Abstract
The purpose of this study was to characterize the molecular phenotype that occurs during the profound morphological shift of cultured osteogenic cells upon treatment with fibroblast growth factor-2 (FGF2). A time course of treatment with FGF2 was performed on an osteoblast cell line, primary bone marrow stromal cells and an osteocyte-like cell line. Morphologic changes were recorded, and gene profiling was carried out by real time PCR. By 8 hours of FGF2 treatment, there is a striking morphological shift of osteoblast and stromal cells to an elongated dendritic-like morphology that is remindful of osteocytes. In osteoblasts treated with FGF2, this morphologic shift is preceded by an induction of several osteocyte markers, including dentin matrix protein 1 (>20-fold) and E11 (>5-fold). There is a transient increase in the gene expression of sclerostin (3.5-fold) and PHEX (2.5-fold). Sclerostin regulation by FGF2 is complex, as gene expression becomes markedly inhibited by FGF2 at times points after 8 hours of treatment before rebounding at day 12. Analogous modulation of osteocyte markers is seen in bone marrow stromal cells and MLO-Y4 osteocyte-like cells. In conclusion, this study shows that FGF2 can regulate the transition of osteogenic cells towards the osteocyte lineage, as well as, regulate the expression of critical genes in osteocytes.
Keywords (<6): Osteoblast, Bone Marrow Stromal Cell, Osteocyte, Dentin Matrix Protein 1, Sclerostin, Connexin43
1. Introduction
Fibroblast growth factor-2 (FGF2) plays an important role in bone formation and osteoblast function (Reviewed in [1; 2]). Deletion of the FGF2 gene in mice leads to reduced bone mass as well as an impaired ability to respond to the osteoanabolic effects of PTH [3; 4; 5; 6]. In a transgenic mouse model, overexpression of the 18kDa-isoform of FGF2 in cells of the osteogenic lineage markedly enhances bone mineral density [7]. The short-term, systemic administration of FGF2 has been shown to increase bone formation in rats [8; 9]. FGF2 has been shown to restore bone mass in ovariectomized rats [10; 11]. Moreover, FGF2 accelerates fracture repair in multiple animal models [12; 13; 14]. The mechanism by which FGF2 enhances bone formation has long been hypothesized to be via the recruitment and expansion of osteoprogenitor cell populations rather than via enhancement of osteoblast differentiation. Indeed, for the most part, in vitro analysis of the effects of FGF2 on osteoblasts and osteoprogenitors has led to the conclusion that FGF2 is antagonistic to osteoblast differentiation. FGF2 has potent mitogenic effects on osteoprogenitors and markedly downregulates the expression of markers of osteoblasts, such as alkaline phosphatase, type I collagen and osteocalcin [15; 16]. However, the effects of FGF2 are stage specific, as treatment of mature osteoblasts with FGF2 can have opposing outcomes from those arising from FGF2 treatment of immature osteoblasts [17].
The terminally differentiated cell of the osteoblast lineage is the osteocyte (Reviewed in [18; 19; 20]). These cells are the most abundant cell type in bone and are found in the newly deposited osteoid or embedded in the mineralized skeleton. Osteocytes have a dendritic-like morphology and lack many of the key molecular markers of their preceding osteoblasts, including greatly reduced expression of type I collagen and alkaline phosphatase (ALP). Further, these cells are unlikely to be able to mineralize. Osteocytes are of great importance to skeletal physiology, as data continues to accumulate to implicate these cells in the mechanical and hormonal responsiveness of bone, as well as, phosphate homeostasis and the regulation of Wnt signaling. Osteocytes are thought to be vital to the orchestration of bone remodeling. However, osteocytes have proven to be difficult to study due to the fact that they are embedded in the mineralized skeleton and have low mitogenic potential. Importantly, little is known about the factors that regulate the transition of osteoblasts to osteocytes. In this study, we explore the hypothesis that FGF2 can induce an osteocyte-like phenotype in osteogenic cells, and regulate the gene expression patterns of osteocytes.
