissues. For example, NMDA-Rs play important physiological as well as pathological roles within the central nervous system (CNS). Several groups, including our own (9, 10), have shown that plasminogen activators (PAs) initiate signal transduction through NMDA-Rs. Overstimulation of NMDA-Rs by glutamate released as a consequence of head trauma or stroke contributes to its deleterious effect on outcome by causing neuronal apoptosis and cerebral vasodilation (10, 11). Moreover, tissue-type PAs (tPAs) and urokinase-type PAs (uPA) act through NMDA-Rs to impair cerebral vasodilation in response to hypercapnea in the setting of hypoxia–ischemia (12).
The molecular basis of the interaction between PAs and NMDA-Rs is incompletely understood. tPA forms a complex with the NMDA-R1 expressed by cortical neurons in culture (10, 13). This precedes the cleavage of its NR1 subunit, which is followed by enhancement of intracellular Ca++ concentrations and cell death (10, 13). It is uncertain whether cell death results from occupancy or cleavage of the NR1 subunit or events downstream of receptor binding (10). It has recently been shown that tPA also causes vasodilatation in the mouse whisker barrel cortex due to phosphorylation of neuronal nitric oxide synthase (NOS), leading to release of NO (14). Even less is known about the interaction between uPA and NMDA-R1.
PAs have been implicated in the development of asthma. The levels of uPA and PA inhibitor (PAI)–1 are elevated in the sputum of patients with asthma (15–18), and associations between asthma and polymorphisms in the uPA and PAI-1 genes have been identified (18, 19). uPA is induced in human bronchial epithelial cells by mechanical activation in vitro and in vivo (18). uPA is activated by mast cell tryptase (20), promotes the adhesion of airway eosinophils to intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 after allergen challenge (21), and potentiates platelet-derived growth factor (PDGF)-induced chemotaxis of human airway SMCs (22). uPA and PAI-1 have also been implicated in the deposition of extracellular matrix and in other aspects of airway remodeling (18, 23). Each of these outcomes suggest that chronic activation of the PA system may contribute to the development of asthma. On the other hand, subepithelial fibrin, which is characteristic of chronic asthma, enhances airway hyperresponsiveness, and aerosolized tPA and uPA have been reported to remediate bronchial reactivity and reduce airway remodeling through their fibrinolytic activity (24, 25).
The explanation for this dichotomy might lie in the fact that PAs are multifunctional proteins with beneficial and deleterious effects, again perhaps most clearly exemplified by their activities in the CNS. For example, in the case of stroke, the beneficial effects of fibrinolysis may be offset by neuronal apoptosis and vasodilation. We have shown that the beneficial and deleterious effects of PAs in the CNS are mediated through different pathways, and involve different domains within the molecules (26, 27). Furthermore, we have shown that it is possible to dissociate the salutary and deleterious effects, and to use these differences to improve outcome (26).
The expression of NMDA-Rs in airways (6) suggests a mechanism by which airway contractility may be regulated physiologically, or may be disordered in reactive airway disease in response to endogenous PAs. However, a direct role for either uPA or tPA in regulating bronchial contractility and their role, if any, in the activation of NMDA-Rs in this process have not been demonstrated to our knowledge. In this article, we examined the effect of PAs on airway SMCs by measuring contractility of tracheal rings from rodents. Our findings indicate that both tPA and uPA promote the contractility of the isolated tracheal rings through an interaction with NMDA-R1. We also report that PAs inhibit NMDA-R1 activity through a two-step process that involves docking accompanied by proteolysis of the receptor. Enhancement of tracheal contractility by PAs can be inhibited either by targeting their docking sites or by neutralizing their catalytic activity, thereby identifying potential means to selectively enhance the salutary effects of PAs in the treatment of asthma.
MATERIALS AND METHODS
Materials
The PAI-1–derived peptide, glutamic acid-blutamic acid–isoleucine-isoleucine–methionine-aspartic acid (EEIIMD), was synthesized by Peptisyntha (Brussels, Belgium and San Diego, CA), as described previously (28). MK-801, acetylcholine (Ach), and glutamate were purchased from Sigma (Rehovot, Israel). Anti–NMDA-R1 antibodies were purchased from Biotest (Tel-Aviv, Israel), monoclonal anti-tPA antibody was the kind gift of American Diagnostica Inc. (Greenwich, CT), and polyclonal anti-uPA was provided by UMTEK (Moscow, Russia). Recombinant tPAs and uPAs were synthesized in our laboratory as described subsequently here. Wild-type (WT) uPA was also provided by American Diagnostica Inc. WT tPA was also provided by American Diagnostica Inc., with results confirmed with tPA purchased from Genentech (South San Francisco, CA) and activase from Boehringer (Ingelheim, Germany).
PAs
cDNAs encoding mature human tPA or single-chain uPA were cloned into the pMT/BiP/V5-HisA plasmid (Invitrogen Corp., Carlsbad, CA). To generate catalytically inactive variants, the S481A mutation in tPA and the homologous S356A mutation in scuPA were generated by PCR with the QuickChange Mutagenesis kit (Stratagene, La Jolla, CA), and the complete sequences were verified. Each protein contains two extra amino acids, arginine and serine (RS-), at the N terminus, resulting from introduction of the Bgl II cloning site. Proteins were expressed in S2 Drosophila Expression System (Invitrogen) according to the manufacturer's protocol, and were purified by antibody affinity chromatography with anti-tPA or anti-uPA coupled to cyanogen-Br–activated sepharose. The final products migrated as single bands on SDS-PAGE at the expected sizes, and their PA activity was confirmed with the plasmin chromogenic substrate, Spectrazyme PL (gift of American Diagnostica), as previously described (29). Proteins were stored at −70°C or lyophilized before use.
PA Activity
PA activity was determined as previously reported by us (30) by adding WT tPA, WT uPA, or PA variants (20 nM) to a reaction mixture containing glutamate–plasminogen (100 nM) and the plasmin chromogenic substrate, Spectrazyme PL (500 μM) in phosphate-buffered saline (PBS) at 37°C. The light absorbance at 405 nm was measured continuously over time. The initial velocity of plasmin generation was optical density per minute.
