Abstract
Serotonin (5-HT) is a neuro-modulator–transmitter influencing global brain function. Past and present findings illustrate a prominent role for 5-HT in the modulation of ponto-medullary autonomic circuits. 5-HT is also involved in the control of neurotrophic processes during pre- and postnatal development of neural circuits. The functional implications of 5-HT is particularly illustrated in the alterations to the serotonergic system, as seen in a wide range of neurological disorders. This article reviews the role of 5-HT in the development and control of respiratory networks in the ponto-medullary brainstem. The review further examines the role of 5-HT in breathing disorders occurring at different stages of life, in particular, the neonatal neurodevelopmental diseases such as Rett, sudden infant death and Prader-Willi syndromes, adult diseases such as sleep apnoea and mental illness linked to neurodegeneration.
Keywords: 5-HT, respiratory rhythm, neurodegeneration, development, respiratory disorders
1. Introduction
In the 1930s, the Italian pharmacologist Vitorio Erspamer became interested in the amine substances that are involved in smooth muscle constriction in the intestinal tracts of a variety of species including rabbits, frogs and mollusks. While studying the enterochromaffin cells of the gut, Erspamer discovered an unknown indole and named it enteramine (Erspamer and Vialli, 1937). The structure of the same indole via investigation of the blood serum was determined to be 5-Hydroxytryptamine (Rappaport et al., 1948). However, at a time when the theory of ‘neurotransmitters’ itself was controversial, it was Twarog working with Page at the Cleveland Clinic, who showed that the same substance which they named as serotonin or 5-hydroxytryptamine (5-HT) was actually found in the brain (Twarog and Page, 1953; Twarog, 1954). Finally, the emergence of 5-HT as an important chemical constituent of the central nervous system (CNS) was demonstrated by Wooley (1963), who made the best case for the participation of 5-HT in brain development, function and mental illness. In this review, we describe the role of 5-HT on breathing control in mammals. It is presently thought that the serotonergic neurons and receptors play a powerful role in setting the ‘gain’ of motor systems. This review will focus on the serotonergic neuronal network in the brainstem and the various pre and postsynaptic 5-HT receptors (5-HTR) that are implicated in both respiratory motor function as well as dysfunction. While it is not possible to isolate the respiratory system from the cardiovascular system (particularly when discussing the effect of 5-HT), owing to limitations of space, this review will focus mainly on breathing control. Regarding the role of 5-HT on cardiovascular function, we recommend the readers to see recent reviews by Ramage and Villalon (2008), Nalivaiko and Sgoifo (2009) and Minson et al (2009).
2.1 5-HT synthesis and neurotransmission mechanisms
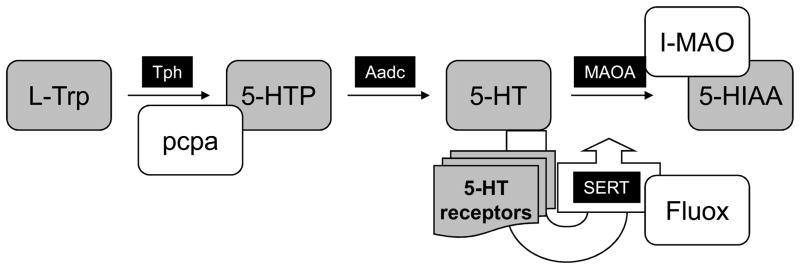
5-HT is a ubiquitous neurotransmitter that is synthesized from L-tryptophan by L-tryptophan hydroxylase (Tph), a marker of 5-HT neurons, first into 5-hydroxy-tryptophan (5-HTP) and then into 5-HT (by aromatic L-amino acid decarboxylase (AaDc, see Fig. 1) (see Gaspar, 2004; Gaspar et al., 2003). After its release, 5-HT acts on a plethora of 5-HTR and is degraded into 5-hydroxyindolacetic acid (5-HIAA) by monoamine oxydase A (MAOA) in the synaptic cleft. In addition, 5-HT is also recaptured by the 5-HT membrane transporter (SERT) and stored in vesicles by the vesicular monoamine transporter (Vmat2). Tph, the initial and rate-limiting enzyme in 5-HT biosynthesis, is irreversibly inactivated by nitric oxide (Kuhn and Arthur, 1996; 1997) and inhibited by hypoxia (Rahman and Thomas, 2009; Poncet et al., 1997; Hedner et al., 1978). This deserves to be noted as a reduction of 5-HT biosynthesis may contribute, at least in part, to the complex effects of nitric oxide or hypoxia.
Fig. 1. Schematic illustration of 5-HT biosynthesis and neurotransmission.
L-Tryptophan (L-Trp) is transformed in 5-hydroxytryptophan (5-HTP) by the enzyme Tryptophan hydroxylase (Tph) and subsequently into serotonin (5-HT) by the enzyme Aromatic L-amino acid decarboxylase (Aadc). In normal conditions, the rate-limiting step of the 5-HT biosynthesis is Tph and not Aadc. After 5-HT release re-uptake is mediated via the serotonin transporter (SERT) and is degraded in 5-hydroxy-indol acetic acid (5-HIAA) by the enzyme monoamine oxydase A (MAOA). Common pharmacological tools used to alter the 5-HT biosynthesis such as p-chlorophenylalanine (pcpa) which blocks 5-HT synthesis, MAOA inhibitors (I-MAOA) which block the degradation and the antidepressant Fluoxetine which blocks 5-HT re-uptake are also illustrated.
Gaddum and Picarelli (1957) proposed first the existence of two types of 5-HTR subtypes, which they termed M and D. Since then, it has become evident from the pharmacological, electrophysiological and DNA-cloning experiments that the serotonergic system comprises of multiple 5-HTRs. In fact, the 5-HTR subtypes cloned to date represent the largest known neurotransmitter receptor families. These subtypes are expressed in distinct, but often overlapping patterns (Palacios et al., 1990), coupled to different transmembrane signaling mechanisms (Table. 1). The currently accepted classification scheme (Hoyer et al., 1994) proposes seven subfamilies of 5-HTR: The 5-HT1AR coupled to Gi proteins, the 5-HT2R coupled to GQ proteins, the gated ion channel 5-HT3R, and the heterogeneous groups of 5-HT4R - 5-HT7R. In particular, the 5HT1AR are also known to be located at pre-synapses to provide auto-inhibition and fine tuning of serotonergic activity. Moreover, 5-HT may act via non-synaptic mechanisms via 5-HT “en passant” release from varicosities. Varicose axonal arborizations are found in almost every 5-HT immunoreactive fibers in the CNS (Léger et al., 2001) indicating possible non-synaptic action of 5-HT in almost every brain area. Serotonergic neurotransmission via hitherto identified 5-HTRs modulate the activity of brainstem cardio-respiratory circuits (Table. 1).