2. Materials and methods
2.1. Cell culture
MC3T3-E1 clone 4 (MC4) cells were obtained from the American Type Culture Collection (Manassas, VA). These cells were maintained in αMEM media (Cellgro, Herndo, VA), supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), penicillin (50 IU/ml) and streptomycin (50μg/ml). Mouse primary bone marrow stromal cells were prepared as described previously [21]. MLO-Y4 cells were kindly provided by Lynda Bonewald (University of Missouri, Kansas City) and were cultured as described [22]. For FGF2 treatments, cells were cultured in αMEM containing 1% fetal bovine serum, 50 μg/ml ascorbic acid and 3 mM sodium phosphate for 24 hours prior to the addition of FGF2 (10 ng/ml). FGF2 was purchased from Millipore (Temecula, CA). The vehicle diluent for FGF2 (phosphate buffered saline, 0.1% bovine serum albumin, 1mM dithiothreitol) was used as a negative control for FGF2 treatments. For long-term cultures, media with or without FGF2 were replaced every 2–3 days. Cell viability was routinely assessed as described previously [23] and was statistically unaffected among treatment groups.
2.2. Crystal violet staining and microscopy
Following the indicated treatments, cells were fixed in 4% paraformaldehyde for 15 minutes, prior to staining with crystal violet (0.1% crystal violet in 10% ethanol) for 20 minutes. Cells were observed on a Nikon Eclipse 50i microscope (Melville, NY) and images captured with a Photometrics Coolsnap CCD camera.
2.3. Proliferation assay
MC4 cells were seeded in 24 well plates at 20,000 cells/well. Cells were treated with or without FGF2 for 24 hours. Cell numbers were assessed with the Cell Counting Kit-8 assay (Dojindo, Rockville, MD) or Cell Titer-Glo assay (Promega, Madison, WI). The results of three experiments performed in triplicate wells were averaged for analysis.
2.4. Migration assay
MC4 cells (6,000 cells/well) were seeded into the upper chamber of a Corning transwell chamber insert (8.0μm pore size; Corning, NY). FGF2 (10ng/ml) containing medium was added to the lower chamber. Medium lacking FGF2 added to the bottom chamber was used for comparison of migration. The cells were allowed to migrate for 4 or 24 hours. The upper side of the transwell filter was cleaned of cells with a cotton swab. The remaining cells were stained with crystal violet, as above. The number of cells migrating to the underside of the filter was counted. The results of three experiments performed in triplicate wells were averaged together.
2.5. RNA isolation and real time PCR
RNA was extracted from cultured cells at the indicated time points, as we have previously reported [24]. Quantitation of gene expression by real time PCR was performed as described previously [25]. The primers used for PCR are listed in Table I.
TABLE I.
Mouse Real Time PCR Primers
| TARGET | FORWARD | REVERSE |
|---|---|---|
| COL1A1 | CTTCACCTACAGCACCCTTGTG | GATGACTGTCTTGCCCCAAGTT |
| ALP | CCTCCGGATCCTGACCAAA | GTCAATCCTGCCTCCTTCCA |
| DMP1 | TGTCATTCTCCTTGTGTTCCTTTG | AGAGCTTTCAGATTCAGTATTGTGGTAT |
| E11 | TGGCAAGGCACCTCTGGTA | TGAGGTGGACAGTTCCTCTAAGG |
| CX43 | CAGGCCGGAAGCACCAT | GCTGTCGTCAGGGAAATCAAA |
| PHEX | GGAAGAAAACCATTGCCAATTATT | CGCCTGCTGAGGTTTGGA |
| SOST | GGAATGATGCCACAGAGGTCAT | CCCGGTTCATGGTCTGGTT |
| 18S rRNA | CATTAAATCAGTTATGGTTCCTTTGG | TCGGCATGTATTAGCTCTAGAATTACC |
2.6. ALP activity assay
ALP activity was determined from MC4 cells treated with or without FGF2 for 4, 8 and 12 days by monitoring the conversion of p-nitrophenol phosphate to p-nitrophenol as described previously [26]. ALP activity was normalized to protein content for each well.
2.7. Statistical analysis
All experiments were performed in triplicate wells and repeated at least three times. Results are expressed as means +/− standard deviations. Data were assessed for statistical significance by ANOVA followed by a Tukey’s post hoc test. P-values less than 0.05 are considered statistically significant.