Anesthesia
All experimental protocols involving the use of vertebrate animals were approved by the Israeli Board for Animal Experiments. Sprague-Dawley rats (Harlan Laboratories, Jerusalem, Israel) were anesthetized with an intraperitoneal injection of ketamine (75 mg/ml) and xylazine (5 mg/ml; Kepro Holland, Deventer, The Netherlands) before experimentation. tPA−/−, uPA−/−, and uropinase plasminogen activator receptor (uPAR)−/− mice on a C57/black 6 background (C57Bl6) and WT C57Bl6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Male mice (4-mo old) were used in all studies.
Isolation of Tracheal and Aortic Rings
Rats or, where indicated, WT C57Bl6 mice, or tPA−/− or uPA−/− mice on the same background, were killed by exsanguination. The tracheas were removed with care to avoid damage to the epithelium, dissected free of fat and connective tissue, and cut into transverse rings 5 mm in length (31). The tissues were kept in an oxygenated (95% O2, 5% CO2) solution of Krebs-Henseliet (KH) buffer (144 mM NaCl, 5.9 mM KCl, 1.6 mM CaCl2, 1.2 mM Mg2SO4, 1.2 mM KH2PO4, 25 mM NaHCO3, and 11.1 mM D-glucose). To study the role of epithelial cells, a stainless steel rod was gently rotated along the surface of the tracheal rings to remove the epithelium (denuded rings) as described elsewhere (27). In other experiments, the contractile response of isolated aortic rings to phenylephrine in the presence or absence of 20 nM tPA or 20 nM uPA was measured (27, 32, 33).
Contractile Response of Isolated Tracheal Rings
To record isometric tension, tracheal rings were mounted in a 10-ml bath containing an oxygenated (95% O2/5% CO2) solution of KH buffer. The rings were equilibrated for 1.5 hours at 37°C and maintained under a resting tension of 2 g throughout the experiment. Each ring was then contracted by adding Ach in stepwise increments from 10−10 to 10−5 M. The absolute maximal contraction was determined by adding 30 mM KCl; the term 100% contraction used in the graphs represents the maximal tension induced by Ach, defined by the concentration at which tension reached a plateau. Where indicated, tPA or uPA was added 10 minutes before adding Ach. In other experiments, the NMDA-R agonist, glutamate (150 μM), receptor antagonists (100 nM), the PAI-1–derived peptide (1 μM), or the NOS inhibitor, l-NAME (50 μM), was added alone or 5 minutes before addition of PAs. In each experiment, rings exposed to KH buffer alone were analyzed in parallel. Isometric tension was measured with a force displacement transducer and recorded online with a computerized system (ExperimentiaÆ, Budapest, Hungary). The effective concentration that results in 50% inhibition of maximal contraction (EC50) was calculated by measuring the response of the rings to increasing concentration of Ach, as previously described (33).
Coimmunoprecipitation
Tracheal rings isolated from tPA−/− mice were incubated in an oxygenated (95% O2/5% CO2) Krebs-Ringer bicarbonate solution (118 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 11 mM glucose, 0.05 mM Na2-EDTA). Where indicated, tPA or uPA was added alone or in the presence of EEIIMD or NMDA-R agonist/antagonist for the indicated times. The rings were homogenized in 5 vol cold RIPA buffer (1% NP40, 0.5% sodium deoxycholate, 10% sodium lauryl sulfate, and 0.5 mM PMSF) in PBS in the absence of protease inhibitors at 4°C for 30 minutes to prevent possible interference by endogenous tPA. The lysates were cleared by centrifugation; the supernatant fractions were then precleared with protein A–agarose beads that had been preblocked with 1% BSA. The supernatants were then incubated for 2 hours with beads containing anti–NMDA-R1 IgG or irrelevant IgG. The beads were washed five times with PBS, the proteins were eluted by three additions of 0.1 glycine buffer for 5 minutes each and centrifuged, and the supernatant was analyzed by Western blotting. Samples were applied to nitrocellulose membranes. The membranes were blocked with horse serum, incubated initially with anti-tPA or -uPA antibodies, and then with a species-specific secondary antibody conjugated to horseradish peroxidase. All experiments were performed in triplicate, and were repeated a minimum of three times. Irrelevant IgG was added in lieu of specific primary antibodies as a control. Western blotting to identify the NR1 subunit of the NMDA-R1 was performed as previously described (10). Briefly, immunoprecipitates were electrophoresed with an 8–10% SDS glycine polyacrylamide gel. Separated proteins were electroblotted onto nitrocellulose membranes. The membranes were blocked with low-fat dry milk in 10 mM Tris·HCl (pH 7.4), 150 mM NaCl, and 0.05% Tween 20, and the NMDA-R1 subunit was detected with the same antibodies. The membranes were incubated with secondary antibodies conjugated with peroxidase and developed with the appropriate colorometric substrates.
Immunohistochemistry
Three unstained recuts were obtained from formalin-fixed, paraffin-embedded tissue blocks of normal-appearing human brain and normal-appearing human lung. Immunostains were performed by an avidin–biotin–peroxidase complex method. Sections from the paraffin blocks were cut at 4-μm intervals, placed on positively charged slides, and deparaffinized with Clear-Rite 3 (Richard-Allan Scientific, Kalamazoo, MI), followed by a series of graded alcohol incubations. The slides were then treated with methanolic H2O2 to quench endogenous peroxidase activity, and rehydrated. No antigen retrieval steps were performed. Sections were incubated with a rabbit polyclonal antibody against NMDA-R1 (1 μg/ml; Abcam, Cambridge, MA) or normal rabbit IgG overnight at 4°C in a humidity chamber. The slides were washed in PBS, incubated with Streptavidin-labeled polyclonal anti-rabbit IgG (Equitech-Bio, Kerrville, TX), and the slides were washed free of unbound antibody in PBS. Biotin streptavidin detection was performed with the Super Sensitive Immunodetection System (BioGenex, San Ramon, CA). The sections were rinsed in PBS rinse (pH 7.4), incubated with chromogenic substrate (Fast Red Chromogen) for 2 minutes, rinsed again in PBS, and incubated with Mayers hematoxylin with a Tissue-Tek slide staining set. The slides were rinsed once more with running water, and incubated with Scott's Bluing Reagent for 10 seconds and dried in a fume hood. After one dip in xylene, coverslips were added using Permount solution. Immunopositivity was scored as mild (weak staining), moderate (moderately intense staining), or strong (markedly intense staining).
