Abstract
Pulmonary arterial hypertension (PAH) is a life-threatening disease that leads to progressive pulmonary hypertension, right heart failure, and death. Endothelial dysfunction and inflammation were implicated in the pathogenesis of PAH. Epoxyeicosatrienoic acids (EETs), products of the cytochrome P450 epoxygenase metabolism of arachidonic acid, are potent vasodilators that possess anti-inflammatory and other protective properties in endothelial cells. We investigated whether gene delivery with the human cytochrome P450 epoxygenase 2J2 (CYP2J2) ameliorates monocrotaline (MCT)-induced pulmonary hypertension in rats. Significant pulmonary hypertension developed 3 weeks after the administration of MCT, but gene therapy with CYP2J2 significantly attenuated the development of pulmonary hypertension and pulmonary vascular remodeling, without causing changes in systemic arterial pressure or heart rate. These effects were associated with increased pulmonary endothelial NO synthase (eNOS) expression and its activity, inhibition of inflammation in the lungs, and transforming growth factor (TGF)-β/type II bone morphogenetic protein receptor (BMPRII)-drosophila mothers against decapentaplegic proteins (Smads) signaling. Collectively, these data suggest that gene therapy with CYP2J2 may have potential as a novel therapeutic approach to this progressive and oftentimes lethal disorder.
Keywords: arachidonic acids, cytochrome P450 epoxygenase, gene therapy, monocrotaline
CLINICAL RELEVANCE.
Primary pulmonary artery hypertension is a fatal disease, and response to current treatments is poor. We investigated whether cytochrome P450 epoxygenase 2J2 gene delivery ameliorated monocrotaline-induced pulmonary hypertension in rats. Our results suggest that gene therapy with cytochrome P450 epoxygenase 2J2 may have potential as a novel therapeutic approach to this progressive and often lethal disorder.
Pulmonary arterial hypertension (PAH) is characterized by vascular obstruction and the variable presence of vasoconstriction, leading to increased pulmonary vascular resistance and right heart failure (1). The pathologic changes of PAH include endothelial cell injury and apoptosis, medial hypertrophy, infiltration by inflammatory cells, and thrombosis in small pulmonary arteries (2, 3). Endothelial dysfunction is characterized by an imbalance of vasodilator and vasoconstrictor factors, including an altered ratio of thromboxane and prostacyclin, decreased expression of endothelial NO synthase (eNOS), and increased endothelin-1 (ET-1) (4). Cytokines and growth factors, such as IL-6 and TGF-β1, which are released by macrophages and lymphocytes, are also involved in the development of pulmonary vascular remodeling (5). Thrombosis leads to a narrowing of the pulmonary vessel lumen, thus worsening PAH (6). Thus, a therapeutic strategy to target these abnormalities may be effective in the treatment of PAH.
Cytochrome P450 epoxygenase 2J2 (CYP2J2), a predominant human P450 arachidonic acid epoxygenase, is widely expressed in heart, lung, blood vessels, liver, kidney, ileum, jejunum, and colon in humans (7). This enzyme metabolizes arachidonic acid to biologically active cis-epoxyeicosatrienoic acids (5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET) (8). EETs have diverse biological properties within the cardiovascular system, and were identified as endothelium-derived hyperpolarizing factors that activate calcium-sensitive potassium channels to result in the hyperpolarization of resting membrane potential and the relaxation of vascular smooth muscle cells (9). EETs also inhibit vascular smooth muscle cell migration (10), and reduce pulmonary vascular resistance in newborn piglets and dogs (11, 12). We observed that the overexpression of cytochrome P450 epoxygenases or the addition of synthetic EETs upregulated endothelial nitric oxide synthase expression and activity (13), protected endothelial cells from apoptosis (14), and stimulated angiogenesis via the activation of both mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways (15). In addition, EETs inhibit platelet aggregation (16), increase tissue plasminogen activator expression (17), and exert anti-inflammatory effects via the suppression of NF-κB and IκB kinase activity (18). These findings suggest that CYP2J2, via the production of EETs, may attenuate pulmonary hypertension through multiple vasoprotective mechanisms. Thus, we investigated whether the overexpression of CYP2J2 attenuates MCT-induced pulmonary hypertension in rats, and determined the impact of CYP2J2 gene delivery on important signaling pathways involved in the pathogenesis of PAH.
MATERIALS AND METHODS
Preparation of pCB6-2J2
The CYP2J2 cDNA and anti-CYP2J2 antibodies were from Dr. Darryl C. Zeldin at the National Institute of Environmental Health Sciences of the National Institutes of Health. The CYP2J2 cDNA was cloned into the eukaryotic expression vector pCB6 (Invitrogen, Carlsbad, CA) using Bam HI and Kpn I restriction sites. The resulting plasmid (pCB6-2J2) was purified using a kit from Qiagen (Hilden, Germany).
Animal Treatment and Gene Delivery
Experimental protocols were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by the Academy of Sciences of China. Thirty-six male Sprague-Dawley rats weighing 250–280 g were randomly divided into two groups. The control rats ( n = 8) received a subcutaneous injection of 0.9% saline, whereas the model rats (n = 28) received a subcutaneous injection of 60 mg/kg monocrotaline (MCT, Sigma, St. Louis, MO). MCT was dissolved in 0.1 N HCl, and the pH was adjusted to 7.4 with 1.0 N NaOH. Three weeks after the injection of MCT, all model rats were randomly divided into three treatment groups: the MCT group (MCT alone, n = 10) received 0.5 ml of saline solution intravenously. The pCB6-treated group (MCT + pCB6, n = 10) and the CYP2J2-treated group (MCT + CYP2J2, n = 8) were injected either with a single intravenous dose of empty pCB6 vector or with pCB6-2J2. Animals were anesthetized via spontaneous inhalation of 1% isoflurane before injection. Purified plasmid DNA was dissolved in 0.9% NaCl at 1 mg/ml, and injected into the sublingual vein at a dose of 3 mg/kg body weight.
We previously demonstrated that both endogenously produced EETs and an exogenous addition of EETs upregulated eNOS and promoted its activity (13, 19). To investigate the role of eNOS further in the CYP2J2/EETs-mediated lowering of PAH, an additional set of experiments was performed. Sixty male Sprague-Dawley rats weighing 250–280 g were randomly divided into six groups (n = 10 for each) that received a subcutaneous injection of 0.9% saline (normal control rats, n = 10), and a subcutaneous injection of 60 mg/kg MCT (Sigma) (n = 50), respectively. The animals receiving MCT were further divided 3 weeks later (45 surviving rats) into five groups, i.e., MCT, MCT + pCB6, MCT + CYP2J2 receiving an injection of pCB6-2J2, MCT + CYP2J2 + NG-l-nitro-arginine methyl ester (l-NAME) receiving an injection of pCB6-2J2 injection and l-NAME added to its drinking water (50 mg/100 ml), and MCT + l-NAME (l-NAME water–drinking group), as described previously (20). Three weeks afterward, all animals underwent hemodynamic measurements, and the eNOS and NOS activity of their lung tissue was determined after they were killed.