Table 1.
Serotonin receptors found in the brainstem cardio-respiratory networks
| subtype | signalling cascades | Physiological effects | Localization | cartdiorespiratory sites | Central Function |
|---|---|---|---|---|---|
| 5HT1A | Gi/o proteins | increase K+ conductance | pre & post synaptic | raphe nuclei hypoglossal nuclei ponto-medullary networks |
inhibit cell firing depresses excitability of respiratory neurons |
| 5HT1B* | Gi/o proteins | increase K+ conductance | pre & post synaptic | raphe nuclei | inhibit cell firing neurotransmitter release control |
| 5HT1D | Gi/o proteins | increase K+ conductance | axon terminals of both 5HT and non-5HT neurons | dorsal raphe midbrain PAG | behavioral breathing control? |
| 5HT1E/1F | Gi/o proteins | unknown | unidentified | unidentified | not known |
| 5HT2A/2B/2C | GQ/11 proteins | decrease K+ conductance | unidentified | ponto-medullary networks (2A/2B ) | neuronal excitation |
| 5HT3 | ionotropic | Gating of K+, Na+ | somato-dendritic/sub-cellular? | NTS area prostrema dorsal motor nucleus of vagus |
neuronal excitation vomitting reflex |
| 5HT4 | G s proteins | decrease K+ conductance | post synaptic | pre-Botzinger complex | neuronal excitation neurotransmitter release control |
| 5HT5 | G i/o or Gs proteins | unknown | unidentified | unidentified | not known |
| 5HT6 | Gs proteins | unknown | post synaptic in higher brain areas | unidentified | not known |
| 5HT7 | Gs proteins | decrease K+ conductance | unidentified | dorsal raphe pre-Botzinger complex |
neuronal excitation |
2.2. Location of serotonergic neurons in the brain
5-HT content was first related to specific neuronal elements in the brain based on lesion and sub-cellular fractionation studies (see Cooper et al., 1991). The direct examination of 5-HT containing cellular processes became possible via the application of formaldehyde-induced fluorescence histochemistry (known as the Falck and Hillarp method; Falck 1962; Falck et al., 1982). Despite this application, the mapping of 5-HT cells was slow (as compared to say, the mapping of catecholamine) because the 5-HT fluorophore develops less efficiently and also fades rapidly under fluorescent microscopy (see Cooper, Bloom and Roth, 1991). In the recent years, the mapping of 5-HT in the brain has greatly improved through combined application of immunohistochemistry (via both direct purification of tryptophan hydroxylase as well as 5-HT), of orthograde axoplasmic transport of radiolabelled amino acids microinjected into identified 5-HT cells via fluorescence histochemistry and via retrograde isolation of 5-HT neurons from suspected terminal fields. It should be specially mentioned here that the basic organization of 5-HT cells originally described byAnnica Dahlström and Kjell Fuxe (1964) stands to this day. As a result of subsequent extensive studies in the last decade, a good map of 5-HT neurons in the brain has been developed (Steinbusch, 1981; Steinbusch et al., 1981; Steinbusch and Mulder, 1984; Tork and Hornung, 1990; Jacobs and Azmitia, 1992; Hornung, 2003). 5-HT cells are found exclusively in the brainstem in distinct cell groups classified as the raphe (Fig. 2). The dorsal raphe nuclei and superior central nucleus are located in the midbrain, the pontine raphe nuclei and inferior central nucleus in the pons, while the caudal raphe, namely the raphe pallidus, raphe magnus and raphe obscurus are located in the medulla. Immunocytochemical localization of 5-HT has also identified 5-HT reactive neurons in the area postrema, in the caudal locus coeruleus, around the interpeduncular nucleus and in the parapyramidal region of the ventral medulla (Steinbusch and Mulder, 1984).
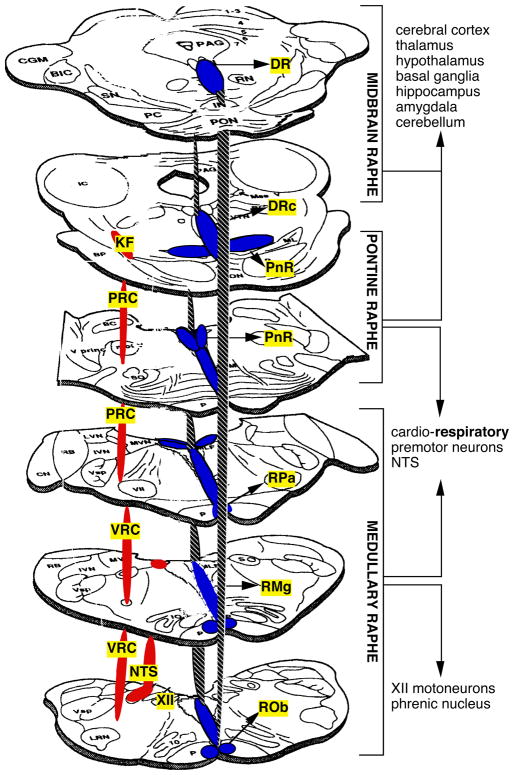
Fig. 2. Distribution and connectivity of serotonin neurons in the brainstem.
Neurons synthesizing 5-HT are found in distinct cell groups called as the raphe in the brainstem. These cells form a column along the midline extending from the caudal medulla to the midbrain. The raphé neurons project to virtually all regions of the neuraxis. The hashed line is intended to elucidate the columnar structure of the raphe system along the midline. The red regions indicate the respiratory columns. Rob: raphe obscurus; RMg: raphe magnus; RPa: raphe pallidus; PnR: pontine raphe nuclei; DRc: caudal dorsal raphe; DR: dorsal raphe; VRC: ventral respiratory column; NTS: nucleus tractus solitari; PRC: pontine respiratory column; KF: Kölliker-Fuse; XII: hypoglossal motor nuclei; PAG: periaqueductal gray.