Statistical Analysis
In vitro and ex vivo experiments were performed in triplicate, and were repeated a minimum of three times on different tissues. All data are presented as means (±SD) of the three experiments.
RESULTS
We have previously reported that uPA and tPA regulate the contractility of systemic and cerebrovascular arterial tone ex vivo and in vivo (27, 33). Furthermore, we have found that tPA (34) and uPA (32) increase mobilization of Ca++ into the cytoplasm of SMCs. SMCs are found in the proximal components of the respiratory tract, where their contraction contributes to the development and morbidity of reactive airway disorders, such as asthma. However, a direct role of tPA or uPA in bronchial contractility has not been demonstrated to our knowledge. Therefore, we examined the effect of PAs on airway SMCs by first measuring their effect on the contractility of tracheal rings isolated from rats.
Effect of PAs on Tracheal Contractility
The addition of uPA to isolated rat tracheal rings increased contractility induced by Ach (Figure 1A). Addition of 10 nM uPA decreased the EC50 of Ach fivefold, from 40 to 8 nM. The effect of uPA was dose dependent and saturable, with an EC50 of approximately 5 nM (Figure 1B). Addition of uPA alone had no effect on the tracheal contractility (data not shown).
Figure 1.
Effect of urokinase-type plasminogen activator (uPA) on the contraction of tracheal rings. (A) Effect of increasing concentrations of acetylcholine (Ach). Contraction of tracheal rings was induced by Ach at the indicted concentrations in the absence (open squares) or presence of 20 nM uPA (closed squares). In this and in each figure, the molar concentration of Ach is displayed in logarithmic units, and the response expressed as a percent of the maximum contraction observed with KCl. (B) Effect of increasing concentrations of uPA. The mean (±SD) of six experiments is shown.
We have also reported that tPA also modulates vascular contractility in vivo and ex vivo (27). Therefore, we next examined the effect of tPA on tracheal contractility. tPA increased the contractility of isolated tracheal rings from rats induced by Ach even when added at physiological concentrations (Figure 2). Addition of tPA alone had no effect on the tracheal contractility (data not shown). We had previously found that tPA exerted opposing effects on the contraction of isolated aortic rings depending on the exogenous concentration of ligand (i.e., low concentrations [1 nM] inhibited, whereas higher concentrations [20 nM] stimulated, vascular tone). In contrast to its effect on aortic tissue, tPA enhanced tracheal contractility at both concentrations tested (Figure 2).
Figure 2.
Effect of tissue-type PA (tPA) on the contraction of rat tracheal rings. Contraction of tracheal rings was induced by Ach at the indicated concentrations in the absence (closed squares) or presence of 1 nM (open squares) or (open triangles) 20 nM tPA. The mean (±SD) of six experiments is shown.
In previous studies, we observed that the effect of tPA and uPA on the contractility of systemic blood vessels was independent of its catalytic activity (27, 33). To examine the requirement for catalytic activity on tracheal contraction induced by Ach, we measured the effect of catalytically inactive variants, tPA-Ser481Ala and uPA-Ser356Ala. Neither catalytically inactive PA variant (designated uPA and tPA) expressed procontractile activity on tracheal rings (Figure 3A). In contrast, both PA variants enhanced the contractility of isolated aortic rings (Figure 3B), as previously reported (27, 32, 33).
Figure 3.
Involvement of PA catalytic activity in the contraction of tracheal and aortic rings. (A) Catalytically inactive PA variants fail to stimulate contractility of tracheal rings. Contraction of tracheal rings was induced by Ach in the absence (Cont) or presence of 20 nM catalytically inactive tPA-ser481ala (mut tPA) or uPA-ser356ala (mut tPA). The mean (±SD) of three experiments is shown. (B) Catalytically inactive PA variants stimulate contractility of aortic rings. Contraction of aortic rings was induced by phenylepherine, as previously reported (28, 33, 34), in the absence (Cont) or presence of 20 nM catalytically inactive mut tPA or mut uPA. The mean (±SD) of three experiments is shown. PE, phenylephrine; WT, wild-type.
Role of NMDA-Rs in Tracheal Contractility
The most extensively studied signal-transduction activity of tPA that is dependent on its catalytic activity involves cleavage of NMDA-R1 (10, 13). NMDA-Rs, including NMDA-R1, have been identified in the respiratory system (6, 7), but their function and agonists have not been fully characterized. Therefore, we next asked whether the procontractile activities of the PAs are mediated through activation of NMDA-Rs. To do so, we first examined the effect of the NMDA-R antagonist, MK-801, on tracheal contraction. The NMDA-R antagonist, MK-801, stimulated the contraction of tracheal rings induced by Ach (Figures 4A and 5A), which suggests that NMDA-Rs contribute to the constitutive relaxation of tracheal SMCs. To examine the role of NMDA-Rs in the contraction of tracheal rings in greater detail, we next examined the effect of the NMDA-R agonist, glutamate. Glutamate inhibited tracheal contraction induced by Ach (Figures 4A and 5A), supporting the hypothesis that activation of NMDA-Rs inhibits contractile activity or induces bronchodilation directly.
Figure 4.
Involvement of N-methyl-D-aspartate receptors (NMDA-Rs) in the contraction of tracheal rings. (A) NMDA-Rs mediate the relaxation of tracheal smooth muscle cells (SMCs) induced by glutamate. Contraction of tracheal rings was induced by Ach in the absence (Cont.) or presence of 20 nM tPA, 20 nM uPA, the NMDA-R antagonist, MK801 (100 nm), or the NMDA-R agonist, glutamate (Glut; 150 μM). The mean (±SD) of three experiments is shown. (B) Involvement of nitric oxide synthase (NOS) in NMDA-R–mediated relaxation of tracheal SMC induced by glutamate. Contraction of tracheal rings was induced by Ach in the absence (Cont.) or presence of l-NAME (50 μM) alone or together with 20 nM tPA, 20 nM uPA, MK801 (100 nm), or glutamate (150 μM). The mean (±SD) of three experiments is shown. (C) Involvement of the epithelium in NMDA-R–mediated relaxation of tracheal SMC induced by glutamate. Contraction of denuded tracheal rings (Den) was induced by Ach in the absence (Cont.) or presence of 20 nM tPA, 20 nM uPA, MK801 (100 nM), or glutamate (150 nM). The mean (±SD) of three experiments is shown.