Hemodynamic Measurements
Two weeks after gene transfer, the animals were anesthetized with sodium pentobarbital (30 mg/kg, intraperitoneal). A polyethylene catheter (PE-100; Clay Adams, Parsippany, NJ) was inserted into the right ventricle (RV) through the jugular vein for the measurement of RV systolic pressure (RVSP). Another polyethylene catheter was inserted into the right carotid artery to measure heart rate and mean systemic arterial pressure (mSAP). Hemodynamic variables were measured using a polygraph system (AP-601G; Nihon Kohden, Tokyo, Japan).
Morphometric Analysis of the Pulmonary Artery and Ventricular Weight Measurement
After hemodynamic analysis, all rats were killed. Lungs were flushed with ice-cold saline through the main pulmonary artery. The right lung was removed and snap-frozen in liquid nitrogen for biochemical studies. The left lung was preserved in 4% PBS-buffered formaldehyde solution and embedded in paraffin. Sections (4-μm) were cut and stained with elastic van Gieson stain. Sections were examined using light microscopy, and morphometric analysis was performed in pulmonary arteries with an external diameter of 25–50 μm and 51–100 μm. The medial wall thickness was calculated with the following formula: medial thickness (%) = medial wall thickness/external diameter × 100. For the quantitative analysis, 30 vessels of each rat were measured and averaged randomly by investigators who were blinded to experimental group assignment. Each ventricle was excised, dissected free, and weighed. The weight ratio of the right ventricle to the left ventricle (LV) plus the septum (S), i.e., RV/(LV + S), was calculated as an index of right ventricular hypertrophy (RVH).
Serum and Urine Collection
Two days before the end of the experiment, 24-hour urine samples were collected in metabolic cages with triphenylphosphine to prevent the decay of urine and the oxidation of EETs. Approximately 1 ml of blood was drawn from the inferior vena cava after hemodynamic analysis, and sera were collected after coagulation and centrifugation. All samples were stored at −80°C before being assayed.
Preparation of Tissue Extracts
Protein samples were prepared by homogenization of the frozen heart, liver, lung, kidney, and aorta tissues in lysis buffer (10 μmol/L Tris/Cl, pH 8.0, 0.2% NP-40, and 1 μmol/L EDTA, pH 7.6) supplemented with protease inhibitor. After centrifugation of the homogenates (3,000 × g for 10 minutes), the supernatants were extracted and used immediately or stored at −80°C before being used. Protein concentrations were estimated by the method of Bradford.
Determination of IL-6/IL-10 in Lung Extracts and Sera
Levels of IL-6 and IL-10 in sera and lung extracts were measured using ELISA kits (Rapidbio Laboratories, Calabasas, CA). The minimum detectable level was 10 pg/ml. The concentration of IL-6 in the lung was corrected for tissue weight, and expressed as picograms per milligram of protein.
Determination of eNOS Activity and NO Synthase Activity
After hemodynamic measurements were performed and the animals were killed, the lung tissue of rats was prepared as already described. The supernatant was extracted and stored at −80°C until used. The NO synthase (NOS) activity was assayed by the conversion of L-arginine to NO with a commercial kit, according to the manufacturer's instruction (R&D Systems, Minneapolis, MN) (21). Briefly, supernatant was incubated with 1 mmol/L reduced nicotinamideadenine dinucleotide phosphate, 1 μmol/L flavin adenine dinucleotide, 1 μmol/L flavin mononucleotide, 3 μmol/L tetrahydrobiopterin, 0.6 mmol/L CaCl2, and 100 nmol/L arginine. Reactions were conducted at 37°C for 15 minutes and then terminated by a stop buffer from the kit. NO in oxygen-containing solutions is chemically unstable, and undergoes rapid oxidation to nitrite/nitrate (stable NO metabolites). To reflect the activity of NOS, the produced nitrite/nitrate was measured using the Griess reagent and a spectrophotometer at 530 nm, as previously described. NOS activity was expressed according to enzyme activity units, as defined by 1 nmol of NO production per milligram of tissue protein per minute at 37°C. The protein content of the supernatant was measured using Coomassie brilliant blue, and was used to normalize the activities of NOS. The determination of eNOS was similar.
Expression of CYP2J2 and Detection of 14,15-Dihydroxyeicosatrienoic Acid
Western blotting was performed to detect levels of CYP2J2 expression in the heart, liver, lung, and aorta. Briefly, samples containing equal amounts of total protein were loaded in 10% sodium dodecyl sulfate (SDS)–polyacrylamide gels, and transferred onto polyvinylidene difluoride (PVDF) membranes after electrophoresis. After incubation in blocking buffer (5% nonfat dry milk in Tris-buffered saline, pH 7.6, and 0.1% Tween-20), membranes were incubated with anti-CYP2J2 antibody (1:1,000 dilution) or anti–β-actin antibody (1:1,000) (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Immunoreactive proteins were visualized by enhanced chemiluminescence, using horseradish-peroxidase–labeled anti-mouse IgG (1:8,000 dilution; Amersham Life Science, Arlington Heights, IL) and quantified by densitometric analysis, using the Bio Image System (Syngene, Cambridge, UK).
For the measurement of 14,15-dihydroxyeicosatrienoic acid (14,15-DHET), rat urine and tissue extracts of liver, lung, and kidney were used. Eicosanoids were extracted from samples thrice with ethyl acetate after acidification with acetic acid, as described previously (14, 15). After evaporation, samples were dissolved in N,N-dimethylformamide (AMRESCO, Solon, OH), and the concentration of 14,15-DHET was determined using an ELISA kit (Detroit R&D, Detroit, MI), according to the manufacturer's instructions.
Analysis of Protein Expression in Lung
To investigate the effects of CYP2J2 gene delivery on the expression of lung protein, proteins were extracted, quantified, and separated by SDS-PAGE, as described above. Western blots were probed with rabbit anti–phospho-Smad1 (Ser463/Ser465), rabbit anti-BMPRII, rabbit anti–TGF-β1, rabbit anti-eNOs, or mouse anti-Smad1 (Santa Cruz Biotechnology), rabbit anti–phospho-Smad2 (Ser465/Ser467), rabbit anti-Smad2 (cell signal), or mouse anti-β-actin, which served as a loading control.
Statistical Analysis
Results are expressed as mean ± SEM. Data were analyzed using either an unpaired Student t test or ANOVA, with the use of SYSTAT software (SYSTAT, Inc., Evanston, IL). Values were considered statistically significant at P < 0.05.