3. Results and discussion
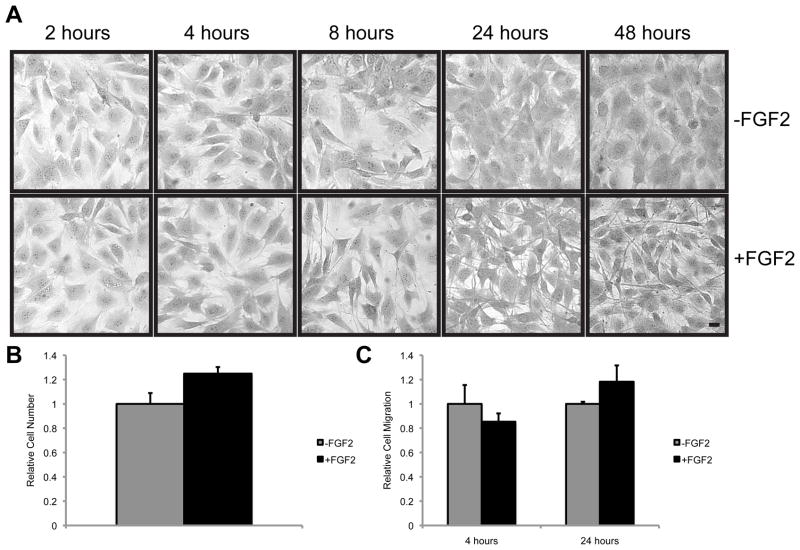
We observed that treatment of MC4 osteoblasts with FGF2 (10 ng/ml) induces a distinct change in cellular morphology (Fig. 1A). Within 8 hours of FGF2 treatment, MC4 cells begin to take on a dendritic-like morphology with development of long cell processes that are remindful of osteocytes. By 24 hours, long cellular processes are seen to extend from the cell body of FGF2 treated MC4 cells. While the occasional cell with this osteocyte-like morphology is seen in MC4 cells that have not been treated with FGF2, nearly every cell in the FGF2 treated culture has adopted this morphology by 24 hours. Further, the osteocyte-like morphology remains persistent for at least 12 days, as long as FGF2 remains in the culture media. The cells maintain this osteocyte-like morphology even at high densities. Notably, in the MC4 cells used in this study, FGF2 has only a modest effect on cell proliferation (Fig. 1B) and no significant impact on cell migration (Fig. 1C). The limited impact of FGF2 on the proliferation of these cells may not be too surprising. MC4 cells are considered a differentiated osteoblast with a high mineralizing capacity [27], and FGF2 has diverse effects on osteoblasts dependent upon their differentiation stage [17].
Figure 1.
FGF2 induces an osteocyte-like morphology in MC4 osteoblasts. (A) Photomicrographs of crystal violet stained MC4 cells treated with or without FGF2 (10ng/ml) for the indicated time. Scale bar, 10 μm. (B) The relative number of viable cells were determined in MC4 cells cultured in the presence or absence of FGF2 (10ng/ml) for 16 hours. (C) The relative number of MC4 cells migrating through an 8 μm transwell chamber in response to the presence or absence of FGF2 (10ng/ml) for the indicated time was assessed by and counting crystal violet stained cells under a light microscope.
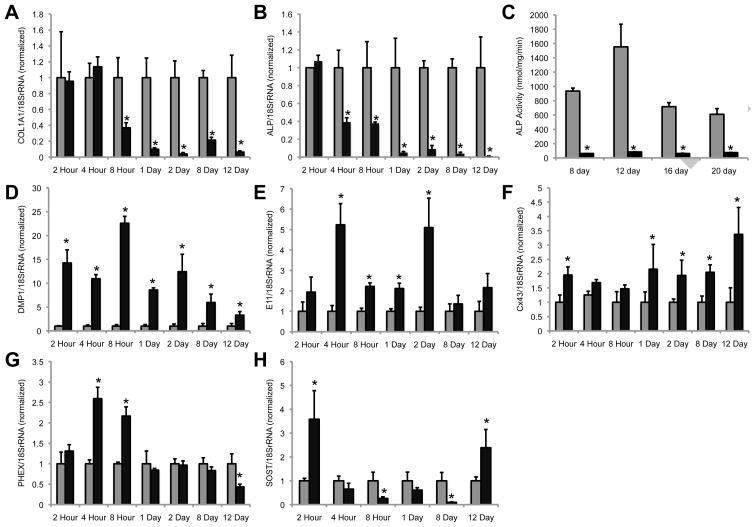
Given the striking morphological changes that were reminiscent of osteocytes, we examined the expression of osteocyte marker genes in these cells. We wanted to test the hypothesis that FGF2 was not functioning to inhibit osteoblast differentiation but rather that it was enhancing progression towards an osteocyte-like phenotype. In fact, others and we have shown that treatment of MC4 osteoblasts with FGF2 can enhance osteocalcin gene expression, a marker of mature osteoblasts and osteocytes [23; 28]. To our knowledge, no one has looked to see if FGF2 could induce the expression of osteocyte markers. Indeed, osteocytes, like osteoprogenitor cells, are characterized by their low expression of both ALP and type I collagen relative to osteoblasts [22; 29; 30; 31]. Consistent with previous studies in osteoblasts [15; 16], we observe a rapid and sustained decrease in the expression of COL1A1 and ALP gene expression caused by FGF2 treatment of MC4 cells as assessed by real time PCR (Fig. 2A and B). Likewise, ALP activity assays reveal an FGF2-dependent decrease in alkaline phosphatase enzymatic activity (Fig. 2C). We observe a rapid (within 2 hours) and sustained (up to 12 days) elevation in dentin matrix protein 1 (DMP1), with expression levels as high as 22-fold greater than those observed in MC4 cells cultured in the absence of FGF2 (Fig. 2D). DMP1, the acid phosphoprotein of