Figure 5.
Model of regulation of tracheal ring contractility by NMDA-R and l-NAME. (A) Intact tracheal rings. Contraction of isolated tracheal rings induced by Ach is inhibited (−) by glutamate (Glut, gray) and stimulated (+) by tPA and uPA (PA) and by MK-801 (black); EpC, epithelial cells. (B) NOS mediates contraction of intact tracheal rings: (1) the NOS inhibitor, l-NAME, inhibits (−) the prorelaxation effect of glutamate (gray) and the procontractile (−) activity of MK-801 (gray); (2) in the presence of l-NAME, the procontractile effect of tPA and uPA (PA) (black) is thereby enhanced (+), and glutamate acquires procontractile activity (black +). (C) Role of epithelial cells in tracheal contractility. In tracheal rings denuded of epithelium (dotted lower rectangle with “X” indicating deletion of EpC), loss of SMC relaxation (X, top rectangle): augments stimulation (black +) of contractility by PA and inhibition contractility by MK-801 (gray −); and glutamate stimulates Ach-induced contractility.
Role of Epithelial Cells in Tracheal Contractility
Glutamate-induced activation of NMDA-Rs in lungs, as well as in blood vessels, induces pulmonary edema and vasodilation through an NOS-dependent mechanism (5, 14). Therefore, tPA and uPA may stimulate contractility by: (1) inhibiting NMDA-R–mediated induction of NOS activity, lowering the threshold for contraction; or (2) acting directly on NMDA-Rs to initiate NOS-independent contractile mechanisms, as described in other organs (35). To distinguish between these possibilities, we first examined the effect of the NOS inhibitor, l-NAME. l-NAME by itself stimulated the contraction of isolated tracheal rings induced by Ach (Figures 4B and 5B), as was seen after the addition of the NMDA-R antagonist, MK-801 (Figure 4A). Second, MK-801 lost its procontractile effect (and indeed, somewhat inhibited Ach-induced contractility) in the presence of l-NAME (Figures 4B and 5B). Third, in the presence of l-NAME, glutamate stimulated the contractile effects of Ach (Figure 4B and 5B). Fourth, l-NAME enhanced the procontractile effect of tPA and uPA, reducing the EC50 from 3.4 to 2.2 nM and 5.8 to 2.5 nM, respectively (Figures 4B and 5B). Taken together, these data strongly suggest that the anticontractile effect of NMDA-R activation is mediated through NOS, similar to the role of endothelial NOS (36) in NMDA-R–mediated vasorelaxation (14, 37). These results also suggest that the contractile activity exerted by tPA and uPA is partially counteracted by NOS activity.
NOS is present in the airway epithelial cells (37, 38). Therefore, to examine the role of NOS in NMDA-R–mediated bronchial contractility in situ, we next studied tracheal rings denuded of epithelium. In contrast to the procontractile effect of l-NAME on intact rings, the NOS inhibitor had no effect on the contractility of denuded tracheal rings induced by Ach (Figures 4C and 5C). Moreover, the procontractile activity of uPA, tPA, and glutamate on denuded tracheal rings was inhibited by MK-801 (Figures 4C and 5C).
Proteolysis of Airway NMDA-R1 by PAs
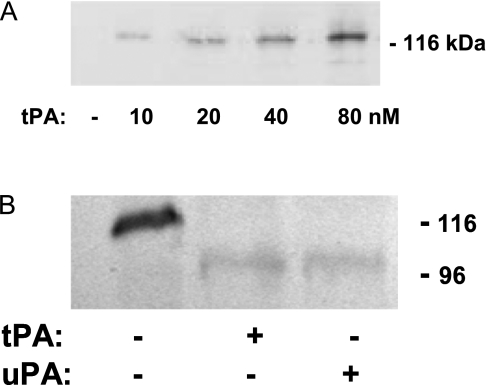
Binding of tPA to NMDA-R1 in cortical cells has been demonstrated by coimmunoprecipitation (10). Therefore, we examined the possibility that tPA and uPA interact with this receptor in the trachea by a similar approach. Homogenates of tracheas isolated from tPA−/− mice were preincubated at 4°C with increasing concentrations of tPA (0–80 nM) and precipitated with an antibody against tPA, followed by immunoblotting with an antibody against NR1. A single band, relative molecular mass of approximately 120 kD, corresponding to the estimated mass of the NR1 subtype, was seen (Figure 6A). This indicates that tPA forms a complex with NMDA-R1 in tracheal tissue. Similar results were obtained with uPA (data not shown).
Figure 6.
Two-step interaction between NMDA-R1 and tPA or uPA in the trachea. (A) tPA and uPA form a complex with NMDA-R1 from tracheal tissue. Homogenates of tracheal rings isolated from tPA−/− mice were preincubated with the indicated concentrations of tPA at 4°C and precipitated with an antibody against tPA, followed by immunoblotting with an antibody against the NR1 subunit of NMDA-R1. The results of an experiment representative of three are shown. (B) tPA and uPA cleave the NR1 subunit of NMDA-R1. Tracheal rings from tPA−/− mice were incubated in buffer alone or containing 20 nM tPA for 120 minutes with or without the PA inhibitor (PAI)–1–derived peptide, EEIIMD (1μM) at 37°C. A tissue homogenate was analyzed by SDS-PAGE and Western blotting using antibody against the NR1 subunit of NMDA-R1. The results of an experiment representative of three are shown.