RESULTS
Expression of CYP2J2 in Rats after Gene Delivery and Its Effect on Survival
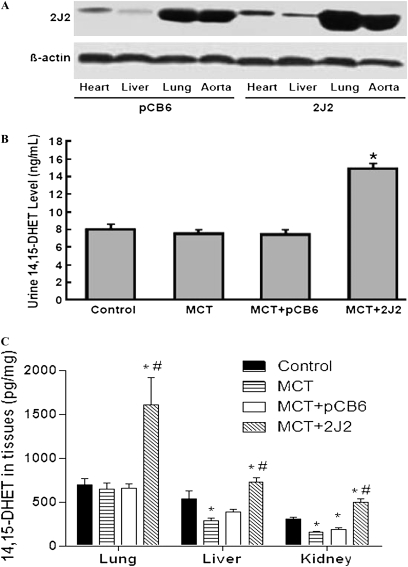
To analyze the expression of CYP2J2, Western blot analysis was performed with the use of an antibody to CYP2J2 that cross-reacts with both human and rat isoforms, 2 weeks after gene delivery (5 weeks after MCT injection, or the end of the study). Compared with rats injected with empty pCB6 vector, the expression of CYP2J2 increased in the heart, liver, lung, and aorta after a single injection of pCB6-2J2 (Figure 1A).
Figure 1.
Expression of cytochrome P450 epoxygenase 2J2 (CYP2J2) and detection of 14,15-dihydroxyeicosatrienoic acid (14,15-DHET) in urine and tissues, 2 weeks after gene delivery. (A) Representative immunoblotting results show increased CYP2J2 expression in heart, liver, lung, and aorta of pCB6-2J2–treated rats compared with empty pCB6 vector–treated rats. Experiments were repeated three times. (B) Urinary concentrations of 14,15-DHET according to ELISA in saline control rats (Control) and rats treated with monocrotaline (MCT), either alone (MCT) or together with pCB6 empty vector (MCT + pCB6) or pCB6-2J2 (MCT + 2J2). (C) Concentrations of 14,15-DHET according to ELISA in tissues of lung, liver, and kidney in various groups. Data shown are mean ± SEM of 6– 8 rats/group. *P < 0.05 versus control. #P < 0.05 versus MCT + pCB6.
In the saline-treated control group, all eight rats survived (100%). Of the 10 animals that received MCT alone or the 10 that received MCT together with empty pCB6 vector, only six (60%) and seven (70%) survived to the end of the study, respectively. However, seven (87.5%) rats survived in the group with CYP2J2 overexpression. When we combined the data from the two experiments, the survival rates were 100% (18/18) in control rats, and 63.2% (12/19), 68.4% (13/19), 88.2% (15/17), 77.8% (7/9), and 66.7% (6/9) in groups receiving MCT alone, MCT + pCB6, MCT with CYP2J2, MCT + CYP2J2 + l-NAME, and MCT + l-NAME, respectively. These data suggest that CYP2J2 gene delivery increases survival in MCT-treated rats.
Effects of CYP2J2 Gene Transfer on 14,15-DHET Levels in Urine and Tissue Extracts
We measured 14,15-DHET levels in urine and local tissues by ELISA. Low levels of immunoreactive 14,15-DHET were detected in the urine of saline-treated control rats (8.02 ± 0.63 ng/mL), MCT-treated rats (7.50 ± 0.46 ng/ml), and MCT + empty pCB6 vector-treated control rats (7.42 ± 0.55 ng/mL). In contrast, in MCT + pCB6-2J2-treated animals, the urinary level of 14,15-DHET was 14.9 ± 0.51 ng/mL, which was significantly higher than in the control groups (Figure 1B). Similarly, tissue contents of 14,15-DHET levels in the liver, lung, and kidney were also increased by CYP2J2 gene delivery (Figure 1C). Notably, the concentration of 14,15-DHET in the lung was higher than in all other tissues, especially in CYP2J2-treated animals, in parallel with CYP2J2 expression, as shown by Western blotting (Figure 1A). These results demonstrate that CYP2J2 gene delivery achieves functional CYP2J2 overexpression in vivo.
Effects of CYP2J2 Gene Transfer on Hemodynamic Parameters and RVH
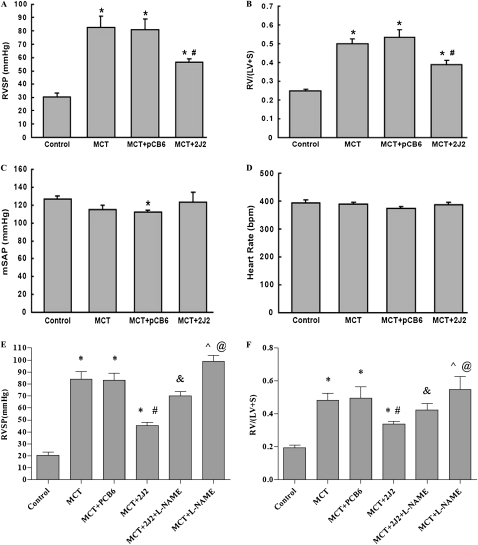
Five weeks after the injections of MCT, rats in the MCT group developed severe PAH, with an RVSP of 82.4 ± 8.4 mm Hg, compared with 30.5 ± 2.8 mm Hg in the saline-treated control group (P < 0.01, Figure 2A). Treatment with pCB6-2J2 but not with empty pCB6 vector significantly inhibited the elevation of RVSP (56.5 ± 2.3 mm Hg versus 80.8 ± 7.8 mm Hg, P < 0.01, Figure 2A). Moreover, the RVSP in pCB6-2J2–treated animals was also lower than in those measured 3 weeks after MCT treatment in three animals (69.3 ± 3.5 mm Hg versus 56.5 ± 2.3 mm Hg, P < 0.05), suggesting partial reversal by pCB6-2J2 treatment. CYP2J2 overexpression also exerted a beneficial effect on RVH. RV/(LV + S) was 0.24 ± 0.01 in the saline-treated control rats (Figure 2B), whereas rats receiving MCT either alone or with empty pCB6 vector developed severe RVH, with an increase in RV/(LV + S) to 0.50 ± 0.03 and 0.53 ± 0.04, respectively (P < 0.01). In contrast, animals receiving CYP2J2 gene transfer together with MCT injection developed significantly less RVH (0.39 ± 0.02, P < 0.01). However, neither mean systemic arterial pressure nor heart rate differed between the four groups (Figures 2C and 2D). These results indicate that CYP2J2 overexpression reversed the MCT-induced PAH and RVH, independent of changes in heart rate or blood pressure.
Figure 2.