the SIBLING (small, integrin binding ligand, N-linked glycoprotein) family, is expressed in osteocytes and pre-osteocytes, but not osteoblasts, in vivo [32]. DMP1 is present in the pericellular bone matrix surrounding osteocytes as well as along their cellular processes. Underscoring its physiologic relevance in osteocyte and skeletal biology, genetic ablation of DMP1 in mice results in osteomalacia and hypophosphatemia, as well as, defective osteocyte differentiation and a disorganized osteocyte lacuno-canalicular network [33].
Figure 2.
FGF2 induces the expression of several osteocyte markers in MC4 osteoblasts. Real time PCR was performed on cDNA prepared from MC4 cells cultured in the presence (black bars) or absence (gray bars) of FGF2 (10ng/ml) for the indicated time periods. Gene specific primers were used to determine the expression of (A) COL1A1, (B) ALP, (D) DMP1, (E) E11, (F) Cx43, (G) PHEX, and (H) SOST. Gene expression data are normalized to 18S rRNA. ALP enzymatic activity (C) is shown for cultures treated with (black bars) or without (gray bars) FGF2 for the indicated time periods. Each time point is normalized relative to the respective -FGF2 control. Asterisks (*) indicate a p-value <0.05 relative to the -FGF2 control.
Similarly, another important osteocyte marker, E11, is increased 2- to 5-fold in MC4 osteoblasts upon FGF2 treatment (Fig. 2E). This increase is statistically significant by 4 hours and remains elevated for the first two days. Like DMP1, E11 is associated with the dendritic processes of osteocytes and short interfering RNA mediated knockdown of E11 has been shown to block the growth of these cell processes [34]. Consistent with the notion that E11 (and perhaps DMP1) may be involved in dendrite formation, the expression of these genes precedes the formation of the dendritic cell processes, which become prominent only at ~8 hours of exposure to FGF2. The FGF2-induced expression of E11 is no longer statistically significant at 8 and 12 days of culture. As E11, is known to be an early marker of osteocyte differentiation, this may suggest that these cells continue to progress towards terminal differentiation while exposed to FGF2.
While not specifically a marker of osteocytes, the importance of the gap junction protein connexin43 in osteocytes is well documented [35; 36; 37]. Connexin43 gene expression is modestly upregulated following FGF2 treatment in MC4 cells (Fig. 2F). Similarly, there is a transient increase in the expression of the osteocyte-associated gene PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) at 4 and 8 hours after FGF2 treatment (Fig. 2G). Like DMP1, PHEX plays an important role in the skeletal control of phosphate homeostasis [38].
Next, we examined the late osteocyte marker, sclerostin (SOST). SOST is a Wnt antagonist that serves as a negative regulator of bone formation [39]. Following FGF2 treatment of MC4 cells, SOST gene expression is complex (Fig. 2H). At 2 hours, there is a 3.5-fold induction of SOST gene expression. By 8 hours, SOST expression is markedly diminished (~ 4-fold reduction) by FGF2 treatment and remains so at 8 days (10-fold reduction). However, by 12 days in culture with FGF2, MC4 cells again demonstrate an increase in SOST gene expression (2.4-fold induction) relative to vehicle-treated controls.
