Abstract
Prime-boost regimens are frequently used to increase the number of memory CD8+ T cells and thus the protective capacity of experimental vaccinations; however, it is currently unknown how the frequency and phenotype of primary (1°) memory CD8+ T cells impact the quantity and phenotype of secondary (2°) memory CD8+ T-cell populations. Here, we show that 2° infections of mice that received different 1° infections and/or immunizations generated similar numbers of 2° effector and memory CD8+ T cells. Remarkably, this result was independent of the numbers and phenotype of 1° memory CD8+ T cells present at the time of rechallenge. However, after adoptive transfer of low numbers of 1° memory CD8+ T cells, a linear correlation between 1° memory CD8+ T-cell input and 2° memory CD8+ T-cell numbers was observed. These data suggest that, above a very low threshold, boosting of 1° memory CD8+ T-cell populations elicits 2° immune responses of similar magnitude. Therefore, our study has important implications for the design of prime-boost regimens that aim to generate protective CD8+ T-cell-mediated immunity.
Keywords: Memory CD8+ T cells, Primary and secondary immune responses, Vaccination
Introduction
Following acute infection with cell-invading pathogens, Ag-specific CD8+ T cells expand exponentially for 5–12 days. Of these effector CD8+ T cells, only 5–10% survive the ensuing contraction phase to form a stable memory population [1, 2]. A number of studies suggest that the protective capacity of these memory CD8+ T-cell populations is dependent on both their absolute number and phenotype [3, 4]. Due to extensive research in this field, many of the parameters that impact the abundance and phenotype of 1° effector and memory CD8+ T-cell populations have been elucidated. These studies have shown that for most infections the number of 1° memory CD8+ T cells correlates with the number of 1° effectors [5]. Therefore, the generation of memory CD8+ T cells is indirectly dependent on Ag presentation, expression of co-stimulatory molecules, signal three cytokines and other parameters that control effector T-cell expansion and survival [6–8].
In addition, naïve precursor frequency and the percentage of naïve T cells that are recruited into the response impact the magnitude of 1° immune responses and the phenotypic properties of the ensuing memory CD8+ T-cell populations [5, 9–11]. Interestingly, most experimental conditions that affect effector CD8+ T-cell expansion also influence the expression of phenotypic markers on memory CD8+ T cells. As an example, effector CD8+ T cells that are exposed to systemic inflammation reach higher peak numbers and simultaneously acquire a memory CD8+ T-cell phenotype much more slowly than effectors primed under non-inflammatory conditions [12–15].
Although some of the mechanisms that increase 1° memory CD8+ T-cell numbers and shape their phenotype are known, it is unclear how these parameters can be altered in CD8+ T-cell populations that have undergone additional rounds of Ag stimulations. Increasing evidence suggests that many protective CD8+ T-cell responses require memory CD8+ T-cell numbers than can only be obtained from prime-boost regimens [3]. However, the experimental conditions that control these 2° immune responses have not been analyzed in detail. Some studies suggest that, similar to naïve CD8+ T cells, memory CD8+ T cells require co-stimulatory signals from DC for optimal expansion [16]. This assumption is supported by studies that show an essential role for the co-stimulatory molecules CD27, 4-1BB and OX40 in the induction of 2° immune responses [17]. In contrast, Ag curtailment seems to affect memory CD8+ T cells less than memory CD4 and naïve CD8+ T cells [18]. The fact that 1° and 2° effector CD8+ T differ in their proliferative capacity [19] and their sensitivity to apoptosis [20, 21] suggests that these two populations could respond differently to the proliferative stimuli and the pro- and anti-apoptotic signals they receive following an infection.
Compared with 1° immune responses, the study of 2° immune responses adds an additional level of complexity since 2° effector/memory CD8+ T cells originate from 1° memory CD8+ T-cell populations that are much more heterogeneous than naïve CD8+ T cells. This heterogeneity is dependent on the 1° immunization/infection and includes differences in both 1° memory CD8+ T-cell numbers and phenotype. Whether this heterogeneity can be harnessed to generate 2° memory T cells of a desired quantity and quality is currently unknown.
In this study, we generated 1° memory T-cell populations using different immunization and infection protocols. These 1° memory CD8+ T cells were then re-challenged in situ and the fate of the ensuing 2° memory CD8+ T-cell populations was analyzed. To specifically address how differences in 1° memory CD8+ T-cell populations impact 2° immune responses and to limit the number of experimental variables, all groups were challenged with Listeria monocytogenes, a pathogen that has been used in preclinical [22, 23] and clinical [24] trials. We show that above a very low threshold, booster infections generate similar quantities of 2° memory CD8+ T cells independent of 1° memory CD8+ T cell frequency and phenotype. These boosted T-cell populations display a nearly homogenous phenotype even when derived from heterogeneous 1° memory CD8+ T cells. Our results provide new insights into the regulation of 2° memory CD8+ T-cell quantity and quality and have important implications for the design of vaccination regimens.
Results
Kinetics of 2° CD8+ T-cell responses is independent of 1° memory CD8+ T-cell phenotype
1° memory CD8+ T-cell numbers and phenotype are shaped by the environmental conditions that naïve CD8+ T cells encounter during the first days after priming [8, 25, 26]. To selectively alter the phenotype of 1° memory CD8+ T cells and study the impact of phenotypic differences on 2° immune responses in situ, we elicited 1° immune responses under low or high inflammatory conditions.