Reversal effects of CYP2J2 gene delivery on right ventricular systolic pressure (RVSP) (A), right ventricle/left ventricle + septum (RV/LV + S) (B), mean systemic arterial pressure (mSAP) (C), and heart rate (D), and effects of NG-l-nitro-arginine methyl ester (l-NAME) on RVSP (E) and RV/LV + S (F). Hemodynamic measurements were performed 2 weeks after gene transfer, and results showed that CYP2J2 overexpression reversed the MCT-induced changes in RVSP and RVH, but had no effects on mSAP and heart rate. l-NAME attenuated the beneficial effect of CYP2J2 overexpression in the model of PAH induced by MCT. Data shown are mean ± SEM of 6–8 rats/group. *P < 0.05 versus control. #P < 0.05 versus MCT + pCB6.
In a second set of experiments, we produced similar results. The overexpression of CYP2J2 partially reversed PAH and RVSP as well as RVH induced by MCT, and mean systemic arterial pressure and heart rate did not differ in all six groups (data not shown). More importantly, l-NAME treatment further attenuated the reduction in pulmonary artery pressure and RVSP in CYP2J2-treated PAH rats (56.5 ± 2.3 mm Hg versus 71.8 ± 4.1 mm Hg, P < 0.01), and l-NAME administration further promoted MCT-induced PAH (98.8 ± 5.2 mm Hg versus 82.4 ± 8.4 mm Hg, P < 0.05; Figure 2E). In the similar way, l-NAME treatment significantly attenuated the beneficial effect of RVH, as evaluated by the ratio of RV/(LV+S) weight in CYP2J2-treated PAH animals (0.42 ± 0.04 versus 0.34 ± 0.02, P < 0.05. Figure 2F), and l-NAME treatment further aggravated RVH in MCT-treated rats (0.58 ± 0.07 versus 0.42 ± 0.04, P < 0.05. Figure 2F). These data suggest that the overexpression of CYP2J2 partly reversed the MCT-induced PAH and RVH, and that eNOS was involved in the mechanism of the beneficial effects of CYP2J2 to a great extent.
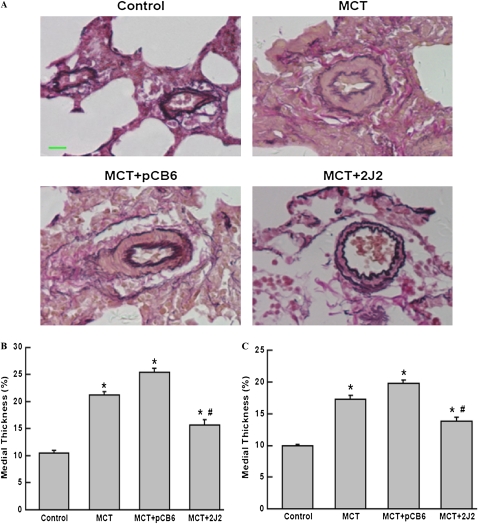
Morphometric Analysis of Pulmonary Arteries
Representative photomicrographs showed that hypertrophy of the pulmonary vessel wall was markedly increased in the MCT group and the empty pCB6 vector + MCT group, compared with the saline-treated control group. However, vessel-wall hypertrophy was significantly reduced in the CYP2J2-treated group (Figure 3A). We also separately quantified the medial- wall thickness of pulmonary arteries at size ranges of 25–50 μm and 51–100 μm in external diameter. A significant increase occurred in percentage of medial-wall thickness after the injection of MCT, whereas CYP2J2 transfer caused a marked reduction in the MCT-induced medial thickening of both size ranges of pulmonary arteries (Figure 3B, 25–50 μm; Figure 3C, 51–100 μm).
Figure 3.
Representative photomicrographs of peripheral pulmonary arteries stained with elastic van Gieson stain (A; original magnification, ×400; scale bar, 20 μm). Quantification of percent medial thickness for vessels 25–50 μm (B) and 51–100 μm (C) in external diameter. CYP2J2 gene delivery attenuated hypertrophy of vessel walls in MCT rats. Data shown are mean ± SEM of 6–8 rats/group. *P < 0.05 versus control. #P < 0.05 versus MCT + pCB6.
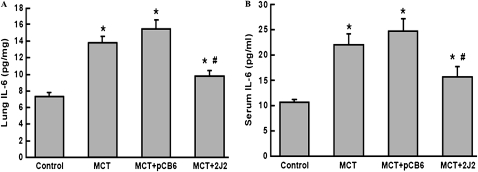
Effects of CYP2J2 Gene Transfer on Lung and Serum IL-6/IL-10 Levels
Five weeks after the injection of MCT, IL-6 concentrations were significantly increased in MCT-treated rat lung extracts compared with saline-treated control lung extracts (13.8 ± 0.7 pg/mg versus 7.3 ± 0.4 pg/mg, P < 0.01). Treatment with MCT + empty pCB6 vector had little effect on lung extract IL-6 concentrations (15.44 ± 1.16 pg/mg, P = NS versus MCT alone). In contrast, after CYP2J2 gene delivery, IL-6 levels were significantly decreased (9.8 ± 0.7 pg/mg, P < 0.01 versus MCT alone, Figure 4A). The effect of CYP2J2 gene transfer on serum IL-6 concentrations was similar in that treatment with CYP2J2, but not with empty pCB6 vector, significantly inhibited the MCT-induced elevation of serum IL-6 concentrations (15.7 ± 2.0 pg/mL versus 24.8 ± 2.5 pg/mL, P < 0.05, Figure 4B). Our measurement of IL-10 concentrations also showed a significant decrease in MCT-treated animals (43.84 ± 1.808 pg/mL in normal rats versus 35.25 ± 2.106 pg/mL in MCT rats and 34.01 ± 3.098 pg/mL in MCT + pCB6 rats, P < 0.05), but with restoration to normal levels after CYP2J2 treatment (51.26 ± 4.155 pg/mL in the MCT + CYP2J2 group, P < 0.05, compared with the MCT-treated rats). These data suggest that MCT induced marked inflammation, whereas CYP2J2 treatment inhibited the inflammation process.
Figure 4.
Concentrations of IL-6 in lung extracts and serum were determined by ELISA, 2 weeks after gene transfer. Treatment with CYP2J2 significantly inhibited the MCT-induced elevation of IL-6 in lung extracts (A) and in serum (B). Data shown are mean ± SEM of 6– 8 rats/group. *P < 0.05 versus control. #P < 0.05 versus MCT + pCB6.