Of interest, the effects of FGF2 on MC4 osteoblast gene expression/osteocyte differentiation are reversible. Within 24 hours of the removal of FGF2 from the culture media, the cells revert to an osteoblast-like spindle-shaped/cuboidal morphology (data not shown). Likewise, removal of FGF2 from the culture media results in the reversion of the gene expression to the same levels as cultured MC4 cells without FGF2 within 24 hours (data not shown). This data demonstrates that FGF2 is not sufficient to permanently alter the cell phenotype towards the osteocyte lineage. It does support the notion that FGF2 is, at minimum, capable of “pushing” osteoblastic cells towards the osteocyte lineage. At present, it is unknown whether additional factors may be required to terminally and irreversibly differentiate these cells into the osteocyte lineage. Regardless, this data is the first to demonstrate that FGF2 may be a factor influencing the transition of osteoblast to an osteocyte lineage.
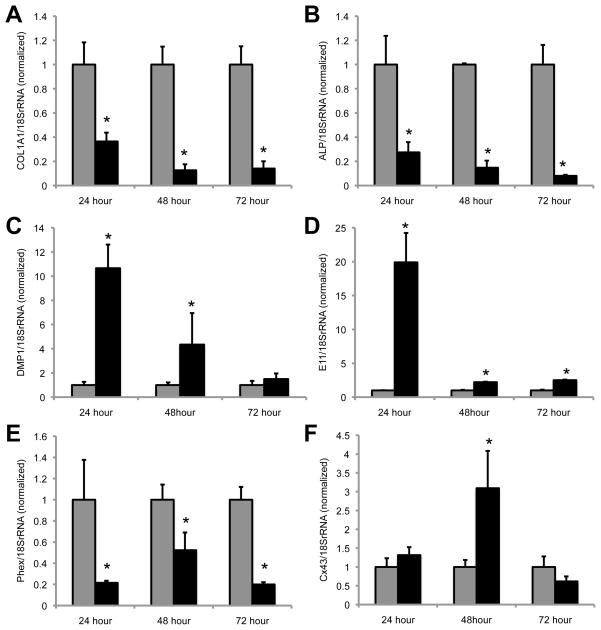
To see if the effects of FGF2 on the development of an osteocyte-like phenotype are specific to MC4 cells, we examined the morphology and gene expression patterns of primary murine bone marrow stromal cells (BMSCs) that had been treated with FGF2. Unlike the response in MC4 cells, there is a robust stimulation of cell proliferation among the BMSCs upon FGF2 treatment. However, we observe a subset of cells that develop an osteocyte-like appearance (not shown). Further, we observe a similar increase in the expression of osteocyte markers, including DMP1, E11, Cx43 and a decrease in COL1A1 and ALP expression at 24, 28 and 72 hours of treatment with FGF2 (Fig. 3). In contrast to the observed effect in MC4 cells, PHEX was noticeably diminished by FGF2 treatment in BMSCs. SOST expression was undetected in the BMSCs. At time points later than 72 hours, the population of cells that proliferate in response to FGF2 overgrows the plate. It is likely that this population of cells blunts the apparent changes in gene expression in the subset of cells that have taken on a osteocyte-like morphology. Future studies will need to be undertaken to separate the sub-populations of cells in the BMSC cultures to fully appreciate the effect of FGF2 on this subset of responsive cells. Regardless, it is apparent that FGF2 can rapidly induce osteocyte-like changes in the gene expression and morphology in BMSCs, and thus is not solely specific to MC4 osteoblasts.
Figure 3.
FGF2 induces the expression of several osteocyte markers in BMSCs. Real time PCR was performed on cDNA prepared from mouse BMSCs cultured in the presence (black bars) or absence (gray bars) of FGF2 (10ng/ml) for the indicated time periods. Gene specific primers were used to determine the expression of (A) COL1A1, (B) ALP, (C) DMP1, (D) E11, (E) PHEX, and (F) Cx43. Gene expression data are normalized to 18S rRNA. Each time point is normalized relative to the respective -FGF2 control. *, p-value <0.05 relative to the -FGF2 control.