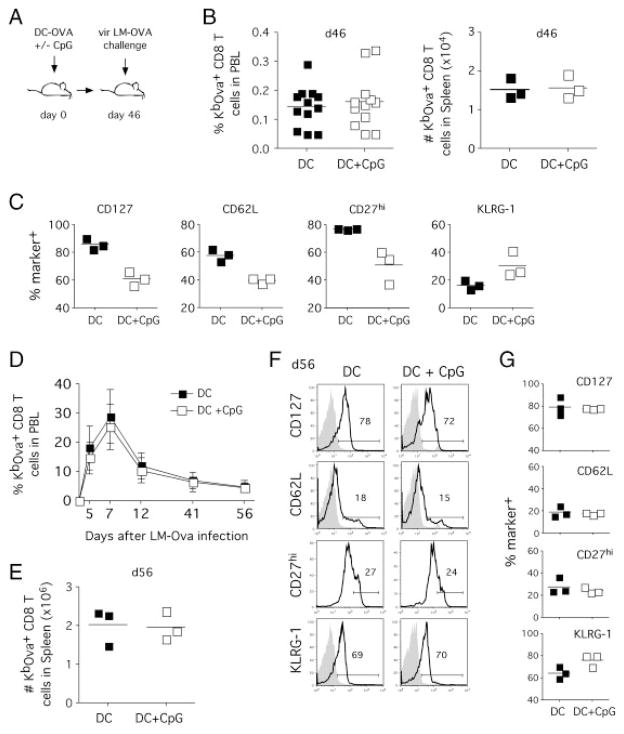
Mature splenic DC were pulsed with Kb-restricted SIINFEKL peptide from OVA and injected into naïve C57BL/6 mice to stimulate a CD8+ T-cell response under low inflammatory conditions [13, 27]. A second group of mice received the same number of Ag-pulsed DC and a single intraperitoneal injection of the TLR9 agonist CpG [28] to induce systemic inflammation (Fig. 1A). Consistent with the previous reports [12, 13], co-injection of CpG led to an increase in the number of OVA-specific CD8+ T cells during the effector phase (data not shown). After the ensuing contraction phase, both groups formed memory CD8+ T-cell populations with similar frequencies of OVA-specific endogenous memory CD8+ T cells in peripheral blood (Fig. 1B, left panel). Similarly, spleens of mice from both groups contained equal absolute numbers of memory CD8+ T cells (Fig. 1B, right panel). In contrast to these similar numbers, the phenotype of the memory CD8+ T cells in the two groups differed for numerous markers (CD127, CD27, CD62L, KLRG-1; Fig. 1C). Compared with mice receiving DC only, CpG injection delayed the rate of memory phenotype acquisition as described previously [12, 13, 29] and induced the expression of killer-cell lectin-like receptor KLRG. Thus, DC priming with or without injection of adjuvants generates 1° memory CD8+ T-cell populations of similar quantity but different phenotype.
Figure 1.
1° memory CD8+ T cells of different phenotype induce similar 2° immune responses. (A) Experimental setup. Mice were injected with Ova257–264-coated DC with or without co-injection of CpG (1826 ODN). In total, 46 days after DC immunization, both groups were challenged with vir LM-OVA (1 × 105 CFU). (B) Frequency of endogenous OVA-specific CD8+ T cells among total PBL (left panel, n = 12) and total numbers of endogenous OVA-specific CD8+ T cells in the spleen (right panel, n = 3) 46 days after immunization. (C) Phenotype of 1° memory CD8+ T cells was analyzed in the spleen 46 days after infection. Numbers show the percentage of marker-positive OVA-specific CD8+ T cells for three individual mice and the mean for each group. (D) Kinetics of 2° OVA-specific CD8+ T-cell responses in PBL after vir LM-OVA challenge. Data show mean ± SD for each group (n = 12). (E) Total numbers of OVA-specific CD8+ T cells in the spleen (day 56 after booster infection). Data show numbers for three individual mice and the mean for each group. (F) Analysis of 2° memory CD8+ T-cell phenotype in the spleen. Histograms show representative marker expression in individual mice compared with isotype controls (shaded histograms). (G) Frequency of marker-positive OVA-specific CD8+ T cells in individual mice and the mean for a group of three mice. Data are representative of two independent experiments.
To test how the differences in 1° memory phenotype affected the 2° immune response both groups were challenged with virulent L. monocytogenes expressing the OVA peptide SIINFEKL (vir LM-OVA). LM-OVA infection resulted in rapid expansion of OVA-specific memory CD8+ T cells. Despite the differences in phenotype, longitudinal analyses in peripheral blood showed similar kinetics for both groups (Fig. 1D). The equal frequencies of effector CD8+ T cells in both groups were maintained during expansion and contraction phase and resulted in similar frequencies (data not shown) and absolute numbers (Fig. 1E) of 2° memory CD8+ T cells in the spleen. Strikingly, the phenotypic differences of the 1° memory CD8+ T cells were not preserved in the ensuing 2° memory CD8+ T-cell populations (Fig. 1F and G). Thus, in situ LM-OVA booster infections of 1° memory CD8+ T-cell populations with different phenotype generate similar absolute numbers of 2° effector and memory CD8+ T cells. Similarly, the phenotype of 2° memory CD8+ T-cell population is independent of the phenotype of the 1° memory CD8+ T-cell population they derive from.