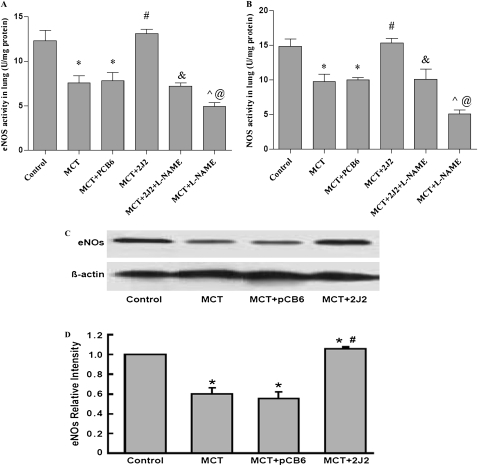
Effects of CYP2J2 Gene Transfer and l-NAME on eNOS and NOS Activity
Treatment with MCT significantly decreased the activity of eNOS (7.60 ± 0.80 U/mg protein versus 12.33 ± 1.17 U/mg protein, P < 0.05) and NOS (9.81 ± 1.07 U/mg protein versus 14.84 ± 1.05 U/mg protein, P < 0.05) compared with normal control rats. As expected, CYP2J2 gene transfer but not empty pCB6 vector significantly increased the activity of both eNOS (13.11 ± 0.52 U/mg protein versus 7.82 ± 0.95 U/mg protein, P < 0.01) and NOS (15.32 ± 0.65 U/mg protein versus 10.00 ± 0.38 U/mg protein, P < 0.01), compared with MCT treatment. In contrast, l-NAME treatment markedly attenuated the CYP2J2-induced upregulation of activity in eNOS (7.2 ± 0.39 U/mg protein versus 13.11 ± 0.52 U/mg protein, P < 0.01) and NOS (10.15 ± 1.42 U/mg protein versus 14.84 ± 1.05 U/mg protein, P < 0.01), and further aggravated the reduction in eNOS and NOS activity in MCT-treated rats (Figures 5A and 5B). When we subtract eNOS activity from total NOS activity, the data that involve the activity of iNOS and nNOS show no significant differences. All these data indicate that eNOS plays an important role in the CYP2J2-mediated reversal of PAH and RVH in the model of MCT-induced PAH..
Figure 5.
Activity of eNOS (A) and NOS (B) in lung tissue of rats, and Western blotting for eNOS and β-actin expression in MCT-treated rat lungs (C and D). A significant decrease occurred in eNOS activity (A) and expression (C and D) in rats treated with MCT, either alone or together with pCB6 empty vector. In contrast, in MCT-treated rats receiving pCB6-2J2, eNOS activity and protein levels were near normal. l-NAME significantly weakened the CYP2J2-induced increase in eNOS activity. Results are representative of 3–5 independent experiments. (D) Blots were scanned, and relative eNOS expression was normalized to β-actin . Data shown are mean ± SEM of 6–8 rats/group. *P < 0.05 versus control. #P < 0.05 MCT + CYP2J2 versus MCT + pCB6. &P < 0.05 MCT + CYP2J2 + l-NAME versus MCT + CYP2J2. ^P < 0.05 MCT + CYP2J2 + l-NAME versus MCT + l-NAME. @P < 0.05 MCT + l-NAME versus MCT
Effects of CYP2J2 Gene Transfer on Expression of Endothelial NOS
To evaluate the possible impact of CYP2J2 on pulmonary endothelium, we measured lung eNOS protein levels by immunoblotting. eNOS protein levels were significantly downregulated after an injection of MCT (Figures 5C and 5D). However, CYP2J2 gene delivery, and not treatment with empty pCB6 vector alone, increased lung-tissue expression of eNOS in MCT-treated rats.
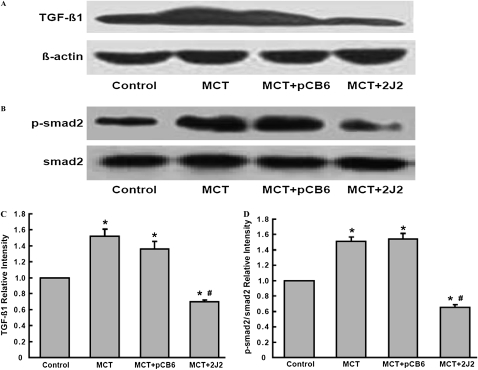
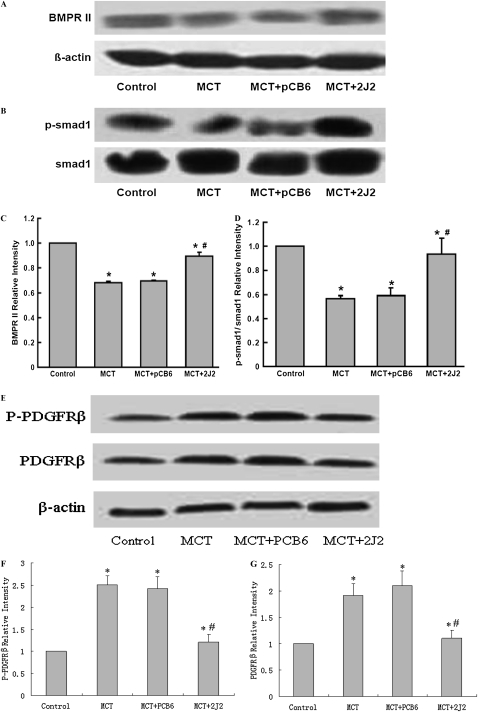
Effects of CYP2J2 Gene Transfer on TGF-β/BMPRII and PDGF Receptor Signaling
The lung expression of TGF-β/BMPRII–Smads signaling was determined by Western blotting to examine the effects of MCT injection and CYP2J2 transfer on these pathways. MCT injection resulted in significant increases in TGF-β1 and p-Smad2 protein in rats injected with MCT alone and MCT with empty pCB6 vector (Figures 6). In contrast, these changes were significantly attenuated in rats injected with pCB6-2J2. We also found a small but significant downregulation of BMPRII in MCT-treated rats. However, the delivery of CYP2J2 prevented the downregulation of BMPRII protein in MCT-treated rats (Figures 7A and 7C). Similarly, the delivery of CYP2J2 restored p-Smad1 to nearly normal levels, compared with empty pCB6 vector-treated rats (Figures 7B and 7D). Together, these results suggest that CYP2J2 gene transfer prevents MCT-induced impaired TGF-β/BMPRII–Smads signaling.
Figure 6.
Protein expression of TGF-β/Smads signaling in MCT-treated rat lungs assessed by Western blotting. A significant increase occurred in TGF-β1 (A) and p-Smad2 (B) expression in rats treated with MCT, either alone or together with pCB6 empty vector. However, in MCT-treated rats receiving pCB6-2J2, TGF-β1 and p-Smad2 protein levels were increased. Results are representative of three independent experiments. Blots were scanned, and relative TGF-β1 (C) and p-Smad2 (D) were normalized to β-actin and Smad2, respectively. Data shown are mean ± SEM of 6–8 rats/group. *P < 0.05 versus control. #P < 0.05 versus MCT + pCB6.