2.3. Afferent inputs and projections of serotonergic neurons in the brain
The brainstem raphe nuclei receive afferents from a large number of brain structures (Hermann et al., 1996, 1997). Significantly, these projections include inputs to the nucleus raphe magnus, nucleus raphe pallidus and the dorsal raphe from the prelimbic, infralimbic and precentral cortices, dorsal hypothalamic area, the midbrain periaqueductal gray, pre-optic area, bed nucleus of the stria terminalis, amygdala, parabrachial nucleus, Kolliker-Fuse and the subcoerulus area in the pons. The caudal raphe receives a small number of projections from the insular and perirhinal cortices, the periaqueductal gray and the pontine reticular nuclei. The brainstem 5-HT cell groups project virtually to all areas of the brain (Steinbusch and Mulder, 1984). The caudal raphe projects mainly to the medulla and the spinal cord, the pontine raphe projects both to the medulla along the ventral and dorsal tegmental field, the pontine respiratory column and the Kölliker-Fuse nucleus (KF), as well as to the midbrain and cerebellar areas, while the dorsal raphe in the midbrain provides extensive 5-HT innervation of the telencephalon and the diencephalon. In particular, the caudal raphe (raphe pallidus, raphe magnus and raphe obscurus) cell projections have been well identified (and are of special interest as they modulate brainstem cardiorespiratory output). They project extensively to the pontine and ventral respiratory columns (PRC & VRC), in particular to the parabrachial area, KF, nucleus ambiguous (NA), retrotrapezoid nucleus (RTN), Botzinger (BotC) and preBotzinger (PreBotC) regions, hypoglossal motor nucleus in the medulla and phrenic nucleus in the cervical spinal cord. (Steinbusch, 1981; Steinbusch et al., 1981; Steinbusch and Mulder, 1984; Holtman et al., 1986, 1987; Holtman, 1988; Connely et al., 1989; Smith et al., 1989; Zhan et al., 1989; Pilowsky et al., 1990; Voss et al., 1990; Jacobs and Azmitia, 1992, Manaker and Tischler, 1993). In general, the serotonergic termination fields tend to overlap, this may be because many investigations used electrolytic or chemical lesions to study cell projections. This can also interrupt fibers of passage. Also, in the past, attempts to localize 5-HT terminals relied mainly on the 5-HT analogue uptakes, 5-HT selective toxins or radiolabelling of 5-HT. These techniques depend heavily on the specificity of the uptake processes. Hence, for discrete mapping of the 5-HT neuronal terminations more orthograde and retrograde studies are required. The main projections of the serotonergic cell groups to the brainstem cardio-respiratory nuclei are summarized in Fig. 2.
2.4. Role of 5-HT in the modulation of respiratory rhythm and motor pattern
Reid and Rand (1951) were perhaps the first to note the direct involvement of 5-HT in the central regulation of breathing. They reported that intravenous injection of 5-HT causes apnea in the cat. A follow-up study extended these findings showing that small amounts of 5-HT injected into the carotid sinus region induced a brief apnea followed by respiratory stimulation and a fall in blood pressure (Ginzel and Kottegoda, 1954). Similar experiments attempted in dogs (Douglas and Toh, 1953) reported the contrary. In dogs, intravenous injection of 5-HT caused stimulation of breathing. Interestingly, in their experiments (Douglas and Toh, 1953) while intra-arterial injection of 5-HT did not cause any respiratory effect, direct injections into the heart (either into the right auricle or left ventricle) or into the carotid body region caused a respiratory increase. Vagotomy, however, did not influence the respiratory increase caused by intravenous 5-HT injection. Combined, these two studies showed that there could be a species difference in the respiratory responses to 5-HT (Page 1952, Page and McCubbin, 1953; Shneider and Yonkman, 1954). However, denervation of carotid sinus abolished the respiratory (and cardiovascular) effects originally induced via intravenous injection. Hence, a general consensus evolved that 5-HT acts either on the chemo or baroreceptors and that they may not be centrally mediated. This was corroborated by evidence obtained in humans at that time (Page and McCubbin, 1953; Michelson et al., 1958; Parks et al., 1960) that respiratory changes due to intravenous injection of 5-HT were due to a direct action of the drug on the receptors located on the arterial side of systemic circulation.
Subsequently, several studies (Olsen et al., 1979; McCrimmon et al., 1982; Dempsey et al., 1987; Morin et al., 1990; Monteau and Hilaire, 1991; Lindsay and Feldman, 1993) established that 5-HT is an important neuromodulator of breathing. Perhaps a definitive role for the direct action of 5-HT on the CNS was demonstrated by Fallert et al (1979) where microelectrophoretic application of 5-HT directly on the medullary respiratory neurons changed the neuronal activity pattern. They showed that while neurons showing inspiratory-expiratory phase spanning (IE cells) were excited following 5-HT microinjection, other inspiratory neurons became inhibited. Lalley et al (1994a) reported that the 5-HT agonists 5-MeODMT had a differential action on the respiratory system depending upon the dose and route of administration. For example, small dosage of 5-MeODMT intravenously injected increased the discharge frequency of inspiratory and expiratory neurons while larger dosage iontophoretically administered onto respiratory neurons caused acute hyperpolarization and reduced action potential discharges. Another study (Lalley et al., 1995) reported that iontophoresis of α-Me-5-HT a 5-HT2R agonist depolarized membrane potential and increased the frequency of action potentials in most of the ventrolateral medullary respiratory neurons and in particular the E2 and postinspiratory neurons. Later investigations showed that depression of expiratory neural discharges arising from stimulation of the raphe obscurus involved both pre-and post-synaptic 5-HT1AR (Lalley et al., 1997). Recent studies from Richter’s laboratory has revealed that the inhibitory or excitatory effects of systemic 5-HT administration strongly depend on the expression profile of 5-HTR on specific populations of respiratory neurons (Manzke et al., 2003, 2009). In particular, the expression of Gi coupled 5-HT1AR on glycinergic interneurones within the ventral respiratory column (VRC) could explain the paradoxical excitatory effect of the putative inhibitory 5-HTR subtype (Manzke et al., 2009).