We then asked whether tPA and/or uPA cleaves the NR1 subtype of the NMDA receptor, which, in the CNS, may increase responsiveness to its agonist, glutamate (10). To address this question, we examined the effect of exogenous tPA at a concentration that stimulates tracheal contraction (Figure 1) on the cleavage of the NR1 subunit. Tracheal rings from tPA−/− mice were incubated with 20 nM tPA or 20 nM uPA for 60 minutes at 37°C, and a tissue homogenate was analyzed by SDS-PAGE and Western blotting. Addition of tPA or uPA to tracheal homogenates shifted the molecular mass of NR1 from approximately 120 to approximately 100 kD, consistent with cleavage and release of the NR1 subunit (10) (Figure 6B).
Mechanism of Interaction of PAs with NMDA-R1: Role of the Docking Site
We previously observed that the effect of tPA and uPA on vascular contractility is inhibited by a PAI-1–derived peptide, EEIIMD, that binds to the docking site of the PAs, but does not affect PA enzymatic activity (27, 33). Therefore, we next examined the effect of this peptide on PA-induced tracheal contractility. EEIIMD inhibited the procontractile activity of tPA and uPA (Figure 7A). In view of our previous finding that EEIIMD inhibits tPA- and uPA-mediated docking and signal transduction without inhibiting PA activity (27, 33), this observation raised the question of how the peptide inhibits contractility, which is accompanied by proteolytic cleavage of NMDA-R1. To address this question, we examined the effect of the peptide on the binding of tPA and uPA to the NMDA-R1, as described in Figure 6A. We found that EEIIMD inhibited the binding of WT tPA to NMDA-R1 (Figure 7B). Similar results were obtained with WT uPA (data not shown).
Figure 7.
The PAI-1–derived peptide inhibits the effect of PAs on tracheal contractility. (A) The PAI-1–derived peptide inhibits the procontractile activity of tPA and uPA. Contraction of tracheal rings was induced by Ach in the absence or presence of 20 nM tPA or 20 nM uPA with or without the PAI-1–derived peptide, EEIIMD (1 μM). The mean (±SD) of three experiments is shown. (B) The PAI-1–derived peptide inhibits the binding of tPA to the NMDA-R. Homogenates of tracheal rings isolated from tPA−/− mice were preincubated with 20 nM tPA with or without 1 μM EEIIMD, and precipitated with an antibody against NR1 subunit of the NMDA-R, followed by immunoprecipitation and immunoblotting with an antibody against tPA, as in Figure 6. The results of an experiment representative of three are shown.
EEIIMD is derived from the docking site of PAI-1, and binds to amino acid residues 296–299 in tPA and 179–184 in uPA (33, 39–41). Therefore, we next examined the role of the PA docking site on the interaction of tPA with NMDA-R1 and its subsequent effect on tracheal contractility in greater detail. To do so, we developed a tPA variant in which each of the amino acid residues (296–299) that comprises the docking site were mutated to alanine (K296A/H297A/R298A/R299A) (mut D tPA). This docking site tPA variant failed to enhance the contractility of the tracheal rings in response to Ach (Figure 8A), although it maintained PA activity (Figure 8B). To elucidate the reason behind the failure of the tPA docking site mutant to affect the contractility of the tracheal rings, we examined its capacity to interact with the NMDA-R1. The mutant docking site tPA variant failed to bind to NMDA-R1 (Figure 8C), mimicking the effect of the PAI-1–derived peptide, EEIIMD. These results indicate that binding of PAs to their NMDA-R1 through their respective docking sites is required for subsequent proteolytic cleavage, and is responsible for its effect on tracheal contractility.
Figure 8.
The docking sites in tPA mediate their interactions with NMDA-R1. (A) Mutation of the docking site inhibits tPA-induced tracheal contractility. Contraction of tracheal rings was induced by Ach in the absence or presence of 20 nM WT tPA or 20 nM of the tPA variant in which each of the amino acid residues (296–299) that comprise the docking site was mutated to alanine (K296A/H297A/R298A/R299A) (mut d tPA). The mean (±SD) of three experiments is shown. (B) Mutation of the docking site does not affect tPA catalytic activity. WT tPA (20 nM) or the tPA variant (mut D tPA) with mutations of the docking site as in A was incubated with glu-plasminogen (100 nM) and the plasmin chromogenic substrate (500 μM), and the optical density was measured continuously over the next 20 minutes. Control A (Cont A) was performed in the absence of plasminogen; control B (Cont B) was performed in the absence of tPA. The mean (±SD) of three experiments is shown. (C) Mutation of the docking site impedes the binding tPA to the NMDA-R. Homogenates of tracheal rings isolated from tPA−/− mice were preincubated with 20 nM WT tPA or tPA variant with mutations of the docking (tPA m) and immunoprecipitated with an antibody against the NR1 subunit. The results of an experiment representative of three are shown. OD, optical density.
Effect of Endogenous PAs on Tracheal Reactivity
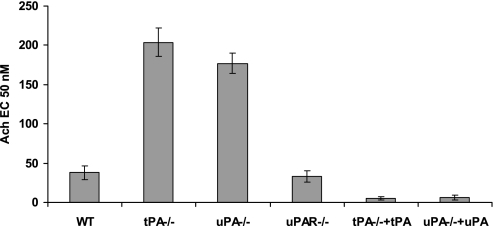
The data presented to this point demonstrate that the contractile activity of the trachea is regulated in vitro by exogenous tPA and uPA, which is accompanied by cleavage of NMDA-R1. The fact that tPA and uPA exert similar effects suggests that the signal transduction events leading to tracheal contraction do not require uPAR. Therefore, we next asked whether tPA and uPA operate similarly in vivo. To begin to address this question, we compared the contractility of tracheal rings isolated from WT, tPA−/−, uPA−/−, and uPAR−/− mice. Contractility of tracheal rings from both PA knockout mice was reduced relative to WT animals (Figure 9), whereas the contractility of rings from uPAR−/− mice was intact. These findings support the involvement of endogenous PAs in regulating airway contractility in situ, and indicate that their effect is independent of uPAR. Contractility was fully restored by the addition of 20 nM tPA or 20 nM uPA, respectively, excluding an off-target effect of gene deletion (Figure 9).
Figure 9.
