Abstract
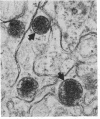
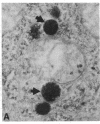
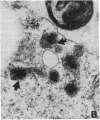
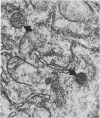
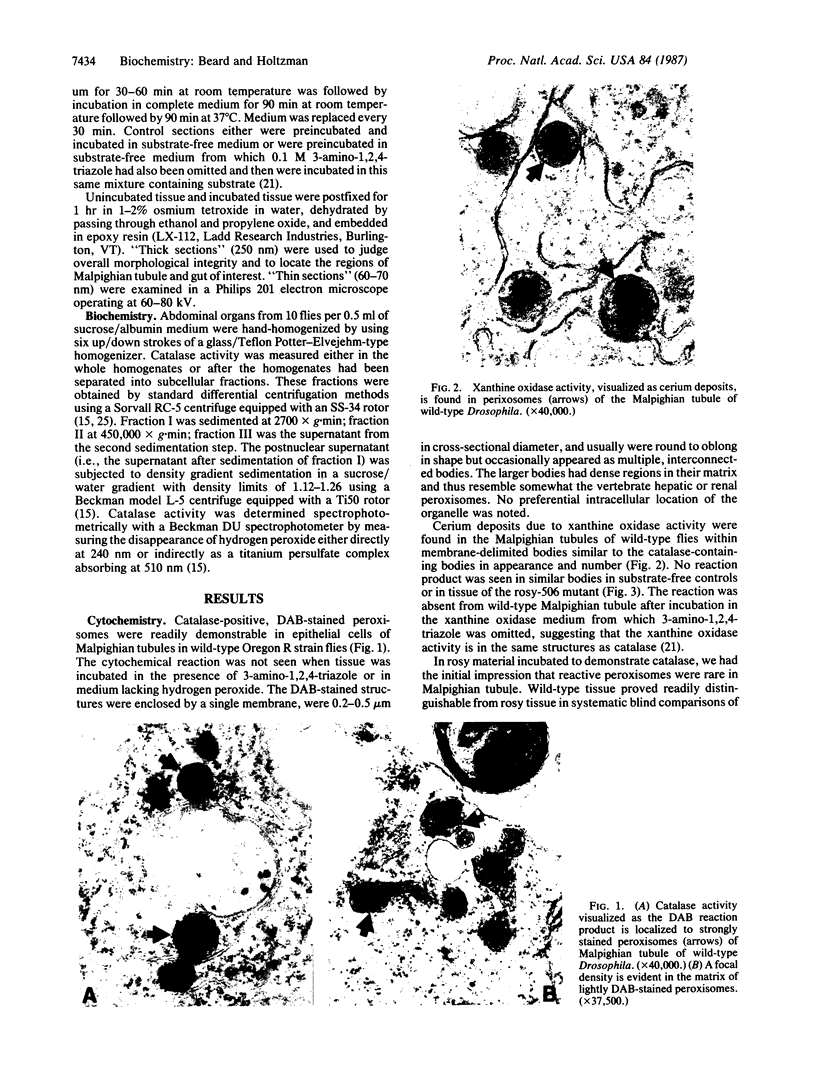
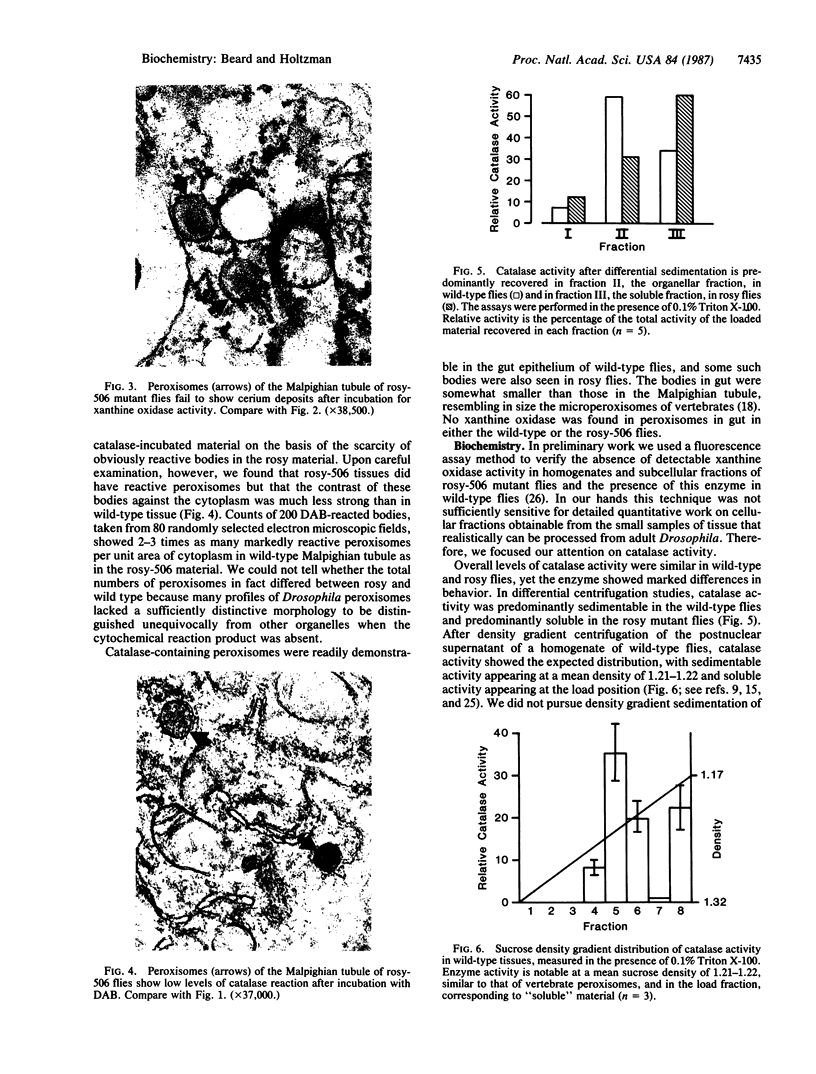
This study shows that peroxisomes are abundant in the Malpighian tubule and gut of wild-type Oregon R Drosophila melanogaster and that the peroxisomal population of the rosy-506 eye-color mutant differs from that of the wild type. Catalase activity in wild-type flies is demonstrable in bodies of appearance and centrifugal behavior comparable to the peroxisomes of vertebrate tissues. Xanthine oxidase (xanthine:oxygen oxidoreductase, EC 1.1.3.22) activity of the Malpighian tubule of wild-type flies is demonstrable cytochemically in bodies like those containing catalase. The rosy-506 mutant flies, with a deletion in the structural gene for xanthine dehydrogenase (xanthine:NAD+ oxidoreductase, EC 1.1.1.204), lack cytochemically demonstrable peroxisomal xanthine oxidase activity. In addition, peroxisomes in the rosy-506 mutants show less intense cytochemical staining for catalase than those in wild-type flies, and biochemical assays indicate that catalase in the rosy mutant is much more accessible to substrate in the absence of detergent than in the wild type. Thus, the rosy-506 mutation appears to affect peroxisomes and may mimic aspects of the defects of peroxisomes in some human metabolic disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. M., Beard M. E., Kleinbergs S. The localization of alpha-hydroxy acid oxidase in renal microbodies. J Exp Zool. 1965 Dec;160(3):329–344. doi: 10.1002/jez.1401600310. [DOI] [PubMed] [Google Scholar]
- Angermüller S., Fahimi H. D. Ultrastructural cytochemical localization of uricase in peroxisomes of rat liver. J Histochem Cytochem. 1986 Feb;34(2):159–165. doi: 10.1177/34.2.3080517. [DOI] [PubMed] [Google Scholar]
- Arnold G., Holtzman E. Ultrastructural localization of alpha-OH acid oxidase in peroxisomes with the CeCl3 technique. J Histochem Cytochem. 1980 Sep;28(9):1025–1028. doi: 10.1177/28.9.6997367. [DOI] [PubMed] [Google Scholar]