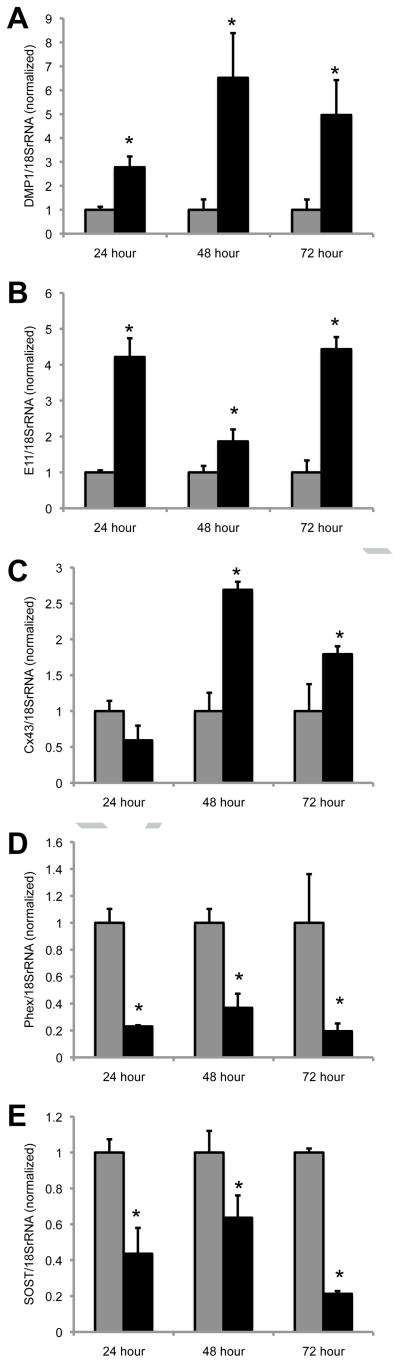
Finally, we examined whether FGF2 could impact the phenotype of osteocytes directly. Accordingly, we used the MLO-Y4 osteocyte-like cell line, which was derived from osteocytes isolated from transgenic mice overexpressing the SV40 large T-antigen under control of the osteocalcin promoter [22]. In contrast to MC4 osteoblasts and BMSCs, DMP1 is the only tested gene induced during the early time points, with FGF2 increasing DMP1 expression 3.7-fold at 8 hours of treatment in the MLO-Y4 cells (data not shown). However, at the 24, 48 and 72 hour time points, FGF2 induced the expression of DMP1 (Fig. 4A) and E11 (Fig. 4B) in MLO-Y4 osteocyte-like cells. The expression of connexin43 was increased ~2 fold at the 48 and 72 hours in FGF2 treated MLO-Y4 cells (Fig. 4C). The expression of PHEX (Fig. 4D) and SOST (Fig. 4E) were both inhibited by FGF2 treatment at the 24, 48 and 72 hour time points. The inhibition of SOST expression by FGF2 in osteocytes is particularly intriguing. SOST is a critical inhibitor of bone formation by antagonizing Wnt signaling [39]. Paradoxically, FGF2 has been shown to be osteoanabolic in vivo despite being largely seen as antagonistic towards osteoblast differentiation and mineralization. However, the inhibition of SOST expression by FGF2 may suggest that the osteoanabolic actions of FGF2 are not solely mediated by regulating osteoblasts/osteoprogenitor proliferation, but may also extend to regulation of mineralization signals (e.g., SOST) by osteocytes.
Figure 4. FGF2 modulates gene expression in MLO-Y4 osteocytes.
Real time PCR was performed on cDNA prepared from MLO-Y4 cells cultured in the presence (black bars) or absence (gray bars) of FGF2 (10ng/ml) for the indicated time periods. Gene specific primers were used to determine the expression of (A) DMP1, (B) E11, (C) Cx43, (D) PHEX, and (E) SOST. Gene expression data are normalized to 18S rRNA. Each time point is normalized relative to the respective -FGF2 control. *, p-value <0.05 relative to the -FGF2 control.
4. Conclusions
The current study shows that FGF2 induces changes in morphology and gene expression in an osteoblast cell line and a subset of BMSCs that are consistent with progression towards an osteocyte like phenotype. Importantly, these data support the hypothesis that FGF2 influences the transition of osteoblasts to osteocytes. Further, this study presents a complimentary model to study osteocyte biology in vitro. Additionally, the data reveal that FGF2 regulates the expression of genes by osteocytes that are involved in the development of the osteocyte cellular process, the formation of the elaborate canalicular network, Wnt signaling and phosphate homeostasis. Lastly, this study also reveals that FGF2 may inhibit expression of SOST, an important negative regulator of bone formation, by osteocytes and may add to the current understanding of the potent osteoanabolic effects of FGF2 in vivo.