Effect of endogenous tPA and uPA on tracheal reactivity. Contraction of tracheal rings isolated from tPA−/−, uPA−/−, or uPAR−/− mice was induced by Ach, as described in Figure 1, in the absence or presence of 20 nM tPA or 20 nM uPA. The mean (±SD) of three experiments is shown.
Expression of NMDA-R1 in Human Trachea
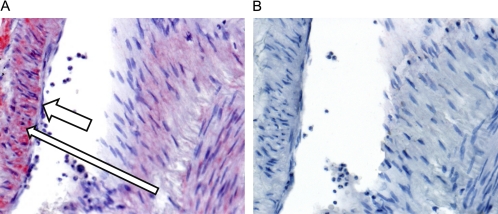
Expression of NMDA-R1 has been reported in rodent airways (6, 7). To help assess the pathophysiological relevance of our findings in humans, we examined the expression of the NMDA-R1 in tracheal tissue from human lungs; staining of sections from human brain was used as a positive control. Immunostaining to detect NMDA-R1 showed moderate staining of perivascular smooth muscle in the brain and normal lung, and mild staining of peribronchial/peribronchiolar smooth muscle in the normal lung sections (Figure 10). No immunopositivity was seen in any tissues with normal IgG as a control. These data show that NMDA-R1 is expressed in human perivascular and airway SMCs (Figure 10).
Figure 10.
Expression of NMDA-R1 in human airways. (A) High-power image of an airway in a normal lung showing NMDA-R1 immunopositivity in bronchial SMCs (long thin arrow) and immunonegativity in overlying epithelial cells (short thick arrow). (400×) (B) High-power image of the negative control of the airway showing immunonegativity in both SMCs and overlying bronchial mucosal cells (40×). Results are representative of sections taken from three separate sources of lung tissue.
DISCUSSION
The results in this article provide the first data, to our knowledge, that implicate tPA and uPA in airway contractility. Our findings are in line with data from previous studies showing that both PAs are involved in diverse lung functions. For example, uPA−/− and tPA−/− mice show increased mortality when challenged with pneumonia (42, 43) or agents that cause pulmonary fibrosis (44, 45). We now extend our understanding of PA function in the lung to the regulation of airway contractility. Both PAs augment Ach-stimulated contraction of isolated tracheal rings in a dose-dependent manner, and rings from both uPA−/− and tPA−/− mice exhibit decreased contraction in response to Ach compared with rings from WT mice, suggesting a role for the PA system in the endogenous regulation of airway contractility.
These results extend studies by others, as well as our own, showing that tPA mediates signal transduction in vascular SMCs to those lining airways, and they provide insight into the cellular and molecular basis of the interaction with NMDA-R1. Our data indicate that the NMDA-R1 is expressed by SMCs in human airways, extending observations in rats (6, 7). We also found that NMDA-R1 plays a constitutive role in mediating relaxation of tracheal SMCs. This conclusion is supported by the findings that the NMDA-R agonist, glutamate, inhibits airway contraction, whereas its antagonist, MK-801, stimulates contractility in response to Ach. NMDA-R1–related anticontractile activity is blocked by the NOS inhibitor, l-NAME, as well as by denuding the epithelial layer from the tracheal rings. Together, these findings suggest that inhibition of airway contractility is mediated by NOS derived from the airway epithelial cells (Figure 11). This interpretation is analogous to the role of endothelial NOS in NMDA-R–mediated vasorelaxation (14, 36), and is supported by the identification of NOS in airway epithelial cells (37, 38).
Figure 11.
Model of tracheal contractility regulation by NMDA-R1. Pathway in red: binding of glutamate (Glut) to NMDA-R1 expressed by SMCs increases intracellular calcium and induces the synthesis of NO by the overlying epithelium (EpC). NO promotes SM relaxation. Pathway in blue: binding of tPA or uPA (PA) to the NMDA-R1 increases intracellular calcium and overrides the inhibitory effect of epithelial NOS and stimulates contractility.
The finding that the procontractile activities of uPA and tPA, like glutamate, are inhibited by MK-801 in denuded tracheal rings strongly suggests stimulation of NMDA-R activity. We did not identify NMDA-Rs in airway epithelium in contrast to clear staining of the underlying SMC. This suggests that production of NOS by the epithelium might be initiated by activation of NMDA-Rs in the underlying SMC layer (Figure 11), similar to what has been described for production of endothelial NOS in the vasculature (36). The pathways underlying this paracrine activation system are under investigation.
Thus, glutamate acting as an NMDA-R agonist leads to NO synthesis by the epithelium, likely through paracrine pathways, which decreases Ach-induced contractility. In contrast, activation of NMDA-Rs by tPA and uPA enhances Ach-induced SMC contractility directly. The basis for this difference in the contractile effect of activating NMDA-R by these two sets of agonists is uncertain. One explanation may lie in the capacity of tPA and uPA, but not glutamate, to cleave the receptor, which may augment or terminate intracellular regulatory pathways. Nicole and colleagues (10) have reported that recombinant tPA evoked more intense calcium influx and caused greater excitotoxic damage than the glutamate analog, NMDA, alone. These authors attributed potentiation of NMDA-R–mediated signaling to t-PA–mediated cleavage of the NR1 subunit of the receptor (10). However, additional study will be needed to clarify the difference in receptor-mediated signaling pathways and their implication for airway function.
The effect of tPA and uPA on NMDA-R1–mediated airway contractility is a multistep process partially elucidated by our studies. Both PAs bind to NMDA-R1 through their respective docking sites, forming a complex that facilitates proteolytic binding and cleavage of the NR1 subunit. Blocking binding of tPA and uPA to NMDA-R1 by a PAI-1–derived peptide or mutating the amino acids within the docking sites to alanines both inhibit receptor cleavage and lead to loss of their constitutive prorelaxation functions to the same extent as inhibiting the respective catalytic sites. These results extend previous studies involving an extensively mutated tPA molecule by identifying the residues responsible for receptor binding as lying within the four to six amino acids that form the docking site (13). Thus, to exert their effect on airway contractility, PAs must first bind to NMDA-R1, which is followed by receptor activation and signal transduction (Figure 11). Whether receptor cleavage itself is involved in the activation process requires additional study.