Despite in vivo investigations generalizing the excitatory or inhibitory effects of 5-HT on medullary respiratory neurons, the precise presynaptic action which induces either excitation or inhibition as well as the selective post-synaptic modulation that fine tunes the responses of respiratory neurons to various neurotransmitters are yet to be elucidated. Owing to the highly diversified effect of the 5-HT system on respiratory neurons, its direct impact on the medullary respiratory rhythm generator has still not been fully defined. In this regard investigations undertaken on highly reduced in vitro preparations have started to provide emerging principles of 5-HT action. Of significance are investigations conducted using in vitro models by Morin et al (1990, 1992, 1992) and Lindsay and Feldman (1993). Morin et al (1990) used an in vitro brainstem-spinal cord preparation of the newborn rat to study the central effects of 5-HT. They reported that 5-HT facilitates the respiratory rhythm generating networks in the medulla via 5-HT1R and the motoneurons through 5-HT2R. In a follow-up study Di Pasquale et al.(1992) reported a landmark finding that 5-HT acts at the medullary level and its effect is due to its specificity on the rostral VRC areas and not due to any diffuse action on all medullary respiratory centers, although the A5 area in the caudal pons may also contribute. In addition, spinal 5-HT1BR located at the pre-synaptic level modulates the transmission of the central drive to phrenic motoneurons (Di Pasquale et al., 1997). Further studies examined the effect of 5-HT on both hypoglossal and phrenic motoneurons (Morin et al., 1992; Lindsay and Feldman 1993). While 5-HT depressed hypoglossal activity (Morin et al., 1992), its influence increased phrenic motoneuronal activity (Morin et al., 1992; Lindsay and Feldman, 1993) with several cells firing tonically even during expiration. The in vitro studies also allowed for a selective analysis of the influence of 5-HT on central respiratory activity during pre- and postnatal development (for details see below). For example, three different effects of 5-HT via medullary 5-HT1AR, spinal 5-HT2AR and spinal 5-HT1BR modulating the frequency and amplitude of the phrenic motoneuron discharge in neonatal rats (Fig. 3) have been confirmed in neonatal mice (Bou-Flores et al., 2000).
Fig. 3. 5-HT modulation of neonatal respiratory activity.
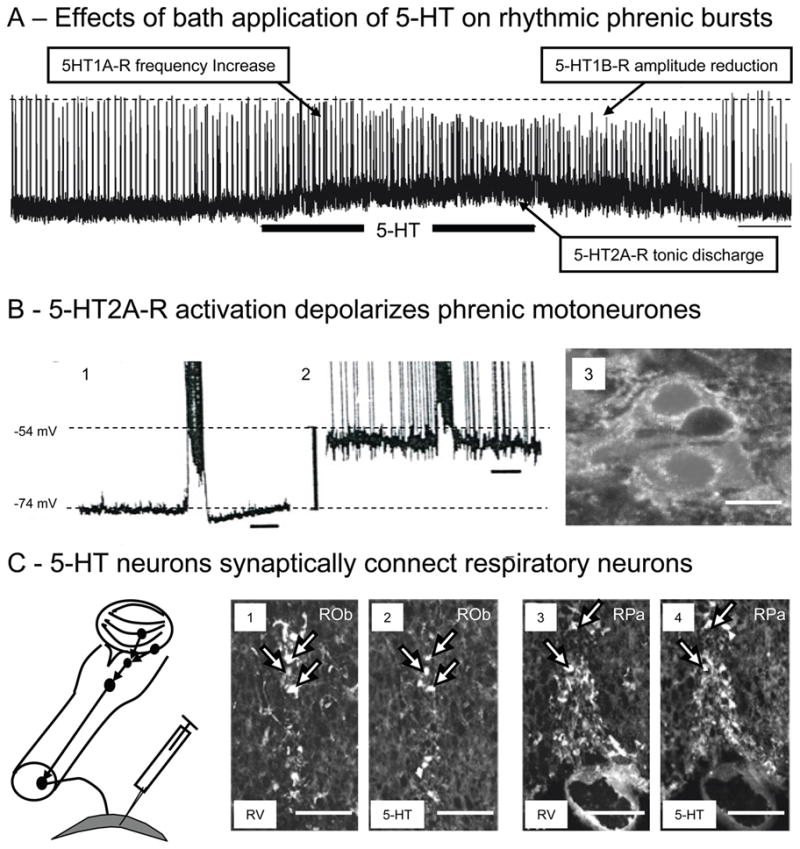
A) Traces showing in vitro phrenic bursts recorded from en bloc preparations of neonatal mice and changes evoked following bath application of 25μM 5-HT (horizontal bar). Note that 5-HT application induced complex effects characterized by an increase in phrenic burst frequency (due to activation of medullary 5-HT1AR), a tonic discharge of motoneurons (due to activation of spinal 5-HT2AR) and a reduction in phrenic burst amplitude (due to a possible presynaptic activation of spinal 5-HT1BR). Scale bar: 1 min (see Bou-Flores et al., 2000).
B) 5-HT2AR activation depolarizes phrenic motoneurons. Intracellular recording of a phrenic motoneuron before (B1) and after 5-HT application (B2). 5-HT induced a 20mV depolarization and a tonic firing of the motoneuron (time scale: 1 s; spikes truncated). B3: Immunohistochemistry reveals that neonatal phrenic motoneurons (retrogradely labelled with rhodamine) widely express 5-HT2AR (white dots). Calibration bar: 20 μm. (see also Bras et al., 2008)
C) 5-HT neurons synaptically coupled with respiratory neurons. Schematic drawing illustrates medullary neurons that project to phrenic motoneurons in neonatal mice after rabies virus (RV) inoculated in the diaphragm at P1. After RV inoculation in the diaphragm, RV progressively infected neurons controlling phrenic motoneuronal output. Thirty hours after inoculation phrenic motoneurons were labeled. After 36 hours neurons in the VRC revealed labeling and after 42 hours infected neurons were observed in histochemically identified 5-HT neurons of the raphe nuclei obscurus (1–2) and pallidus (3–4). These experiments illustrate the synaptic connectivity of raphe 5-HT neurons with phrenic premotor neurons in the VRC. Calibration bars: 100 μm. (also see Zanella et al., 2008)
Intrinsic ionic mechanisms (for example calcium activated potassium conductance) regulate the firing of serotonergic neurons (Burlhuis andAghajanian, 1987; see Aghajanian et al., 1990) A recent study (Rockhill et al., 2009) has found that that spontaneous activity propagation in the mouse midbrain originates from the midline serotonergic cell bodies during early embroyonic stage. These findings suggest that 5-HT cells may possess tonic pacemaker properties, which may in turn be modulated by two neurotransmitters: (1) norepinephrine acting through adrenergic receptors and (2) 5-HT acting through somatodendritic 5-HT1A autoreceptors. While norepinephrine action excites the pacemaker, 5-HT action is inhibitory (see Aghajanian et al., 1990). Thus, the coupling of raphe nuclei serotonergic pacemakers to medullary respiratory rhythm generators should be explored in the future.