Kinetics of 2° memory CD8+ T-cell responses is independent of 1° memory CD8+ T-cell numbers
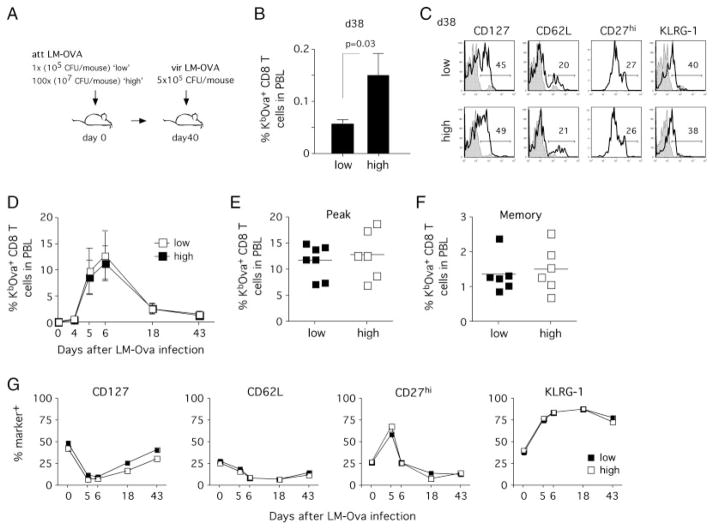
To analyze the influence of 1° memory CD8+ T-cell numbers on 2° effector/memory CD8+ T cells, we next sought to generate different numbers of 1° memory CD8+ T cells with similar phenotype. Since the use of different pathogens and the manipulation of systemic inflammation affect both memory T-cell numbers and phenotype [13], we chose to alter the dose of infection while using a single pathogen. Mice were infected with doses of att LM-OVA that differed by 100-fold (“high” dose: 107 CFU/mouse and “low” dose: 105 CFU/mouse, Fig. 2A). The difference in the infectious dose resulted in higher CD8+ T-cell numbers in the “high” group at the effector stage (data not shown) and a threefold increase in the frequency of 1° memory CD8+ T cells in peripheral blood (Fig. 2B). Lowering the infectious dose to 103 CFU/mouse did not result in further decreases in the frequency of 1° memory CD8+ T cells (data not shown). Despite the differences in numbers, the phenotype of both groups (CD127, CD27, CD62L and KLRG-1 expression) was similar at the memory stage (Fig. 2C).
Figure 2.
Altering the dose of the 1° infection results in minor differences in the numbers of 2° memory CD8+ T cells after re-challenge. (A) Experimental setup. Groups of mice were infected with 1 × 105 (low dose; 1 ×) or 1 × 107 (high dose; 100 ×) CFU of att LM-OVA and re-challenged with vir LM-OVA 40 days later (5 × 105 CFU/mouse). (B) Percentages of endogenous OVA-specific CD8+ T cells in total PBL on day 38 after 1° infection. Data show mean ± SD for each group (n = 8). (C) Phenotype of 1° memory CD8+ T cells in peripheral blood samples. Open histograms show representative examples of individual mice and shaded histograms represent isotype controls. (D) 2° immune responses of OVA-specific CD8+ T cells in PBL. Mean ± SD for each group are shown. Frequency of OVA-specific (E) 2° effectors (day 6) and (F) 2° memory (day 43). Data show numbers for individual mice and the mean for each group. (G) Phenotype of OVA-specific 2° effector/memory CD8+ T cells in pooled PBL samples. Data are representative of two independent experiments.
Both groups of mice were then challenged with vir LM-OVA and CD8+ T-cell kinetics were analyzed in peripheral blood. Endogenous OVA-specific CD8+ T cells expanded vigorously in the “high” and “low” group (Fig. 2D). During the effector phase, peak numbers did not differ between the groups (Fig. 2E) and at the memory stage percentages of 2° memory CD8+ T cells were similar in peripheral blood (Fig. 2F). Again the phenotype of 2° memory CD8+ T cells did not reveal any differences between mice infected with the “high” versus the “low” dose (Fig. 2G).
These results demonstrate that, under experimental conditions used here, changes in the LM infectious dose alter 1° memory CD8+ T cells numbers but not phenotype. However, these differences in the absolute numbers of 1° memory CD8+ T cells seem to be insufficient to impact 2° memory CD8+ T-cell numbers and phenotype after secondary infection.
A linear correlation between 1° and 2° memory CD8+ T-cell numbers in adoptive transfer model
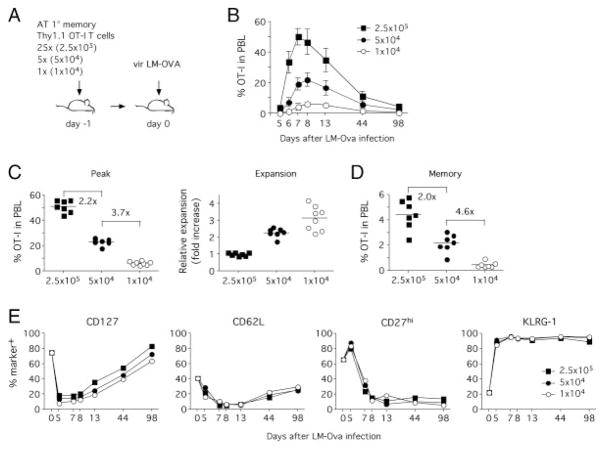
To increase the differences in 1° memory CD8+ T-cell numbers, we employed an adoptive transfer system of OVA-specific TCR transgenic OT-I CD8+ T cells. In contrast to endogenous immune responses, the use of OT-I T cells facilitates the transfer of different numbers of memory CD8+ T cells with identical phenotype. In order to generate 1° memory OT-I T cells, naïve mice were seeded with 1 × 103 Thy1.1 OT-I T cells and infected with vaccinia virus expressing the SIINFEKL peptide (VacV-OVA). VacV-Ova primary infection was used to ensure that known and defined numbers of 1° memory CD8+ T cells are transferred before secondary LM-OVA infection. In total, 40 days after infection, OT-I T cells were isolated by positive selection for Thy1.1 (>70% purity) and 2.5 × 105, 5 × 104 or 1 × 104 1° memory OT-I T cells were transferred into naïve hosts prior to infection with vir LM-OVA (Fig. 3A). As described earlier [30, 31], only a fraction of these transferred cells (approximately 5–10%) survive the adoptive transfer and contribute to subsequent immune responses.
Figure 3.
Adoptive transfer of different numbers of 1° OT-I T cells generates different numbers of 2° memory CD8+ T cells after re-challenge. (A) Experimental setup. Different numbers of 1° memory OT-I T cells (2.5 × 105, 5 × 104 or 1 × 104) from VacV-OVA immune mice were adoptively transferred into naïve hosts (n = 8/group) and mice were challenged with vir LM-OVA. (B) Kinetics of 2° OT-I T cell responses in PBL. Graphs show percentages of OT-I T cells in total PBL (mean ± SD, n = 8). (C) Peak expansion of OT-I T cells in individual mice and mean percentage for each group (left panel). Numbers show fold differences in mean OT-I frequency between individual groups. Relative expansion (right panel) was calculated by normalizing peak expansion of OT-I T cells for input numbers with the expansion in the group receiving 2.5 × 105 1° memory OT-I T cells representing a value of 1. Results from individual mice and mean for each group are shown. (D) 2° memory OT-I frequency in total PBL (individual mice and mean for each group shown). Numbers show fold differences in mean OT-I frequency between individual groups. (E) Phenotype of OT-I T cells in pooled PBL samples. Data are representative of two independent experiments.