The fact that both PAs express procontractile effects supports the concept that each is involved in the development of asthma. The novelty of these data lies in the fact that they show a direct and rapid effect of the PAs on the contractility of the airways. This finding extends previous studies implicating PAs in the long-term sequealae of inflammation, such as the deposition of extracellular matrix, airway remodeling (18, 23), and resolution of subepithelial fibrin that enhances airway hyperresponsiveness (24, 25). Taken together, it appears that PAs may play several roles in asthma that are stage dependent, including deleterious acute effect on airway contractility and a beneficial short- or long-term effect by inhibiting fibrin deposition and aberrant airway remodeling. It is therefore important to further our understanding of the mechanisms and determinants in PAs that mediate each process to develop a novel PA with enhanced benefit:risk ratio for protracted use in patients with reactive airways disease, including asthma.
Additional study is also needed to elucidate: how the contractile function of NMDA-Rs is differentially regulated by PAs and glutamate (Figure 11); the possible involvement of airway innervation; how the receptor is regulated by mediators of inflammation and allergy; the role of tPA and uPA in acute reactive airway disease and smooth muscle hypertrophy; and the role of the epithelium and the NMDA-R/NO pathway in human asthma. Despite these limitations, our studies identify several potential new therapeutic targets and specific means to ameliorate reactive airway disease.
This work was supported by National Institutes of Health grants PO1 HL076406 (S.I., D.B.C., A.A.-A.R.H.) and HL82545 (A.A.-R.H.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0257OC on January 22, 2010
Author Disclosure: S.I. serves on the advisory board for Kluwers (Clinical Pulmonary Medicine) and receives $1,001–$5,000, has received lecture fees from Brahms for $5,001–$10,000, and has also received a sponsored grant from Attenuon, LLC, for $10,001–$50,000. He serves on the CPM editorial board for Williams and Wilkens Publishers, and receives $1001–$5000, wrote a book chapter for Hodder Arnold Publishers for less than $1,000 and Wolters Kluwer Publishers for less than $1,000. He received consultancy fees from National Institutes of Health for less than $1,000 and a sponsored grant more than $100,001 along with sponsored grants from FAMRI for more than $100,001 and the U.S. Army for $10,001–$50,000. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Eder W, Ege M, Von Mutius E. The asthma epidemic. N Engl J Med 2006;355:2226–2235. [DOI] [PubMed] [Google Scholar]
- 2.An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 2007;29:834–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kellner J, Tantzscher J, Oelmez H, Edelmann M, Fischer R, Huber RM, Bergner A. Mechanisms altering airway smooth muscle cell Ca2+ homeostasis in two asthma models. Respiration 2008;76:205–215. [DOI] [PubMed] [Google Scholar]
- 4.Janssen L, Killian K. Airway smooth muscle as a target of asthma therapy: history and new directions. Respir Res 2006;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Said S, Berisha H, Pakbaz H. Excitotoxicity in the lung: N-methyl-D-aspartate–induced, nitric oxide–dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci USA 1996;93:4688–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kathleen G, Dickman J, Youssef G, Mathew M, Said S. Ionotropic glutamate receptors in lungs and airway, molecular basis for glutamate toxicity. Am J Respir Cell Mol Biol 2004;30:139–144. [DOI] [PubMed] [Google Scholar]
- 7.Gill S, Mueller R, Peter P, Mcguire F, Olga O, Pulido M. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol Pathol 2000;28:277–284. [DOI] [PubMed] [Google Scholar]
- 8.Said S. Glutamate receptors and asthmatic airway disease. Trends Pharmacol Sci 1999;20:132–135. [DOI] [PubMed] [Google Scholar]
- 9.Armstead W, Cines D, Higazi AA-R. Plasminogen activators contribute to age dependent impairment of NMDA cerebrovasodilation after brain injury. Brain Res Dev Brain Res 2005;156:139–146. [DOI] [PubMed] [Google Scholar]
- 10.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor–mediated signaling. Nat Med 2001;7:59–64. [DOI] [PubMed] [Google Scholar]
- 11.Vivien D, Buisson A. Serine protease inhibitors: novel therapeutic targets for stroke? J Cereb Blood Flow Metab 2000;20:755–764. [DOI] [PubMed] [Google Scholar]
- 12.Armstead W, Cines D, Higazi AA-R. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke 2005;36:2265–2269. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Atalaya J, Roussel B, Levrat D, Parcq J, Nicole O, Hommet Y, Benchenane K, Castel H, Leprince J, To Van D, et al. Toward safer thrombolytic agents in stroke: molecular requirements for NMDA receptor–mediated neurotoxicity. J Cereb Blood Flow Metab 2008;28:1212–1221. [DOI] [PubMed] [Google Scholar]
- 14.Maiya R, Zhou Y, Norris E, Kreek M, Strickland S. Tissue plasminogen activator modulates the cellular and behavioral response to cocaine. Proc Natl Acad Sci USA 2009;106:1983–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowal K, Bodzenta-Lukaszyk A, Pampuch A, Szmitkowski M, Donati M, Iacoviello L. Plasminogen activator inhibitor–1 plasma concentration in allergic asthma patients during allergen challenge. Int Arch Allergy Immunol 2007;144:240–246. [DOI] [PubMed] [Google Scholar]
- 16.Kowal K, Zukowski S, Moniuszko M, Bodzenta-Lukaszyk A. Plasminogen activator inhibitor–1 (PAI-1) and urokinase plasminogen activator (uPA) in sputum of allergic asthma patients. Folia Histochem Cytobiol 2008;46:193–198. [DOI] [PubMed] [Google Scholar]
- 17.Chu E, Cheng J, Foley J, Mecham B, Owen C, Haley K, Mariani T, Kohane I, Tschumperlin D, Drazen J. Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells. Am J Respir Cell Mol Biol 2006;35:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho S, Ryu C, Oh C. Plasminogen activator inhibitor–1 in the pathogenesis of asthma. Exp Biol Med (Maywood) 2004;229:138–146. [DOI] [PubMed] [Google Scholar]