2.5. Serotonergic neurotransmission in the control of blood gas homeostasis, body temperature and circulation
Besides the neuromodulatory role of 5-HT in shaping and modulating central respiratory function (for details see 2.4.), 5-HT also plays a prominent role in other autonomic circuits linked to breathing. Of particular interest is the 5-HT control of CO2/pH homeostasis. Although it is commonly admitted that the RTN/pFRG neurons have a crucial role in mediating ventilatory responses to CO2/pH changes (Guyenet et al., 2009; Onimaru et al., 2009), 5-HT neurons also contribute to the central chemosensitivity (Corcoran et al., 2009, Richerson, 2004; Hodges and Richerson, 2010). 5-HT neurons located in close proximity to arteries entering the brainstem, may detect changes in arterial CO2 and thus contribute to the adaptation of the central respiratory drive to environmental changes. In in vitro preparations 5-HT neurons have been shown to be intrinsically chemosensitive. 5-HT cells increase their firing rate in response to hypercapnia and focal acidosis of the raphe nuclei n vivo (Bernard et al., 1996; Nattie and Li, 2001; Feldman et al., 2003) while specific lesions of raphe 5-HT neurons reduce the respiratory response to hypercapnia (Nattie et al., 2004; Dias et al., 2007). In the chemosensitive RTN/pFRG area, SERT and 5-HTR expression have been reported, revealing a dense 5-HT innervation that possibly constitutes the anatomical substrate for 5-HT-dependent control of RTN/pFRG chemoceptors (Liu and Wong-Riley, 2010; Mulkey et al., 2007; Rosin et al., 2006). Further, the ventilatory response to hypercapnia is reduced in SERT knockout mice, especially in males (Li and Nattie, 2008). As well, lmx1b and Pet-1 knockout mice causing near-complete deletion of serotonergic neurons in the brain have altered respiratory responses to CO2/pH (Erickson et al., 2007; Hodges et al., 2008). CO2/pH related changes in transgenic mice are potentially also due to compensatory mechanisms occurring during ontogeny and thus may not relate solely to the absence of 5-HT.
5-HT also plays an important role in arterial oxygen sensing of carotid body chemoceptors (see Prabhakar et al., 2006). In MAOA-KO mice, the excess of 5-HT decreases the ventilatory responses to hypoxia (Burnet et al., 2001), suggesting that the excessive 5-HT affects the responsiveness of the central respiratory network to hypoxia. However, microdialysis of the 5-HT1AR agonist 8-OHDPAT in the medullary raphe does not affect the ventilatory responses to hypoxia (Taylor et al., 2005). Perhaps the impaired ventilatory responses to hypoxia of MAOA-KO mice originates from an effect of the excessive 5-HT on the responsiveness of carotid bodies chemoceptors to hypoxia and/or on the central relay of peripheral hypoxic inputs. The latter possibility is more likely as hypoxia induces a release of 5-HT in the NTS area, possibly contributing to the early ventilatory response to hypoxia (Kanamaru and Homma, 2009). Nevertheless, it was also shown that 5-HT is dynamically released within the medullary VRC during the hypoxic respiratory depression (Richter et al., 1999).
The raphé nuclei play a major role in thermoregulation (Blessing and Nalivaiko, 2000; Morrsion et al., 2008; McAllen et al., 2010) which is also substantiated via the use of transgenic mouse line Lmx1b, lacking almost all central 5-HT neurons. The Lmx1b mice become rapidly hypothermic when exposed to cold environment because of impaired shivering and non-shivering thermogenesis, although they retain normal thermosensory perception and heat conservation (Hodges et al., 2008). In these mice, mild cold stress compromises ventilatory control as a result of defective thermogenesis (Hodges and Richerson, 2008, 2010). In the dorsolateral pons, the lateral parabrachial nucleus may be pivotal in processing central chemoreceptor inputs and cutaneous thermoreceptor inputs, with 5-HT inputs contributing to gate the spino-parabrachial thermoafferent pathway for an optimal interaction of respiratory and thermal regulation (Poon, 2009).
3. 5-HT and respiratory disorders
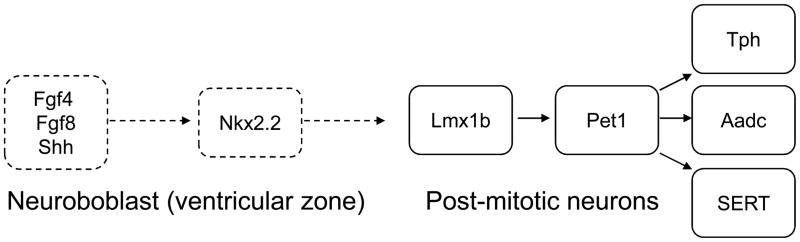
To understand respiratory dysfunction connected with 5-HT it is important to examine the role of 5-HT during prenatal development of neuronal circuits because many respiratory disorders are of neurodevelopmental origin (see 3.2). 5-HT neurons are amongst the earliest to be expressed during embryogenesis. They develop from embryonic days 10–12 (E10-12) in mice and ring the first month of gestation in primates (Gaspar et al., 2003, Gaspar, 2004). They are generated in rhombomeres r1–r3 and r5–r7 caudal to the midbrain-hindbrain organizer under the control of the fibroblast growth factors 4 and 8 (Fgf4 and Fgf8) and sonic hedgehog (Shh) from precursors in the ventricular zone (Fig. 4). The combined action of the secreted positional markers Shh, Fgf4 and Fgf8 defines the dorso-ventral positioning of 5-HT somata within the CNS. Nkx2.2, downstream of Shh, could be implicated in the specification of the caudal 5-HT neurons, probably in conjunction with Gata3.
Fig. 4. Genetic determination and genesis of 5-HT neurons.
During the early embryogenesis 5-HT neurons are generated from ventricular zone precursors controlled by fibroblast growth factors 4 and 8 (Fgf4 and Fgf8) and sonic hedgehog (Shh). In post-mitotic neurons, a complex network of genes such as Pet1, Lmx1b, Nkx2.2, Mash1, Gata2, Gata3, and Phox2b will determine the important proteins for 5-HT biosynthesis and neurotransmission such as Tph, Aadc, SERT. (for details see Fig. 1).