Acknowledgments
This work was supported by a grant from National Institutes of Health (AR052719). MLO-Y4 cells were kindly provided by Dr. Lynda Bonewald (University of Missouri, Kansas City). The authors thank Dr. Jose Moreno for thoughtful discussion and technical assistance.
Abbreviations
- ALP
alkaline phosphatase
- BMSCs
bone marrow derived stromal cells
- COL1A1
collagen I α1
- DMP1
dentin matrix protein 1
- FGF2
fibroblast growth factor 2
- MC4
MC3T3 clone 4
- PHEX
phosphate-regulating gene with homologies to endopeptidases on the X chromosome
- SIBLING
small, integrin binding ligand, N-linked glycoprotein
- SOST
sclerostin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marie PJ. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene. 2003;316:23–32. doi: 10.1016/s0378-1119(03)00748-0. [DOI] [PubMed] [Google Scholar]
- 2.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–65. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 3.Hurley MM, Okada Y, Xiao L, Tanaka Y, Ito M, Okimoto N, Nakamura T, Rosen CJ, Doetschman T, Coffin JD. Impaired bone anabolic response to parathyroid hormone in Fgf2−/− and Fgf2+/− mice. Biochem Biophys Res Commun. 2006;341:989–94. doi: 10.1016/j.bbrc.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Montero A, Okada Y, Tomita M, Ito M, Tsurukami H, Nakamura T, Doetschman T, Coffin JD, Hurley MM. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest. 2000;105:1085–93. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabbieti MG, Agas D, Xiao L, Marchetti L, Coffin JD, Doetschman T, Hurley MM. Endogenous FGF-2 is critically important in PTH anabolic effects on bone. J Cell Physiol. 2009;219:143–51. doi: 10.1002/jcp.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naganawa T, Xiao L, Abogunde E, Sobue T, Kalajzic I, Sabbieti M, Agas D, Hurley MM. In vivo and in vitro comparison of the effects of FGF-2 null and haplo-insufficiency on bone formation in mice. Biochem Biophys Res Commun. 2006;339:490–8. doi: 10.1016/j.bbrc.2005.10.215. [DOI] [PubMed] [Google Scholar]
- 7.Xiao L, Liu P, Li X, Doetschman T, Coffin JD, Drissi H, Hurley MM. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. J Biol Chem. 2009;284:3170–82. doi: 10.1074/jbc.M804900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai H, Tsukuda R, Mayahara H. Effects of basic fibroblast growth factor (bFGF) on bone formation in growing rats. Bone. 1995;16:367–73. doi: 10.1016/8756-3282(94)00049-2. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Hanada K, Tamura M, Shibanushi T, Nigi H, Tagawa M, Fukumoto S, Matsumoto T. Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor in rats. Endocrinology. 1995;136:1276–84. doi: 10.1210/endo.136.3.7867582. [DOI] [PubMed] [Google Scholar]
- 10.Liang H, Pun S, Wronski TJ. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology. 1999;140:5780–8. doi: 10.1210/endo.140.12.7195. [DOI] [PubMed] [Google Scholar]
- 11.Pun S, Florio CL, Wronski TJ. Anabolic effects of basic fibroblast growth factor in the tibial diaphysis of ovariectomized rats. Bone. 2000;27:197–202. doi: 10.1016/s8756-3282(00)00312-4. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Hara Y, Tagawa M, Tamura M, Yuge T, Fukuda H, Nigi H. Recombinant human basic fibroblast growth factor accelerates fracture healing by enhancing callus remodeling in experimental dog tibial fracture. J Bone Miner Res. 1998;13:942–9. doi: 10.1359/jbmr.1998.13.6.942. [DOI] [PubMed] [Google Scholar]
- 13.Radomsky ML, Aufdemorte TB, Swain LD, Fox WC, Spiro RC, Poser JW. Novel formulation of fibroblast growth factor-2 in a hyaluronan gel accelerates fracture healing in nonhuman primates. J Orthop Res. 1999;17:607–14. doi: 10.1002/jor.1100170422. [DOI] [PubMed] [Google Scholar]
- 14.Radomsky ML, Thompson AY, Spiro RC, Poser JW. Potential role of fibroblast growth factor in enhancement of fracture healing. Clin Orthop Relat Res. 1998:S283–93. doi: 10.1097/00003086-199810001-00029. [DOI] [PubMed] [Google Scholar]