- 19.Begin P, Tremblay K, Daley D, Lemire M, Claveau S, Salesse C, Kace S, Montpetit A, Becker A, Chan-Yeung M, et al. Association of urokinase-type plasminogen activator with asthma and atopy. Am J Respir Crit Care Med 2007;175:1109–1116. [DOI] [PubMed] [Google Scholar]
- 20.Stack M, Johnson D. Human mast cell tryptase activates single-chain urinary-type plasminogen activator (pro-urokinase). J Biol Chem 1994;269:9416–9419. [PubMed] [Google Scholar]
- 21.Brooks A, Bates M, Vrtis R, Jarjour N, Bertics P, Sedgwick J. Urokinase-type plasminogen activator modulates airway eosinophil adhesion in asthma. Am J Respir Cell Mol Biol 2006;35:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlin S, Roth M, Black J. Urokinase potentiates PDGF-induced chemotaxis of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2003;284:L1020–L1026. [DOI] [PubMed] [Google Scholar]
- 23.Jeffery P. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 2001;164:S28–S38. [DOI] [PubMed] [Google Scholar]
- 24.Wagers S, Norton R, Rinaldi L, Bates J, Sobel B, Irvin C. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 2004;114:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuramoto E, Nishiuma T, Kobayashi K, Yamamoto M, Kono Y, Funada Y, Kotani Y, Sisson TH, Simon RH, Nishimura Y. Inhalation of urokinase-type plasminogen activator (uPA) reduces airway remodeling in a murine asthma model. Am J Physiol Lung Cell Mol Physiol 2008;114:104–111. [DOI] [PubMed] [Google Scholar]
- 26.Armstead WM, Nassar T, Akkawi S, Smith DH, Chen XH, Cines DB, Higazi AA. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci 2006;9:1150–1155. [DOI] [PubMed] [Google Scholar]
- 27.Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, Heyman S, Higazi AA-R. The in vitro and in vivo effect of tPA and PAI-1 on blood vessel tone. Blood 2004;103:897–902. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Strickland DK, Cines DB, Higazi AA-R. Regulation of single chain urokinase binding, internalization and degradation by a plasminogen activator inhibitor 1-derived peptide. J Biol Chem 1997;272:27053–27057. [DOI] [PubMed] [Google Scholar]
- 29.Higazi AA-R, Barghouti II, Abu-Much R. Identification of an inhibitor of tissue-type plasminogen activator-mediated fibrinolysis in human neutrophils. J Biol Chem 1995;270:9472–9477. [DOI] [PubMed] [Google Scholar]
- 30.Higazi AA-R, Ajawi F, Akkawi S, Hess E, Kuo A, Cines BC. Regulation of the single-chain urokinase–urokinase receptor complex activity by plasminogen and fibrin: novel mechanism of fibrin specificity. Blood 2005;105:1021–1028. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura M, Shibata O, Saito M, Yamaguchi M, Nishioka K, Makita T, Sumikawa K. Selegiline, an MAO-B inhibitor, attenuates airway smooth muscle contraction in the rat trachea. J Pharm Pharmacol 2004;56:935–939. [DOI] [PubMed] [Google Scholar]
- 32.Haj-Yejia A, Nassar T, Sachais BS, Kuo A, Bdeir K, Al-Mehdi AB, Mazar A, Cines DB, Higazi AA-R. Urokinase-derived peptides regular vascular smooth muscle cell contraction in vitro and in vivo. FASEB J 2000;14:1411–1422. [DOI] [PubMed] [Google Scholar]
- 33.Nassar T, Haj-Yeha S, Akkawi S, Kuo A, Bdeir K, Mazar A, Cines DB, Higazi AA-R. Binding of urokinase to low density lipoprotein–related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem 2002;277:40499–40504. [DOI] [PubMed] [Google Scholar]
- 34.Akkawi S, Nassar T, Tarshis M, Cines BC, Higazi AA-R. LRP and avB3 mediate tPA-activation of smooth muscle cells. Am J Physiol Heart Circ Physiol 2006;291:H1351–H1359. [DOI] [PubMed] [Google Scholar]
- 35.Jankovic SM, Jankovic SV, Stojadinovic D, Jackovlevic M, Milovanovic D. Effect of exogenous glutamate and N-Methyl-D-aspartic acid on spontaneous activity of isolated human ureter. Int J Urol 2007;14:833–837. [DOI] [PubMed] [Google Scholar]
- 36.Naseem K. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med 2005;26:33–65. [DOI] [PubMed] [Google Scholar]
- 37.Gaston B, Drazen J, Loscalzo J, Stamler J. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 1994;149:538–551. [DOI] [PubMed] [Google Scholar]
- 38.Asano K, Chee C, Gaston B, Lilly C, Gerard C, Drazen J, Stamler J. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci USA 1994;91:10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madison EL, Goldsmith EJ, Gerard RD, Gething MJH, Sambrook JF, Bassel-Duby RS. Amino acid residues that affect interaction of tissue plasminogen activator with plasminogen activator inhibitor 1. Proc Natl Acad Sci USA 1990;87:3530–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madison E, Goldsmith E, Gething M, Sambrook J, Gerard R. Restoration of serine protease-inhibitor interaction by protein engineering. J Biol Chem 1990;265:21423–21426. [PubMed] [Google Scholar]
- 41.Madison EL, Goldsmith EJ, Gerard R, Gething MJ, Sambrook J. Serpin-resistant mutants of human tissue-type plasminogen activator. Nature 1989;339:721–725. [DOI] [PubMed] [Google Scholar]
- 42.Gyetko M, Chen G-H, McDonald RA, Goodman R, Huffnagle GB, Ilkinson CCW, Fuller JA, Toews GB. Urokinase is required for the pulmonary inflammatory response to Cryptococcus neoformans: a murine transgenic model. J Clin Invest 1996;97:1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor–deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J Immunol 2000;165:1513–1519. [DOI] [PubMed] [Google Scholar]
- 44.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor–1 gene. J Clin Invest 1996;97:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunther A, Lubke N, Ruppert C, Weissma N, Grimmminger F, Seeger W. Prevention of lung fibrosis in a rabbit model of bleomycin-induced fibrosis upon aerosol application of heparin or urokinase. Chest 2001;120:4. [Google Scholar]