In post-mitotic neurons, the 5-HT phenotype is determined by a sequence of genes such as Pet1, Lmx1b, Nkx2.2, Mash1, Gata2, Gata3, and Phox2b forming a transcriptional network (Fig. 4) specifying the differentiation of 5-HT neurons (Alenina et al., 2006). It is interesting to note that Phox2b, a crucial gene for the production of enzymes for catecholamine biosynthesis, also plays a pivotal role in the selection between a motoneuron or a 5-HT neuronal fate (Pattyn et al., 2003).
During development, expression of Lmx1b precedes Pet1. Lmx1b knock-out mice lack all central 5-HT neurons (Ding et al., 2003) whereas Pet1 knock-out mice have a loss of 70–80% of central 5-HT neurons (Hendricks et al., 1999; 2003). In Lmx1b conditional knock-out mice in which Lmx1b was only deleted in Pet1-expressing 5-HT neurons, the initial generation of central 5-HT neurons is normal and the mice survive, although they lack almost all central 5-HT neurons at later stages of development (Zhao et al., 2006). Thus, Lmx1 and Pet1 contribute to establishing the enzymatic machinery required for the production of 5-HT (Zhao et al., 2006). Two isoforms of Tph exist that produce 5-HT in the periphery (Tph1) and CNS (Tph2) (Nakamura and Hasegawa,, 2007). Pet1 expression is strictly limited to the raphe nuclei and appears one day before the 5-HT neurons can be identified as it contributes to the formation of Tph and SERT. SERT and Vmat2 may be expressed in glutamatergic neuronal subpopulations which may take-up and store 5-HT released from neighbouring 5-HT terminals, even though they do not express Tph and do not synthesize 5-HT (Lebrand et al., 1996, 1998). This is mostly the case in developing thalamic neurons of neonatal rodents (Lebrand et al., 1996, 1998) and in the locus coeruleus, the medial and lateral parabrachial, and KF of adult cats where 5-HT containing neurons have been reported (Léger et al. 1979).
As discussed previously (see Gaspar et al., 2003; Gaspar, 2004; Nakamura and Hasegawa, 2007; Kinney et al., 2007), the early expressed 5-HT neurons affect via 5-HT release, several developmental processes such as cell division, migration, differentiation and synaptogenesis. From this view-point it is possible to suggest that 5-HT contributes to the formation and function of all the central networks. As early as E11.5 when rhombomeric segmentation disappears in mice, 5-HT neurons of the developing midline raphe system and 5-HT2AR already play a role in initiating and propagating spontaneous, rhythmic and synchronous activity throughout the hindbrain (Hunt et al., 2005). It cannot be excluded that these early (E11.5) 5-HT-related rhythmic events which occur at a slow rate (about 2 cycles per minute) contribute to the emergence of the embryonic respiratory rhythm a few days later; first at the level of the embryonic parafacial group (E14.5) and one day later at the Pre-Bötzinger complex level (Thoby-Brisson et al., 2009; Fortin and Thoby-Brisson, 2009). Hence, 5-HT may play a role in the maturation of central networks, including the brainstem respiratory network and the respiratory rhythm generator. In MAOA-deficient mice (Cases et al., 1995), the lack of MAOA degradation of 5-HT results in excess of endogenous 5-HT, with levels of 5-HT during the foetal and neonatal periods 5 to 10-fold higher than in control mice (Lajard et al., 1999). This perinatal excess of 5-HT is not lethal, but alters behaviour of MAOA-deficient mice (Cases et al., 1995) and the formation of several neuronal networks. The excess of 5-HT in MAOA-deficient mice affects the formation of somatosensory maps (Cases et al., 1996), retinal projections to the dorsal lateral geniculate nucleus (Upton et al., 1999), spinal locomotor network (Cazalets et al., 2000) and brainstem respiratory network (Bou-Flores et al., 2000). Excessive 5-HT in MAOA-deficient mice alters the organization of the distal dendritic tree of phrenic motoneurons, the expression of 5-HT1AR and 5-HT1BR and the synaptic organisation of the medullary respiratory neurons (Bou-Flores et al., 2000; Lanoir et al., 2006; Bras et al., 2008). Indeed, transient alterations in 5-HT homeostasis during development may modify the wiring of network connections and may permanently affect behaviours (Nakamura and Hasegawa, 2007) and as well, the 5-HTR expression. 5-HTRs are expressed early in embryonic life and are dynamically regulated during the perinatal development by 5-HT availability, the latter being regulated via SERT. SERT is the primary target for widely used antidepressants.
3.1. Neurodevelopmental disorders linked to 5-HT system alterations
Identified alterations of the 5-HT system play a major role in dysfunctions of the central respiratory network associated with neurodevelopmental disorders. This review is focused on the Rett Syndrome (RTT; Katz et al., 2009) and the Prader-Willi Syndrome (PWS; Zanella et al., 2008; 2009) as prime examples for neurodevelopmental diseases while others such as congenital central hypoventilation syndrome are reviewed elsewhere (see Amiel et al., 2009). Finally, despite the lack of an understanding of gene mutation(s) underlying sudden infant death syndrome (SIDS) we review it under the section of neurodevelopmental disorders concerning breathing as 5-HT may play a critical role in the onset of SIDS.
Rett Syndrome
Rett Syndrome (RTT)(OMIM312750) is a neurodevelopmental disease with low prevalence (see Katz et al., 2009) where infants, after an apparently normal perinatal period, enter a stage of regression and develop neurologic symptoms involving respiratory deficits. This includes erratic respiratory rhythm and life threatening apnoeas. RTT mainly concerns girls and approximately 25% of RTT patients may die prematurely from cardio-respiratory failure. RTT results from mutations in the gene encoding methyl-CpG-binding protein 2 (Mecp2), one of many of methyl-binding proteins that regulate gene expression by repressing transcription at methylated promoters.
Breathing abnormalities are amongst the essential criteria prescribed for the diagnosis of RTT. The respiratory symptoms are more severe during wakefulness than sleep (Wesse-Mayer et al., 2006, 2008) and are exacerbated by emotions and behavioural arousal. As mutations in MECP2 are responsible for most cases of RTT, mouse models have been generated in which Mecp2 is either deleted, mutated or overexpressed. Several mouse models for RTT are now available, presenting breathing defects reminiscent of RTT. In Mecp2−/y mice, with extended exonic deletion of the Mecp2 gene, breathing pattern is normal from birth to P30 although they show subtle abnormalities of post-sigh breathing (Voituron et al., 2010) and respiratory responses to environmental challenges (Voituron et al., 2009). After P30 Mecp2−/y develop breathing symptoms, with an erratic rhythm and life-threatening apnoeas aggravating with age until respiratory distress and death occurs at about P60 (Viemari et al., 2005). In situ studies using a perfused brainstem preparation revealed that apnoeas at P45 correlate with discharges of laryngeal adductor motor output causing upper airway closure (Stettner et al., 2007). These findings indicated that apnoeas in Mecp2−/y could be interpreted as breath-hold. Recent data from our labs have now confirmed the occurrence of active breath-holds in Mecp2−/y in vivo (Voituron et al., 2010 under review).