Interestingly, CD8+ T-cell kinetics differed significantly between the three groups (Fig. 3B). At the peak of the effector phase, the initial fivefold differences between the groups had dropped to 2.2-fold and 3.7-fold (Fig. 3C). Thus 1° memory OT-I T cells that were transferred at lower numbers had expanded more than those transferred in higher numbers (Fig. 3C) but not enough to reach similar numbers of effector CD8+ T cells. As a consequence, 2° memory CD8+ T-cell numbers differed significantly between the three groups (Fig. 3D). Of interest, 2° memory OT-I frequency between groups receiving 2.5 × 105 and 5 × 104 differed twofold, whereas the difference in OT-I frequency between groups receiving 5 × 104 and 1 × 104 reflected the fivefold difference in input numbers. As in the previous experiments, the phenotype in the three groups was similar for all phenotypic markers tested (Fig. 3E).
Therefore, the quantity of 1° memory CD8+ T cells correlates with the number of subsequently generated 2° memory CD8+ T cells when low numbers of 1° memory CD8+ T cells are present. Corrected for a 10% “take” of the adoptive transfers, our data suggest that below a threshold of 2.5 × 104 1° memory OT-I T cells, 2° memory CD8+ T-cell numbers start to correlate with the number of adoptively transferred 1° memory OT-I T cells. When primary memory OT-I T-cell numbers drop below 5 × 103, this correlation between 1° and 2° memory OT-I T-cell numbers follows a linear pattern. However, the phenotype (CD127, CD27, CD62L and KLRG-1 expression) of the 2° memory CD8+ T-cell population remains unaffected by these numerical differences.
Magnitude of 2° CD8+ T-cell responses in situ
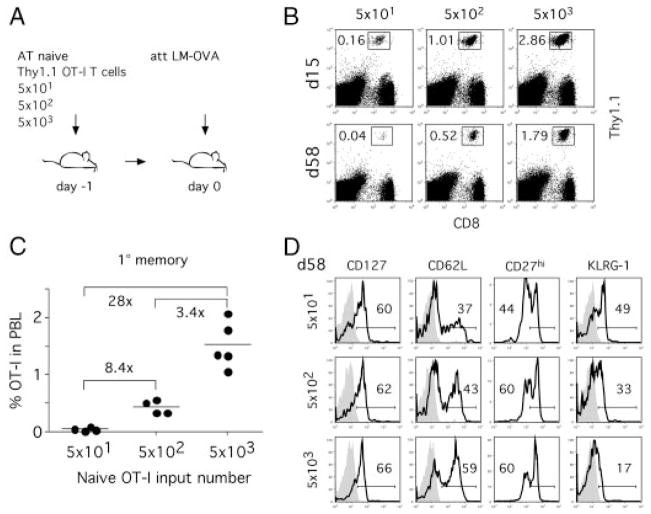
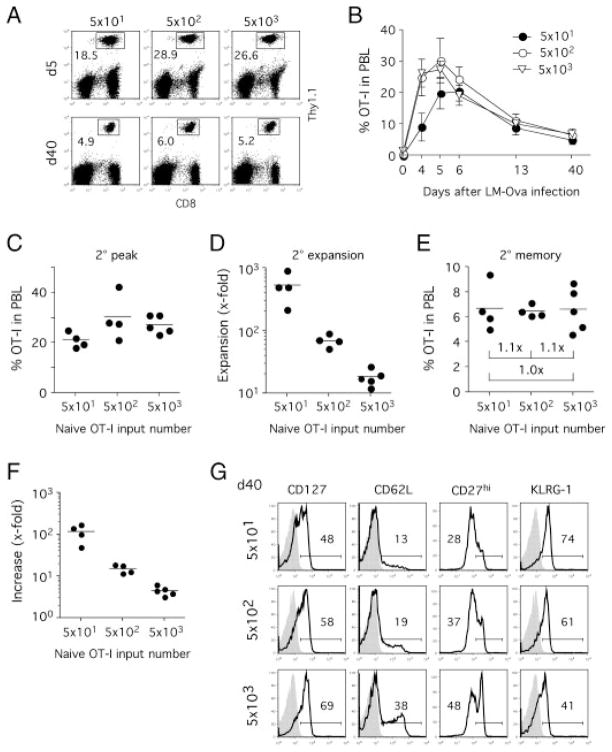
Since only low numbers of memory CD8+ T cells survive the adoptive transfer into naïve hosts, it remains unknown whether adoptive transfer experiments adequately reflect the abundance of memory CD8+ T-cell populations in natural infections. To study the influence of 1° memory CD8+ T-cell numbers on 2° immune responses in an experimental setup that mimics the abundance of 1° memory CD8+ T cells in situ, we generated 1° memory CD8+ T cells with adoptive transfers of different numbers of naïve OT-I T cells prior to att LM-OVA infection (Fig. 4A). Increases in naïve precursor frequency have been shown to increase 1° memory T-cell numbers, alter T-cell phenotype and even memory lineage commitment [5, 32]. Three different groups with low numbers of transferred OT-I T cells (5 × 101, 5 × 102 and 5 × 103) were chosen to stay within the range of natural CD8+ T-cell precursor frequency [11, 30, 33].
Figure 4.