Figure 7.
Bone morphogenetic protein (BMP) and platelet-derived growth factor receptor β (PDGFRβ) signaling in MCT-treated rat lungs assessed by Western blotting. A significant decrease occurred in BMPRII (A) and p-Smad1 (B) expression in rats treated with MCT, either alone or together with pCB6 empty vector. However, in MCT-treated rats receiving pCB6-2J2, concentrations of BMPRII and p-Smad1 protein were increased. Results are representative of three independent experiments. Blots were scanned, and relative BMPRII (C) and p-Smad1 (D) were normalized to β-actin and Smad1, respectively. (E) Concentrations of PDGFRβ and phosphorylated PDGFRβ were upregulated in MCT-treated animals, and treatment with CYP2J2 attenuated the upregulation. (F and G) Relative PDGFRβ and phosphorylated PDGFRβ, respectively, were normalized to β-actin. Data shown are mean ± SEM of 6– 8 rats/group. *P < 0.05 versus control. #P < 0.05 versus MCT + pCB6.
We also probed platelet-derived growth factor receptor β (PDGFRβ) and its activity. Results showed that the expression levels of PDGFRβ and phosphorylated PDGFRβ were upregulated, and that CYP2J2 gene therapy significantly attenuated upregulation, which was restored to basal level (Figures 7E–G).
DISCUSSION
In the present study, we demonstrated the effective delivery of CYP2J2 cDNA, using the pCB6 eukaryotic expression vector, into rats treated with MCT to induce PAH. A marked increase in RV systolic pressure and hypertrophy was evident in rats after the administration of monocrotaline. However, RVSP and the hypertrophy of the RV and pulmonary vessel walls were significantly reduced after a single intravenous injection of pCB6-2J2. Treatment with eNOS inhibitor l-NAME attenuated the beneficial effects in pulmonary artery pressure and RVH in CYP2J2-treated PAH rats. These effects of CYP2J2 are likely attributable to a restoration of pulmonary eNOS expression, increased eNOS activity, an inhibition of inflammation in the lung, and the regulation of TGF-β/BMPRII–Smads signaling. Together, these results suggest that CYP2J2 gene delivery may have potential as a novel treatment of this progressive and often lethal disorder.
The injection of MCT can cause injury and apoptosis in endothelial cells (22), thereby decreasing the number of pulmonary capillaries (23) and resulting in pulmonary hypertension and vascular remodeling. Previous studies demonstrated that the cell-based gene transfer of angiopoietin-1 (24), vascular endothelial growth factor (25), or C-type natriuretic peptide (3) either prevented the apoptosis of endothelial cells, or increased the number of pulmonary capillaries after the administration of MCT, and inhibited the development of pulmonary hypertension. These reports also suggest that endothelial cell injury and the reduction of pulmonary capillary density play an important role in the pathogenesis of MCT-induced PAH. In our study, we demonstrated that the delivery of CYP2J2 significantly attenuated increases in RV systolic pressure and the ratio of RV/LV + S, suggesting that CYP2J2 delivery ameliorates MCT-induced PAH. Furthermore, we showed that the overexpression of CYP2J2 reversed the MCT-induced downregulation of eNOS expression and its activity in the lungs. In contrast, eNOS inhibitor l-NAME blocked the effects of CYP2J2 overexpression to a great extent. These results further support the idea that eNOS is involved in the beneficial effects of CYP2J2 overexpression and the increases in consequential EET production.
We previously reported that CYP epoxygenase overexpression, which is known to increase EET biosynthesis, significantly protected endothelial cells from apoptosis (14), and promoted angiogenesis via effects on MAPK and PI3 kinase/Akt signaling pathways, and to some extent, on the eNOS pathway (15). In addition, we demonstrated that the transfection of bovine aortic endothelial cells with P450 epoxygenases in vitro, or the overexpression of P450 epoxygenases in vivo, resulted in increased eNOS expression, which was attenuated by 17-octadecynoic acid (17-ODYA), a P450 inhibitor (13). Endothelial nitric oxide synthase is an enzyme that produces nitric oxide in vascular endothelial cells (26), and plays a pivotal role in the regulation of pulmonary vascular tone (27). Gene transfer with endothelial nitric oxide synthase attenuated MCT-induced pulmonary hypertension in rats (28). Other pharmacologic treatments that were effective in this PAH model were also associated with an upregulation of eNOS (3, 24, 25). Thus, the therapeutic effects of CYP2J2 delivery on pulmonary hypertension may be mediated by protection of the endothelium through a restoration of the expression of eNOS, at least in a part. In addition, the inhibition of inflammation, as described below, and the relaxing effects of EETs on the artery as an endothelium-derived hyperpolarization factor may also play roles.
Schermuly and colleagues demonstrated that the inhibition of PDGF receptor phosphorylation by the administration of STI571 reversed MCT-induced pulmonary hypertension and its pathophysiologic changes, which suggests that PDGF receptor signaling plays an important role in the development of pulmonary hypertension. In the present study, CYP2J2 overexpression attenuated PDGFRβ expression and its activity, suggesting that EETs may inhibit PDGF receptor signaling (29). The mechanisms of this effect are unknown. However, EET-induced eNOS upregulation may play an important role in this process by inhibiting the activation of Rho A (30).
Pokreisz and colleagues (31) documented that CYP epoxygenase–derived EETs are associated with hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling in mice. Revermann and colleagues proved that EETs and the soluble epoxide hydrolase that metabolized EETs to their less active dihydroxy derivatives were determinants of the acute hypoxic pulmonary vasoconstrictor response (32). However, Keserü and colleagues (33) confirmed that inhibition of the soluble epoxide hydrolase attenuated monocrotaline-induced pulmonary hypertension in rats. These results imply that EETs and the soluble epoxide hydrolase may play different roles in different models of PAH.
IL-6 may play an important role in the pathogenesis of PAH through inflammation, the promotion of vascular smooth muscle cell (VSMC) proliferation (34), and interactions with bone morphogenetic protein pathways (35). Elevated concentrations of IL-6 were also evident in animals treated with MCT (36) and in patients with idiopathic PAH (37). Furthermore, an injection of IL-6 can increase RV pressure and RV hypertrophy in mice (38), and the inhibition of IL-6 with a serotonin receptor antagonist (39) significantly reduced the thickness of the media of small pulmonary arteries and reduced RV hypertrophy. In the present study, we showed that IL-6 was excessively produced in the serum and lung extracts of rats treated with MCT. However, IL-6 concentrations were significantly decreased in CYP2J2-treated rats. This finding may be attributable to the anti-inflammatory nature of CYP2J2-derived eicosanoids. Node and colleagues reported that physiologic concentrations of EETs or the overexpression of CYP2J2 decreased the cytokine-induced expression of endothelial cell adhesion molecules, and EETs prevented leukocyte adhesion to the vascular wall by an inhibition of transcription factor NF-κB and IκB kinase, both in vitro and in vivo (18), supporting the anti-inflammatory effects of EETs.