The breathing symptoms of Mecp2−/y mice are thought to reflect respiratory dysfunction caused by alterations in several neurochemical systems, including the 5-HT system (Katz et al., 2009). In Mecp2−/y mice, the number of 5-HT neurons is normal but HPLC measurements have revealed reduced levels of 5-HT in the brain and the medulla. In addition, comparison of mRNAs encoding 5-HT metabolic enzymes, 5-HTR subtypes and the 5-HT transporter in respiratory related areas of WT and Mecp2−/y mice has revealed significant decreases in mutant mice by P40 (Manzke et al., 2008). As the alterations of the 5-HT system are detected at a rather late developmental stage, they are unlikely linked to initiating the breathing deficits but they may still contribute to the expression of breathing instability.
Prader-Willi Syndrome
Prader-Willi syndrome (PWS) (OMIM 176270) is a rarely occurring (prevalence 1/25000) multi-genetic and neurodevelopmental disorder (Zanella et al., 2009). PWS patients develop complex neurologic deficits that advance with age. Amongst the first deficit is a marked neonatal hypotonia leading to feeding problems followed by hyperphagia at later stages leading to obesity during childhood. In addition, hypothalamic dysfunction results in growth hormone deficiency, hypogonadism, dysautonomy, learning disabilities, behavioral and psychiatric problems. PWS patients also display breathing disorders, including frequent sleep apnoeas and attenuated ventilatory response to hypoxia and hypercapnia (Nixon et al. 2002; Festen et al. 2006). Since these breathing disorders appear well before obesity, and it is hypothetised that the deficits are present at birth (Zanella et al. 2008).
PWS is caused by the loss of the paternal expression of several genes of the 15q11-q13 family. Among several mutant mice generated as model for PWS, Necdin deficient mice (Ndn-KO) display respiratory disorders similar to that seen in PWS (but not obesity) (Zanella et al. 2008). This suggests that the human Necdin gene may be responsible for the breathing symptoms associated with PWS.
In vivo and in vitro approaches revealed dysfunctions of the central respiratory network of Ndn-KO. First, in vivo plethysmography at P2 reveal that Ndn-ko neonates (compared to WT littermates) have a highly variable respiratory rhythm, frequent apnoeas and attenuated responses to hypoxia and hypercapnia. Secondly, the isolated respiratory network of Ndn-ko neonates (compared to WT littermates) produce a variable respiratory rhythm, frequent apnoeas, weak response to hypoxia and a paradoxical reduction of the respiratory rhythm under 5-HT application. The central respiratory network of neonatal Ndn-KO mice does not seem to work appropriately at birth, consistent with results obtained in Ndn-ko foetuses (Ren et al., 2003). In mice, the Necdin allele is expressed in the maturing CNS as early as E10.5, with a peak expression between P0 and P4. Ndn mRNA is expressed in the cortex, the hypothalamus and several brainstem respiratory networks, such as the NTS, the hypoglossal motor nucleus and the VRC, but not in the pre-Bötzinger complex or the RTN/pFRG areas. Interestingly, high levels of Ndn mRNA (Lee et al. 2003) and proteins have been detected in most 5-HT neurons examined in the medulla (Zanella et al. 2008). Using Rabies Virus to retrogradely label the respiratory network after inoculation into the diaphragm revealed that 5-HT neurons expressing Necdin were synaptically connected to the medullary respiratory network controlling the diaphragm. The number of 5-HT neurons in WT and Ndn-KO neonates does not reveal a significant difference, revealing that Ndn is not crucial for 5-HT neurons maturation and survival. However, 5-HT concentrations in the medulla of WT and Necdin-KO neonates are more than 50% increased in Ndn-KO compared to WT (Zanella et al. 2008). These findings suggest that excess in endogenous 5-HT in Ndn-KO mice alters the maturation of the central respiratory network, leading to persistent anomalies in respiratory rhythmogenesis and regulation. The same may occur in PWS patients and is supported by evidence of abnormal levels of 5-HT metabolites in the cerebrospinal fluid and abnormal MAOA activity in platelets (Akefeldt et al. 1998). In addition, treatments targeting 5-HT systems have been used in PWS patients and have shown significant alleviation of some symptoms but nothing is known about the effects of 5-HT treatment on breathing symptoms of PWS patients (Goldstone 2004).
Sudden Infant Death Syndrome (SIDS)
SIDS is defined as the sudden and unexpected death of an infant (<12 months) that remains unexplained despite a complete autopsy and post-mortem examination. SIDS is the leading cause of infant death in industrialized countries, with twice as many boys than girls and the peak incidence occurring at 2–4 months. SIDS is of multi-origin and its actual neurogenic cause remains unknown. However, a recent review (see Paterson et al., 2009) proposes that prenatal alterations of the maturing 5-HT system weaken the robustness of the neonatal respiratory system, which increases the risk of SIDS. The commonly accepted hypothesis is that SIDS occurs when three factors simultaneously impinge on the infant: 1) an underlying vulnerability, 2) a developmental window (the 2–4 months critical period), and 3) several environmental factors heightening the infant vulnerability (e.g. face-down sleeping position, bed sharing, warm environment, prenatal exposure to drugs, nicotine, alcohol). A consensus has been reached that infant vulnerability may be related to an altered respiratory control during sleep, possibly including reduced chemoreceptor sensitivity, abnormal rhythmogenesis causing failure to initiate inspiration and/or gasping. A common scenario is that SIDS is facilitated by hypercapnia/hypoxia from re-breathing exhaled air as a result of sleeping in the face-down position in a warm bedroom. However, the precise nature of the trigger(s) for SIDS remains unknown.