Naïve CD8+ T-cell precursor frequency influences the quantity and phenotype of 1° memory CD8+ T cells after infection. (A) Experimental setup. Different numbers of naïve Thy1.1 OT-I T cells were adoptively transferred into naïve Thy1.2 B6 hosts (n = 5/group) prior to infection with att LM-OVA. (B) Detection of OT-I CD8+ T cells in PBL on indicated days after infection. Numbers represent frequency of OT-I in total PBL. (C) Frequency of 1° memory OT-I T cells in PBL 58 days after infection. Numbers show fold differences in mean OT-I frequency between individual groups. (D) Phenotype of 1° memory OT-I T cells. Histograms show phenotype in pooled PBL samples for each group and isotype controls (shaded histograms). This experiment was repeated once with similar results. Data are representative of two independent experiments.
In accordance with the previous results, high numbers of adoptively transferred naïve precursors resulted in higher abundance of effector and memory OT-I T cells [5] (Fig. 4B). 1° memory CD8+ T-cell numbers differed significantly between the three groups and reached a 28-fold difference between the two groups that received 5 × 101 or 5 × 103 naïve OT-I T cells, respectively (Fig. 4C). In addition, mice seeded with higher numbers of naïve OT-I T cells had higher expression of CD62L and lower expression of KLRG-1, whereas expression of CD127 and CD27 was similar in all groups (Fig. 4D). Therefore, altering the input number of naïve T cells modulates 1° memory CD8+ T-cell numbers and changes the expression of some but not all phenotypic markers when low numbers of T cells are transferred.
Briefly, 60 days after 1° infection, all groups were challenged with vir LM-OVA. At day 5 after the second challenge, all OT-I T cell groups had expanded vigorously in peripheral blood (Fig. 5A). In contrast to the 1° immune response, expansion kinetics were similar between the three groups (Fig. 5B). The group initially seeded with 5 × 101 OT-I T cells peaked 1 day later and at only slightly lower numbers than the other two groups (Fig. 5C). When the numbers of all OVA-specific CD8+ T cells (endogenous and TCR-Tg OT-I CD8+ T cells) were assessed by tetramer staining, the kinetics for all three groups were nearly identical (data not shown). The similar peak numbers could be explained by a much higher expansion in the group receiving 5 × 101 OT-I T cells compared with those initially seeded with 5 × 102 and 5 × 103 OT-I T cells (Fig. 5D). Consequently, all three groups contained identical numbers of 2° memory OT-I T cells at the memory stage (day 40; Fig. 5E). Comparison of 2° with 1° memory CD8+ T-cell numbers revealed that memory T-cell numbers were boosted more than 100-fold in the group initially seeded with 5 × 101 naïve OT-I T cells but only fourfold in the group seeded with 5 × 103 naïve OT-I T cells (Fig. 5F). 2° memory CD8+ T-cell phenotype reflected the differences seen in 1° memory CD8+ T cells with higher expression of CD127, CD62L and CD27 but lower expression of KLRG-1 in the groups with high 1° memory CD8+ T-cell numbers (Fig. 5G).
Figure 5.
Magnitude of 2° CD8+ T-cell responses is independent of 1° memory CD8+ T-cell frequency and phenotype. (A) LM-OVA immune mice (Fig. 4) were challenged with vir LM-OVA and 2° CD8+ T-cell responses were monitored in PBL. Detection of OT-I CD8+ T cells in PBL on indicated days after 2° infection. Numbers represent frequency of OT-I in total PBL. (B) Kinetics of the 2° OT-I T cell responses in PBL. Data show mean ± SD for each group. (C) Peak frequency of 2° OT-I CD8+ T cells in PBL. (D) The fold increase in OT-I T cell numbers was calculated by comparing OT-I frequency in PBL before (1° memory) and at the peak of the 2° immune response. (E) Frequency of 2° memory OT-I T cells in PBL 40 days after vir LM-OVA infection. Numbers represent the fold difference in 2° memory OT-I T cells numbers between groups. (F) The fold increases in memory OT-I frequency were calculated by comparing 1° and 2° memory OT-I frequency in PBL for individual mice. (G) 2° memory OT-I phenotype in pooled PBL samples 40 days after re-challenge. Shaded histograms represent isotype controls. Data are representative of two independent experiments.
These results demonstrate that when studied in situ and not in an adoptive transfer system, 2° memory CD8+ T-cell numbers are independent of 1° memory T-cell numbers and phenotype. Our data also suggest a limit for the number of 2° effector and memory CD8+ T cells for a given booster infection. Interestingly, at the higher absolute number of 1° memory CD8+ T cells, 2° memory CD8+ T cells seem to preserve the phenotypic differences of the 1° memory CD8+ T-cell populations.
Quality of 2° CD8+ T-cell responses in situ
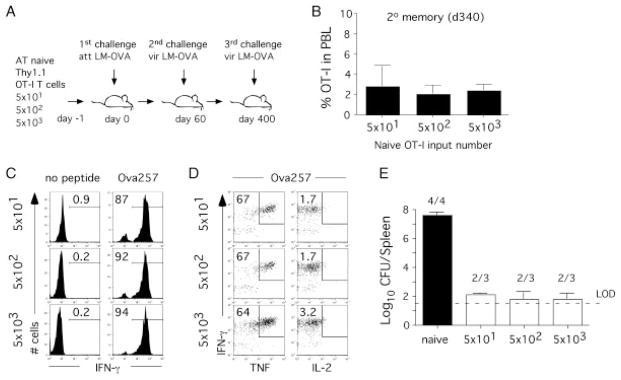
The results shown in Fig. 4 and 5 show that when analyzed in situ 2° memory CD8+ T-cell numbers detected relatively early after booster challenge (day 40) are independent of 1° memory CD8+ T-cell numbers and phenotype. In order to address the long-term maintenance of 2° memory CD8+ T cells and their function (ability to produce cytokines and provide protection), the same groups of mice were analyzed 1 year after secondary challenge (Fig. 6A). Importantly, indistinguishable frequencies of 2° memory OT-I CD8+ T cells were detected in the blood of all groups of mice 340 days after 2° infection, suggesting that the maintenance of 2° memory CD8+ T-cell pool is independent of numbers and phenotype of 1° memory CD8+ T cells (Fig. 6B). In addition, the same frequency of 2° memory CD8+ T cells produces IFN-γ and TNF after short ex vivo peptide stimulation (Fig. 6C and D). As previously described for 2° memory CD8+ T-cell responses [19, 34] and despite their rapid Ag-specific IFN-γ and TNF production, 2° OT-I memory CD8+ T cells were not able to produce IL-2 (Fig. 6D). Thus, the ability of 2° memory CD8+ T cells to produce effector CD8+ T-cell cytokines is similar in all groups analyzed.