- Arnold G., Liscum L., Holtzman E. Ultrastructural localization of D-amino acid oxidase in microperoxisomes of the rat nervous system. J Histochem Cytochem. 1979 Mar;27(3):735–745. doi: 10.1177/27.3.39097. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg H. Organization of purpine degradation in the liver of a teleost (carp; Cyprinus carpio L.). A study of its subcellular distribution. Mol Cell Biochem. 1977 May 31;16(1):17–21. doi: 10.1007/BF01769834. [DOI] [PubMed] [Google Scholar]
- Hand A. R. Cytochemical detection of peroxisomal oxidases. J Histochem Cytochem. 1979 Oct;27(10):1367–1370. doi: 10.1177/27.10.92494. [DOI] [PubMed] [Google Scholar]
- Hanna C. H., Hopkins T. A., Buck J. Peroxisomes of the firefly lantern. J Ultrastruct Res. 1976 Nov;57(2):150–162. doi: 10.1016/s0022-5320(76)80105-0. [DOI] [PubMed] [Google Scholar]
- Hirai K. I. Light microscopic study of the peroxidatic activity of catalase in formaldehyde-fixed rat liver. J Histochem Cytochem. 1969 Sep;17(9):585–590. doi: 10.1177/17.9.585. [DOI] [PubMed] [Google Scholar]
- KELLER E. C., Jr, GLASSMAN E. A THIRD LOCUS (LXD) AFFECTING XANTHINE DEHYDROGENASE IN DROSOPHILA MELANOGASTER. Genetics. 1964 Apr;49:663–668. doi: 10.1093/genetics/49.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. I., Datta N. S., Dobyns W. B., Hajra A. K., Moser A. B., Noetzel M. J., Zackai E. H., Moser H. W. Neonatal adrenoleukodystrophy: new cases, biochemical studies, and differentiation from Zellweger and related peroxisomal polydystrophy syndromes. Am J Med Genet. 1986 Apr;23(4):869–901. doi: 10.1002/ajmg.1320230404. [DOI] [PubMed] [Google Scholar]
- Larsen W. J. Cell remodeling in the fat body of an insect. Tissue Cell. 1976;8(1):73–92. doi: 10.1016/0040-8166(76)90021-5. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Robbi M., Fujiki Y., Wong L. Biogenesis of peroxisomal proteins in vivo and in vitro. Ann N Y Acad Sci. 1982;386:285–300. doi: 10.1111/j.1749-6632.1982.tb21423.x. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M., McMahon J. T. The origin and fate of microbodies in the fat body of an insect. J Cell Biol. 1971 Jan;48(1):61–78. doi: 10.1083/jcb.48.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre R. J., O'Brien S. J. Interacting gene-enzyme systems in Drosophila. Annu Rev Genet. 1976;10:281–318. doi: 10.1146/annurev.ge.10.120176.001433. [DOI] [PubMed] [Google Scholar]