- 15.Rodan SB, Wesolowski G, Yoon K, Rodan GA. Opposing effects of fibroblast growth factor and pertussis toxin on alkaline phosphatase, osteopontin, osteocalcin, and type I collagen mRNA levels in ROS 17/2.8 cells. J Biol Chem. 1989;264:19934–41. [PubMed] [Google Scholar]
- 16.Hurley MM, Abreu C, Harrison JR, Lichtler AC, Raisz LG, Kream BE. Basic fibroblast growth factor inhibits type I collagen gene expression in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993;268:5588–93. [PubMed] [Google Scholar]
- 17.Debiais F, Hott M, Graulet AM, Marie PJ. The effects of fibroblast growth factor-2 on human neonatal calvaria osteoblastic cells are differentiation stage specific. J Bone Miner Res. 1998;13:645–54. doi: 10.1359/jbmr.1998.13.4.645. [DOI] [PubMed] [Google Scholar]
- 18.Noble BS. The osteocyte lineage. Arch Biochem Biophys. 2008;473:106–11. doi: 10.1016/j.abb.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Rochefort GY, Pallu S, Benhamou CL. Osteocyte: the unrecognized side of bone tissue. Osteoporos Int. doi: 10.1007/s00198-010-1194-5. [DOI] [PubMed] [Google Scholar]
- 20.Bonewald LF. Osteocyte messages from a bony tomb. Cell Metab. 2007;5:410–1. doi: 10.1016/j.cmet.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Castro CH, Shin CS, Stains JP, Cheng SL, Sheikh S, Mbalaviele G, Szejnfeld VL, Civitelli R. Targeted expression of a dominant-negative N-cadherin in vivo delays peak bone mass and increases adipogenesis. J Cell Sci. 2004;117:2853–64. doi: 10.1242/jcs.01133. [DOI] [PubMed] [Google Scholar]
- 22.Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12:2014–23. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 23.Lima F, Niger C, Hebert C, Stains JP. Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism. Mol Biol Cell. 2009;20:2697–708. doi: 10.1091/mbc.E08-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stains JP, Weber JA, Gay CV. Expression of Na(+)/Ca(2+) exchanger isoforms (NCX1 and NCX3) and plasma membrane Ca(2+) ATPase during osteoblast differentiation. J Cell Biochem. 2002;84:625–35. [PubMed] [Google Scholar]
- 25.Niger C, Howell FD, Stains JP. Interleukin-1beta increases gap junctional communication among synovial fibroblasts via the extracellular signal regulated kinase pathway. Biol Cell. 2009 doi: 10.1042/BC20090056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–98. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 28.Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002;277:36181–7. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- 29.Becker J, Schuppan D, Benzian H, Bals T, Hahn EG, Cantaluppi C, Reichart P. Immunohistochemical distribution of collagens types IV, V, and VI and of pro-collagens types I and III in human alveolar bone and dentine. J Histochem Cytochem. 1986;34:1417–29. doi: 10.1177/34.11.3772076. [DOI] [PubMed] [Google Scholar]
- 30.Mikuni-Takagaki Y, Kakai Y, Satoyoshi M, Kawano E, Suzuki Y, Kawase T, Saito S. Matrix mineralization and the differentiation of osteocyte-like cells in culture. J Bone Miner Res. 1995;10:231–42. doi: 10.1002/jbmr.5650100209. [DOI] [PubMed] [Google Scholar]
- 31.Sandberg M, Autio-Harmainen H, Vuorio E. Localization of the expression of types I, III, and IV collagen, TGF-beta 1 and c-fos genes in developing human calvarial bones. Dev Biol. 1988;130:324–34. doi: 10.1016/0012-1606(88)90438-1. [DOI] [PubMed] [Google Scholar]
- 32.Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16:2017–26. doi: 10.1359/jbmr.2001.16.11.2017. [DOI] [PubMed] [Google Scholar]
- 33.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–52. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys. 2008;473:188–92. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–62. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stains JP, Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta. 2005;1719:69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Strom TM, Juppner H. PHEX, FGF23, DMP1 and beyond. Curr Opin Nephrol Hypertens. 2008;17:357–62. doi: 10.1097/MNH.0b013e3282fd6e5b. [DOI] [PubMed] [Google Scholar]
- 39.Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–4. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]