Figure 6.
The quality of 2° CD8+ T-cell responses is independent of 1° memory CD8+ T-cell frequency and phenotype. (A) Experimental design. Indicated numbers of naïve Thy1.1 OT-I T cells were adoptively transferred into naïve Thy1.2 B6 hosts prior to infection with att LM-OVA. At day 60 post 1° infection all groups of mice were challenged with vir LM-OVA. A year after 2° challenge, all group of mice were challenged for the third time with high dose of vir LM-OVA (5 × 105 CFU/mouse). (B) Frequency of 2° memory OT-I T cells (mean+SD; three to four mice per group) in PBL 340 days after 2° infection. (C, D) Peptide-stimulated intracellular cytokine staining of OT-I CD8+ T cells from blood. Numbers represent the percentage of OT-I CD8+ T cells that stain positive for a given cytokine combination. Representative staining is shown. (E) On day 3 post 3° LM challenge bacterial numbers were determined in the spleen. Numbers represent the number of mice that had detectable bacteria. LOD represents the limit of detection. Similar data were also obtained in the liver.
In order to confirm that the quality of 2° CD8+ T-cell responses in situ is independent of numbers and phenotype of 1° memory CD8+ T cells, all groups of mice were infected for the third time with high dose of vir LM-OVA (5 × 105 CFU/mouse). On day 3 p.i., bacterial clearance was determined in the spleen (Fig. 6E) and liver (data not shown). As expected, all immune mice showed increased levels of protection when compared with control (naïve) group of mice. Importantly, the degree of protection was similar in all groups of mice that contained 2° OT-I memory CD8+ T cells (Fig. 6A), suggesting that in situ function of 2° memory CD8+ T-cell responses is not influenced by 1° memory CD8+ T-cell numbers and phenotype.
Discussion
Although CD8+ T cells play an important role in the immune response to acute and chronic infections, no current vaccination mediates protection primarily by a CD8+ T-cell-mediated immune response. One reason for this lack of clinical success in CD8+ T-cell vaccinations is that, in contrast to B-cell-mediated antibody responses, the experimental conditions that best generate protective numbers of memory CD8+ T cells are not well defined. This represents an important limitation for the development of CD8+ T-cell-based vaccines since a number of studies suggest that a single immunization is often not sufficient to reach protective CD8+ T-cell thresholds [3]. These studies suggest that prime-boost regimens and therefore 2° memory CD8+ T cells will be required for long-lasting protection [35].
Our study shows that for a variety of different 1° infections and immunizations, similar numbers of 2° effector and memory CD8+ T cells can be generated using a strong booster infection. Unexpectedly, this process was largely independent of the number and phenotype of 1° memory CD8+ T cells. Thus our results suggest that 1° infections/immunizations (e.g. DNA vaccinations, DC immunizations, injections of Ag carrier beads) that generate memory CD8+ T-cell populations with different absolute numbers and phenotype could induce similar numbers of 2° memory CD8+ T cells when followed by a strong booster infection. Why the expansion of 2° effector CD8+ T cells is independent of 1° memory CD8+ T-cell phenotype remains unclear. Interestingly, we have analyzed the proliferative potential of memory OT-I T-cell subsets and these assays confirm the previously published reports that central memory CD8+ T cells (CD62Lhigh) expand more vigorously than effector memory CD8+ T cells (CD62Llow) following LM-OVA challenge (data not shown and [4]). One possible explanation for the similar 2° expansion is that the absolute number of 1° memory CD8+ T cells in our experiments was high enough to achieve similar 2° expansion peaks regardless of the quantity of central memory CD8+ T cells. This assumption would predict that the differential proliferative capacities of memory CD8+ T-cell subpopulations matter most when low numbers of 1° memory T cells (adoptively transferred or endogenous) are present in a host.
The fact that booster infections of heterogeneous primary memory CD8+ T-cell populations evoke similar secondary expansion peaks suggests the presence of regulatory mechanisms that control the magnitude of 2° responses [36]. One of the parameters that most likely has an important effect on 2° expansion is Ag abundance. In the initial days after the booster immunization, high numbers of 1° memory CD8+ T cells could compete with naïve T cells and other memory CD8+ T cells for peptide–MHC complexes [37] and co-stimulatory molecules expressed on DC. Similarly, memory CD8+ T cells could encounter and kill Ag-laden DC [38] and therefore curtail Ag presentation and inhibit their own expansion [39]. In support of this, activated memory CD8+ T cells have been shown to enter reactive lymph nodes and eliminate DC early after infection [40]. In addition to competition for Ag presented on DC, early clearance of pathogens by memory CD8+ T cells could lead to lower systemic inflammation and therefore to decreased effector CD8+ T-cell expansion and memory levels. Interestingly, we have observed higher Ag loads early after booster infection in groups of mice with low numbers of 1° memory CD8+ T cells (our unpublished data) which could indicate that higher Ag abundance leads to sustained T-cell expansion when low numbers of 1° memory CD8+ T cells are present. With this regard, it will be interesting to investigate the role of effector memory CD8+ T cells and their impact on Ag abundance since this cytolytic memory CD8+ T-cell subset could potentially regulate Ag abundance and thus influence secondary memory CD8+ T-cell expansion.