Growth factors of the TGF-β superfamily have emerged as important regulators of both normal cardiovascular development and the onset or progression of vascular diseases, such as PAH (40, 41). Genetic studies of PAH revealed that mutations in the open reading frame of the BMPR2 gene were identified in approximately 40% of familial PAH and in up to 15% of patients with idiopathic PAH (42–44). In addition, animal models of pulmonary hypertension induced by high flow (45), chronic hypoxia (46), or MCT (47) also demonstrated a reduced expression of BMPR-II/Smad. TGF-β1, another member of the TGF-β superfamily, is also involved in the onset and progression of PAH. Abundant data document both increased and decreased TGF-β signaling in the disease (48–51). In our study, MCT significantly increased the expression of TGF-β1 and p-Smad2, whereas it significantly decreased BMPRII and p-Smad1 in the lungs. Furthermore, impaired TGF-β/BMPRII–Smads signaling was restored by a delivery of CYP2J2. Although the precise mechanisms remain unclear, these changes should favor the protection of endothelial cells from injury and PAH. BMPRII is highly expressed in the endothelium and smooth muscle layer of the pulmonary vasculature (52). It promotes pulmonary arterial endothelial cell survival, and protects these cells against apoptosis (53). A pronounced downregulation of BMP/Smad signaling occurred in MCT-induced PAH in rats, and this perturbed signaling had functional consequences for the maintenance of pulmonary arterial smooth muscle cell mass (47). Recently, Sakao and colleagues documented that pulmonary arterial endothelial cell apoptosis induced by high shear stress and vascular endothelial growth factor recepter (VEGFR) blockade led to the production of factors, in particular TGF-β1, that activated the proliferation of vascular smooth muscle cells (54). The failure of BMP signaling via Smad1/5 can increase TGF-β/activin receptor-like kinase-5 (ALK-5)/Smad2/3 signaling (55). This may occur because Smad1/5 signaling competes for the availability of the co-Smad, Smad4. In addition, Smad1 may physically interact with Smad3 and lead to degradation, or may prevent its phosphorylation. In this study, CYP2J2 gene delivery markedly upregulated BMPRII and p-Smad1, and downregulated TGF-β1 and p-Smad2. These changes in expression of TGF-β/BMPRII–Smads signaling may contribute to the observed protective effect of CYP2J2 gene therapy on MCT-induced PAH.
In conclusion, this study showed that gene therapy with CYP2J2 ameliorated MCT-induced PAH. This beneficial effect may be mediated by an increase of pulmonary endothelial NO synthase expression and its activity, an inhibition of inflammation in the lung, and a restoration of TGF-β/BMPRII–Smads signaling. Further studies are needed to determine if a similar approach can be developed for the treatment of PAH in humans.
This work was supported by grants 2007CB512004 and 2006CB503801 from the 973 Program, and by grants 30430320 and 30971247 from National Nature Science Foundation Committee of China. This work was also supported, in part, by grant Z01 ES025034 from the Intramural Research Program of the National Institute of Environmental Health Sciences at the National Institutes of Health.
Luyun Wang's present address is the Institute of Cardiopulmonary Cerebral Resuscitation, Second Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0161OC on January 29, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Newman JH, Fanburg BL, Archer SL, Badesch DB, Barst RJ, Garcia JG, Kao PN, Knowles JA, Loyd JE, McGoon MD, et al. Pulmonary arterial hypertension: future directions: report of a National Heart, Lung and Blood Institute/Office of Rare Diseases workshop. Circulation 2004;109:2947–2952. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh T, Nagaya N, Murakami S, Fujii T, Iwase T, Ishibashi-Ueda H, Yutani C, Yamagishi M, Kimura H, Kangawa K. C-type natriuretic peptide ameliorates monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med 2004;170:1204–1211. [DOI] [PubMed] [Google Scholar]
- 4.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004;109:159–165. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, et al. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res 2007;101:734–741. [DOI] [PubMed] [Google Scholar]
- 6.Farber HW, Loscalzo J. Prothrombotic mechanisms in primary pulmonary hypertension. J Lab Clin Med 1999;134:561–566. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem 1996;271:3460–3468. [DOI] [PubMed] [Google Scholar]
- 8.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res 2000;41:163–181. [PubMed] [Google Scholar]
- 9.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation 1995;92:3337–3349. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome P450 epoxygenase-derived eicosanoids. Circ Res 2002;90:1020–1027. [DOI] [PubMed] [Google Scholar]
- 11.Fuloria M, Smith TK, Aschner JL. Role of 5,6-epoxyeicosatrienoic acid in the regulation of newborn piglet pulmonary vascular tone. Am J Physiol Lung Cell Mol Physiol 2002;283:L383–L389. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson AH, Sprague RS, Lonigro AJ. 5,6-epoxyeicosatrienoic acid reduces increases in pulmonary vascular resistance in the dog. Am J Physiol 1998;275:H100–H109. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Lin L, Jiang J, Wang Y, Lu ZY, Bradbury JA, Lih FB, Wang DW, Zeldin DC. Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase C signaling pathways. J Pharmacol Exp Ther 2003;307:753–764. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, et al. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/AKT signaling pathways. Am J Physiol Heart Circ Physiol 2007;293:H142–H151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, Wang DW, Zeldin DC. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/AKT signaling pathways. J Pharmacol Exp Ther 2005;314:522–532. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick FA, Ennis MD, Baze ME, Wynalda MA, McGee JE, Liggett WF. Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. Influence of stereochemistry. J Biol Chem 1986;261:15334–15338. [PubMed] [Google Scholar]
- 17.Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Galpha S mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem 2001;276:15983–15989. [DOI] [PubMed] [Google Scholar]
- 18.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999;285:1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang JG, Chen RJ, Xiao B, Yang S, Wang JN, Wang Y, Cowart LA, Xiao X, Wang DW, Xia Y. Regulation of endothelial nitric-oxide synthase activity through phosphorylation in response to epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat 2007;82:162–174. [DOI] [PubMed] [Google Scholar]
- 20.Sekiguchi F, Yamamoto K, Matsuda K, Kawata K, Negishi M, Shinomiya K, Shimamur K, Sunano S. Endothelium-dependent relaxation in pulmonary arteries of l-NAME–treated Wistar and stroke-prone spontaneously hypertensive rats. J Smooth Muscle Res 2002;38:131–144. [DOI] [PubMed] [Google Scholar]