- Moser H. W., Goldfischer S. L. The peroxisomal disorders. Hosp Pract (Off Ed) 1985 Sep 15;20(9):61–70. doi: 10.1080/21548331.1985.11703129. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. II. A cytochemical method for light and electron microscopy. J Histochem Cytochem. 1972 Dec;20(12):1006–1023. doi: 10.1177/20.12.1006. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Romer F. Ultrastructural changes of the oenocytes of Gryllus bimaculatus DEG (Saltatoria, Insecta) during the moulting cycle. Cell Tissue Res. 1974;151(1):27–46. doi: 10.1007/BF00222032. [DOI] [PubMed] [Google Scholar]
- Samis H. V., Baird M. B., Massie H. R. Renewal of catalase activity in Drosophila following treatment with 3-amino-1,2,4-triazole. J Insect Physiol. 1972 May;18(5):991–1000. doi: 10.1016/0022-1910(72)90036-4. [DOI] [PubMed] [Google Scholar]
- Schram A. W., Goldfischer S., van Roermund C. W., Brouwer-Kelder E. M., Collins J., Hashimoto T., Heymans H. S., van den Bosch H., Schutgens R. B., Tager J. M. Human peroxisomal 3-oxoacyl-coenzyme A thiolase deficiency. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2494–2496. doi: 10.1073/pnas.84.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens R. B., Heymans H. S., Wanders R. J., van den Bosch H., Tager J. M. Peroxisomal disorders: a newly recognised group of genetic diseases. Eur J Pediatr. 1986 Feb;144(5):430–440. doi: 10.1007/BF00441734. [DOI] [PubMed] [Google Scholar]
- Scott P. J., Visentin L. P., Allen J. M. The enzymatic characteristics of peroxisomes of amphibian and avian liver and kidney. Ann N Y Acad Sci. 1969 Dec 19;168(2):244–264. doi: 10.1111/j.1749-6632.1969.tb43113.x. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969 Jul 25;244(14):3855–3863. [PubMed] [Google Scholar]
- Veenhuis M., Bonga S. D. The cytochemical demonstration of catalase and D-amino acid oxidase in the microbodies of teleost kidney cells. Histochem J. 1977 Mar;9(2):171–181. doi: 10.1007/BF01003629. [DOI] [PubMed] [Google Scholar]