Our experiments with low numbers of adoptively transferred memory CD8+ T cells show that the concept that similar numbers of 2° memory CD8+ T cells can be derived from heterogeneous 1° memory CD8+ T-cell populations has important limitations. Below a certain threshold, 2° memory CD8+ T-cell numbers correlate linearly with 1° memory CD8+ T-cell input numbers. This effect becomes most apparent when 1° memory CD8+ T-cell populations (corrected for the “take” of the adoptive transfer experiments) consist of 5 × 103 or less memory CD8+ T cells. The fact that these 1° memory CD8+ T-cell populations are reduced in their peak expansion after re-challenge could indicate that their proliferative capacity is not sufficient to reach maximum peak numbers. In support of this, comparison of our data with the published kinetics for naïve OT-I T cells [5] show that low numbers of adoptively transferred memory OT-I T cells expand less than the same number of naïve OT-I T cells. Another study has shown reduced expansion of 1° memory CD8+ T cells compared with naïve T cells after heterologous infections [19]. Thus, it seems that 1° memory OT-I T-cell populations bigger than 5 × 103 cells are needed to compensate for this reduced expansion and reach the maximum numbers of 2° effector and memory CD8+ T cells.
In most of our experiments, 2° memory CD8+ T-cell phenotype was independent of 1° memory CD8+ T-cell phenotype and numbers. This was most evident when the numbers of 1° memory CD8+ T cells were low and when 2° effector/memory CD8+ T cells had undergone multiple divisions. In fact, the only differences in 2° memory CD8+ T-cell phenotype were observed when very high numbers of 1° memory CD8+ T cells were present which expanded less than fourfold after booster infection. These findings could indicate that the phenotype of 2° memory CD8+ T-cell populations that are derived from heterogeneous 1° memory CD8+ T-cell populations converges once the cells have undergone a certain number of divisions. Alternatively, high numbers of 1° memory CD8+ T cells could lead to partial recruitment of memory T cells into the response and subsequently to a mixture of 1° and 2° memory CD8+ T cells. Therefore, high numbers of 1° memory CD8+ T cells might be required to effectively modulate 2° memory CD8+ T-cell phenotype.
It is important to notice that our study used a single booster infection and only minor variations in the infectious dose to reduce the number of variables that could influence the 2° immune responses. We chose virulent L. monocytogenes as an example for a strong booster infection but we have obtained similar results using actA- attenuated LM-OVA as a boosting agent. It is possible that the use of different booster infections or changes in the infectious dose could modulate 2° memory CD8+ T-cell phenotype. In fact, a recent study suggests that the use of different booster infections alters the expression of at least some phenotypic markers [41], indicating that the choice of the booster agent is critical for the phenotype of 2° memory CD8+ T cells.
In conclusion, our study provides new insights into the mechanisms that control abundance, phenotype and function of 2° memory CD8+ T cells. Our findings have important implications for the design of 1° and 2° infections/immunizations. For example, our data predict that the use of adjuvants to induce large 1° immune responses might not be needed when this 1° immunization is followed by a strong booster infection. The goal of the initial infection/immunization should be to generate 1° CD8+ T-cell numbers that exceed the threshold below which 1° memory CD8+ T-cell frequency correlates with 2° memory CD8+ T-cell numbers. Further increases in the number of 1° memory CD8+ T-cell numbers would not be of additional benefit to prime-boost regimens since they do not result in higher numbers of 2° memory CD8+ T cells. The modulation of 2° memory CD8+ T-cell phenotype and memory lineage commitment, however, would require substantial numbers of 1° memory CD8+ T cells or the choice of booster infections other than L. monocytogenes. Thus, the results of our study can be used to tailor prime-boost regimens that generate 2° memory CD8+ T-cell populations of the desired quality and quantity.
Materials and methods
Mice, bacteria and viruses
In total, 6–8 wk old C57BL/6 (Thy1.2/1.2 ) mice were obtained from the US National Cancer Institute. TCR-transgenic OT-I Thy1.1 mice were described previously [42]. Mice were bred and maintained in the animal facilities of the University of Iowa at the appropriate biosafety levels. Vaccinia virus expressing OVA (VacV-OVA) was grown and injected as described previously [43]. Attenuated actA-deficient L. monocytogenes expressing OVA (att LM-OVA) and virulent L. monocytogenes (vir LM-OVA) expressing OVA were grown and quantified as described earlier [44]. CFU per spleen or gram of the liver was determined at indicated days after infection as described earlier [27]. Limit of detection was 30 CFU/organ. All animal experiments followed approved Institutional Animal Care and Use Committee (ACURF) protocols.
DC immunizations
Splenic DC (DC) were isolated after subcutaneous injection of C57BL/6 mice with 5 × 106 B16 cells expressing Flt3L as described previously [13]. When tumors were palpable (5 mm × 5 mm), mice were injected with 2 mg LPS (Sigma) i.v. to mature the DC. Spleens were harvested 16 h later and were digested with DNase and Collagenase for 20 min at 37°C/7% CO2 with shaking (120 RPM). Spleen pieces were smashed through a nylon cell strainer (70 μm) to generate a single-cell suspension, RBC were lysed and splenocytes were resuspended in two parts of 10% FBS RPMI-1640 to one part B16-Flt3L conditioned medium+recombinant GM-CSF (1000 m/mL)+2 mM Ova257–264 and incubated 2 h at 37°C/5%CO2 with shaking (100 RPM). Spleen cells were washed three times and CD11c+ cells were isolated using anti-CD11c microbeads (Miltenyi Biotec). The purity and activation status of DC were determined by staining for CD11c, CD86 and MHC class II. Routinely, greater than 90% pure CD11c+ DC were obtained and the yield was approximately 15–20 × 106 DC per mouse. DC were resuspended in saline and injected i.v. To induce systemic inflammation, mice immunized with DC received a single intraperitoneal injection of 200 μg CpG ODN 1826 on the same day.