- 21.Sun XC, Chen WN, Li SQ, Cai JS, Li WB, Xian XH, Hu YY, Zhang M, Li QJ. Fluorocitrate, an inhibitor of glial metabolism, inhibits the up-regulation of NOS expression, activity and NO production in the spinal cord induced by formalin test in rats. Neurochem Res 2009;34:351–359. [DOI] [PubMed] [Google Scholar]
- 22.Thomas HC, Lame MW, Dunston SK, Segall HJ, Wilson DW. Monocrotaline pyrrole induces apoptosis in pulmonary artery endothelial cells. Toxicol Appl Pharmacol 1998;151:236–244. [DOI] [PubMed] [Google Scholar]
- 23.Schraufnagel DE, Schmid A. Pulmonary capillary density in rats given monocrotaline. A cast corrosion study. Am Rev Respir Dis 1989;140:1405–1409. [DOI] [PubMed] [Google Scholar]
- 24.Zhao YD, Campbell AI, Robb M, Ng D, Stewart DJ. Protective role of angiopoietin-1 in experimental pulmonary hypertension. Circ Res 2003;92:984–991. [DOI] [PubMed] [Google Scholar]
- 25.Zhao YD, Courtman DW, Ng DS, Robb MJ, Deng YP, Trogadis J, Han RN, Stewart DJ. Microvascular regeneration in established pulmonary hypertension by angiogenic gene transfer. Am J Respir Cell Mol Biol 2006;35:182–189. [DOI] [PubMed] [Google Scholar]
- 26.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988;333:664–666. [DOI] [PubMed] [Google Scholar]
- 27.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987;327:524–526. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Liu H, Visner G, Fletcher BS. Sleeping beauty-mediated eNOS gene therapy attenuates monocrotaline-induced pulmonary hypertension in rats. FASEB J 2006;20:2594–2596. [DOI] [PubMed] [Google Scholar]
- 29.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 2005;115:2811–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Largiader T, Eto M, Payeli SK, Greutert H, Viswambharan H, Lachat M, Zund G, Yang Z, Tanner FC, Luscher TF. Endothelial nitric oxide synthase gene transfer inhibits human smooth muscle cell migration via inhibition of Rho A. J Cardiovasc Pharmacol 2008;52:369–374. [DOI] [PubMed] [Google Scholar]
- 31.Pokreisz PFI, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, Szelid Z, Vermeersch P, Gillijns H, et al. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension 2006;47:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Revermann MB-SE, Dony E, Schermuly RT, Morisseau C, Geisslinger G, Fleming I, Hammock BD, Brandes RP. Inhibition of the soluble epoxide hydrolase attenuates monocrotaline-induced pulmonary hypertension in rats. J Hypertens 2009;27:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keserü B. B-SEPR, Fisslthaler B, Dietrich A, Gudermann T, Hammock BD, Falck JR, Weissmann N, Busse R, Fleming I. Epoxyeicosatrienoic acids and the soluble epoxidehydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J 2008;22:4306–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Ikeda U. Inflammatory cytokines and cardiovascular disease. Curr Drug Targets Inflamm Allergy 2003;2:257–265. [DOI] [PubMed] [Google Scholar]
- 35.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 2007;292:L1473–L1479. [DOI] [PubMed] [Google Scholar]
- 36.Bhargava A, Kumar A, Yuan N, Gewitz MH, Mathew R. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis 1999;1:126–132. [PubMed] [Google Scholar]
- 37.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151:1628–1631. [DOI] [PubMed] [Google Scholar]
- 38.Golembeski SM, West J, Tada Y, Fagan KA. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest 2005;128(Suppl 6)572S–573S. [DOI] [PubMed] [Google Scholar]
- 39.Miyata M, Ito M, Sasajima T, Ohira H, Kasukawa R. Effect of a serotonin receptor antagonist on interleukin-6–induced pulmonary hypertension in rats. Chest 2001;119:554–561. [DOI] [PubMed] [Google Scholar]
- 40.Eickelberg O, Morty RE. Transforming growth factor beta/bone morphogenic protein signaling in pulmonary arterial hypertension: remodeling revisited. Trends Cardiovasc Med 2007;17:263–269. [DOI] [PubMed] [Google Scholar]
- 41.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol 2006;26:1712–1720. [DOI] [PubMed] [Google Scholar]
- 42.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA III, Newman J, Williams D, Galie N, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet 2001;68:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA III, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 2000;26:81–84. [DOI] [PubMed] [Google Scholar]
- 44.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 2000;37:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rondelet B, Kerbaul F, Van Beneden R, Hubloue I, Huez S, Fesler P, Remmelink M, Brimioulle S, Salmon I, Naeije R. Prevention of pulmonary vascular remodeling and of decreased BMPR-2 expression by losartan therapy in shunt-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 2005;289:H2319–H2324. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of Type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2006;290:L450–L458. [DOI] [PubMed] [Google Scholar]
- 47.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, Peters DM, Dumitrascu R, Seeger W, Knaus P, et al. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2007;27:1072–1078. [DOI] [PubMed] [Google Scholar]
- 48.Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2004;170:1340–1348. [DOI] [PubMed] [Google Scholar]
- 49.Botney MD, Bahadori L, Gold LI. Vascular remodeling in primary pulmonary hypertension. Potential role for transforming growth factor-beta. Am J Pathol 1994;144:286–295. [PMC free article] [PubMed] [Google Scholar]
- 50.Zakrzewicz A, Kouri FM, Nejman B, Kwapiszewska G, Hecker M, Sandu R, Dony E, Seeger W, Schermuly RT, Eickelberg O, et al. The transforming growth factor-beta/Smad2,3 signalling axis is impaired in experimental pulmonary hypertension. Eur Respir J 2007;29:1094–1104. [DOI] [PubMed] [Google Scholar]
- 51.Zaiman AL, Podowski M, Medicherla S, Gordy K, Xu F, Zhen L, Shimoda LA, Neptune E, Higgins L, Murphy A, et al. Role of the TGF-beta/ALK5 signaling pathway in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med 2008;177:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atkinson C, Stewart S, Imamura T, Trembath RC, Morrell NW. Immunolocalisation of BMPR-II and TGF-SS Type I and II receptors in primary plexogenic pulmonary hypertension. J Heart Lung Transplant 2001;20:149. [DOI] [PubMed] [Google Scholar]
- 53.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res 2006;98:209–217. [DOI] [PubMed] [Google Scholar]
- 54.Sakao S, Taraseviciene-Stewart L, Wood K, Cool CD, Voelkel NF. Apoptosis of pulmonary microvascular endothelial cells stimulates vascular smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol 2006;291:L362–L368. [DOI] [PubMed] [Google Scholar]
- 55.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc 2006;3:680–686. [DOI] [PubMed] [Google Scholar]