Antibodies and peptides
For FACS analysis, the following antibodies were used with appropriate combinations of fluorochromes: Thy1.1 (OX-7) and CD62L (MEL-14, both BD Pharmingen), CD8 (53-6.7), CD127 (A7R34), IFN-γ (XMG1.2), TNF (MP6-XT22), IL-2 (JES6-5HG), CD27 (LG.7F9, all Ebioscience), KLRG-1 (2F1, Southern Biotech) and appropriate isotype controls. Ova257–264 epitope was used as described previously [5].
Adoptive transfer of OT-I cells
For adoptive transfer of naïve CD8+ T cells, Thy1.1 OT-I T cells from peripheral blood or from spleen were injected i.v. into naïve Thy1.2 C57BL/6 mice. To generate memory OT-I T cells for adoptive transfer experiments, 1 × 103 naïve Thy1.1 OT-I T cells were transferred into Thy1.2 recipients and mice were immunized with 3 × 106 pfu VacV-OVA by intraperitoneal injection. About 40–60 days after infection, memory OT-I T cells were isolated by positive selection for Thy1.1 to achieve > 70% purity and 2.5 × 105, 5 × 104 or 1 × 104 memory OT-I T cells were injected i.v. into naïve recipients. Mice were immunized 24 h after the adoptive transfer by i.v. injection of 1 × 105 or 5 × 105 CFU (~1.0 and 5.0 LD50) virulent LM-OVA.
Quantification of CD8+ T-cell responses and intracellular cytokine staining
For experiments involving the adoptive transfer of OT-I T cells, immune responses in the blood or spleen were analyzed by FACS analysis for Thy1.1 transgenic OT-I T cells. For quantification of endogenous CD8+ T-cell responses, OVA-tetramers (specific for the SIINFEKL peptide of OVA) were generated by conjugation of biotinylated monomers to streptavidin-APC. Tetramer staining was performed according to the standard protocols as described previously [45]. The ability of memory OT-I CD8+ T-cell populations to produce cytokines (IFN-γ, TNF and IL-2) was determined by peptide (Ova257–264) stimulated intracellular cytokine staining as described previously [46].
Statistical analysis
Statistical significance was assessed using the two-tailed t-test with a confidence interval of > 95%. Data are presented as mean ± SD unless otherwise stated in the figures. All experiments were repeated at least once to ensure reproducibility of the results.
Acknowledgments
This study was supported by start-up funds from the Department of Pathology, University of Iowa (V. P. B.), NIH grants AI83286 (V. P. B.), AI42767, AI46653, AI50073, AI59752 (J. T. H.) and the Deutsche Forschungsgemeinschaft (DFG) fellowship WI 3308/1-1 (T. C. W.).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 2.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci USA. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 8.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Heijst JW, Gerlach C, Swart E, Sie D, Nunes-Alves C, Kerkhoven RM, Arens R, et al. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science. 2009;325:1265–1269. doi: 10.1126/science.1175455. [DOI] [PubMed] [Google Scholar]
- 10.Wirth TC, Pham NL, Harty JT, Badovinac VP. High initial frequency of TCR-transgenic CD8 T cells alters inflammation and pathogen clearance without affecting memory T cell function. Mol Immunol. 2009;47:71–78. doi: 10.1016/j.molimm.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 13.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 18.Ravkov EV, Williams MA. The magnitude of CD4+ T cell recall responses is controlled by the duration of the secondary stimulus. J Immunol. 2009;183:2382–2389. doi: 10.4049/jimmunol.0900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 20.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 21.Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- 22.Paterson Y, Maciag PC. Listeria-based vaccines for cancer treatment. Curr Opin Mol Ther. 2005;7:454–460. [PubMed] [Google Scholar]
- 23.Shahabi V, Reyes-Reyes M, Wallecha A, Rivera S, Paterson Y, Maciag P. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57:1301–1313. doi: 10.1007/s00262-008-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 25.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 26.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 28.Wagner H. Toll meets bacterial CpG-DNA. Immunity. 2001;14:499–502. doi: 10.1016/s1074-7613(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 29.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seedhom MO, Jellison ER, Daniels KA, Welsh RM. High frequencies of virus-specific CD8+ T cell precursors. J Virol. 2009;83:12907–12916. doi: 10.1128/JVI.01722-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pewe LL, Netland JM, Heard SB, Perlman S. Very diverse CD8 T cell clonotypic responses after virus infections. J Immunol. 2004;172:3151–3156. doi: 10.4049/jimmunol.172.5.3151. [DOI] [PubMed] [Google Scholar]
- 34.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Merica R, Khoruts A, Pape KA, Reinhardt RL, Jenkins MK. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J Immunol. 2000;164:4551–4557. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 37.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 39.Cockburn IA, Chakravarty S, Overstreet MG, Garcia-Sastre A, Zavala F. Memory CD8+ T cell responses expand when antigen presentation overcomes T cell self-regulation. J Immunol. 2008;180:64–71. doi: 10.4049/jimmunol.180.1.64. [DOI] [PubMed] [Google Scholar]
- 40.Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, Stein JV, et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 41.Hovav AH, Panas MW, Osuna CE, Cayabyab MJ, Autissier P, Letvin NL. The impact of a boosting immunogen on the differentiation of secondary memory CD8+ T cells. J Virol. 2007;81:12793–12802. doi: 10.1128/JVI.01519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 43.Restifo NP, Bacik I, Irvine KR, Yewdell JW, McCabe BJ, Anderson RW, Eisenlohr LC, et al. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 44.Haring JS, Corbin GA, Harty JT. Dynamic regulation of IFN-gamma signaling in antigen-specific CD8+ T cells responding to infection. J Immunol. 2005;174:6791–6802. doi: 10.4049/jimmunol.174.11.6791. [DOI] [PubMed] [Google Scholar]
- 45.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 46.Badovinac VP, Harty JT. Intracellular staining for TNF and IFN-gamma detects different frequencies of antigen-specific CD8(+) T cells. J Immunol Methods. 2000;238:107–117. doi: 10.1016/s0022-1759(00)00153-8. [DOI] [PubMed] [Google Scholar]