Post-mortem examination have revealed that an important subset of SIDS infants (approximately 70% of studied cases) have abnormalities of different 5-HT markers in the brainstem (Paterson et al., 2006; Paterson et al., 2009, Duncan et al., 2010). As the 5-HT system affects maturation and function of the respiratory network, as well as sleep and thermoregulation, such 5-HT anomalies may induce alterations/dysfunctions of the respiratory system, which may contribute to SIDS during the critical developmental window in conjunction with environmental stressors. A pioneering study reported abnormal levels of 5-HT metabolites found in the cerebrospinal fluids of SIDS infants (Caroff et al., 1992). Since then multiple abnormalities of the 5-HT system have been observed in SIDS victims, such as an increased number of 5-HT neurons, reduced expression of 5-HT1AR and 5-HT2AR, reduced 5-HT binding, abnormal Tph expression, reduced brain 5-HT levels and altered 5-HT turnover (Paterson et al., 2009). As in rodents, 5-HT neurons in humans are expressed early in embryogenesis, as early as the 7th gestational week (Kinney et al., 2007). Post-mortem tract-tracing reveal that human 5-HT neurons send axonal projections to brainstem respiratory-related areas such as the NTS and the hypoglossal motor nucleus, where the presence of SERT, 5-HT1AR, and 5-HT2AR binding sites have been demonstrated. In addition, NK1-positive neurons in the putative PreBötzC area of infants express 5-HT1AR and 5-HT2AR (see Fig. 3 in Paterson et al., 2009). Consistent with the developmental role of 5-HT in central respiratory networks, SIDS can be linked to mal-development of 5-HT circuits during the early foetal life, which may become fatal later after birth.
Recent studies have screened for the genetic basis of 5-HT anomalies that may play a role in SIDS. First, significant differences in genotype distribution and allele frequency of the SERT promoter gene were found in a Japanese SIDS and control infants groups, with more frequent L and XL alleles in SIDS infants (Narita et al., 2001). As the L allele induces a more efficient 5-HT re-uptake than the S allele, 5-HT availability at the synaptic cleft and 5-HT modulation of the respiratory network might be reduced in SIDS victims. SERT gene anomalies in SIDS cases are also observed in other ethnic groups (Weese-Mayer et al., 2003a, 2003b). PHOX2B is an important gene for the production of essential enzymes for catecholamine biosynthesis and for the selection between motoneuron and 5-HT neuronal fate in the embryonic CNS. A study (Rand et al., 2006) suggests a potential relationship between the 5HT system, PHOX2B, and SIDS. However, genetic variations of genes for the 5-HT1AR and 5-HT2AR were reported unlikely to be responsible for the 5-HT system alterations observed in SIDS victims (Morley et al., 2008; Rand et al., 2009). Thus, significant changes in 5-HT1AR and 5-HT2AR expression observed in SIDS victims could be a secondary effect in a global autonomic circuit instability. On the other hand, genetically induced over-expression of 5-HT1AR in mice induce sporadic bradycardia and hypothermia, frequently progressing to death, suggesting that excessive 5-HT auto-inhibition and altered 5-HT homeostasis are risk factors for SIDS (Audero et al., 2008). In addition, besides 5-HT deficits, catecholaminergic deficits may occur, altering the noradrenergic modulation of the respiratory network and therefore increasing SIDS risks (Hilaire, 2006).
3.2. Respiratory dysfunction connected to alteration of 5-HT system with unclear genetic background
Here we summarize the implication of 5-HT in breathing disorders which are not explicitly linked to a genetic cause and show onset during different stages of life. Examples of these disease types include breathing abnormalities linked to neurodegenerative diseases (e.g. dementia), sleep apnoea syndromes and panic disorders. Importantly, all these diseases may have a genetic pre-disposition and with the disease onset and expression influenced by epigenetics. However these genetic/epigenetic factors are not yet well understood.
Sleep apnoea syndrome (SAS)
As already outlined the 5-HT system is a potent modulator of neuronal circuits that control breathing. However, 5-HT has also strong implication in the regulation of the sleep-wake cycle (Popa et al., 2005). Alterations of the 5-HT system that affect both respiration and sleep may contribute to SAS. In fact compelling experimental evidence has accumulated supporting a crucial role of the 5-HT system in the generation of apnoeic events.
First, subjecting anaesthetized neonatal rats to intraperitoneal injection of l-tryptophan, which activates the 5-HT biosynthesis, increases 5-HT levels and induce transient apnoeas. Some of these apnoeas showed airways obstructions (Hilaire et al., 1993). Application of 5-HT or other serotonergic drugs to the IVth ventricle in decerebrated kittens also induces apnoeas (Khater-Boidin et al., 1996). Also, transgenic mice lacking 5-HT2AR showed increased incidence for SAS (Popa et al., 2005). Finally, increasing 5-HT levels in WT mice by pharmacological blockade of the 5-HT degradation also increased the number of sleep apnoeas (Real et al., 2007). MAOA-KO mice linked to excess of 5-HT display frequent apnoeas and their numbers can be reduced by treatment to block 5-HT biosynthesis (Real et al., 2007) or by enhancing 5-HT (Real et al., 2009). C57/BL6 mice spontaneously produce transient breath-holds characterised by glottal closure (Stettner et al., 2008a) and the incidence of these active breath-holds could be decreased with application of the 5-HT1AR agonist 8-OH DPAT (Stettner et al., 2008b).
In humans most of the sleep apnoeas are considered to be obstructive sleep apneas (OSA). This specific type of sleep apnoea is caused by a decreased tone of the upper airway muscles during sleep. In connection with predisposing factors such as unfavourable upper airway anatomy and obesity, upper airway atonia can cause physical obstruction to respiratory airflow thus leading to repetitive apnoeas during sleep. OSA is now recognized as a major disorder and an important cause of medical morbidity and mortality affecting millions of people worldwide. For example OSA is considered to be the most common respiratory disorder in adults and concerns >20 million people in the USA alone (Lavie and Lavie, 2008). OSAs have predispositions to hypertension, cardiovascular disease, stroke, and even sexual dysfunction. Pharmacological management of sleep apnoea remains a major problem (see Abad and Guilleminault, 2006) and modulation and stabilization of the 5-HT system is a prime target. Studies of control subjects and medically healthy recreational users of “ecstasy” (a popular recreational drug and a selective brain 5-HT neurotoxin) suggest that brain 5-HT dysfunction may play a causal role in the pathophysiology of sleep apnoeas (McCann et al., 2009). Another clinical study involving SAS patients and healthy controls in Chinese Han population has suggested that SERT gene may be involved in susceptibility to SAS (Yue et al., 2008).
Neurodegenerative disease
Neurodegenerative diseases and dementia are characterized by cognitive, behavioural and emotional deficits. However, despite breathing dysfunction being a major problem in these neurodegenerative diseases and dementia, respiratory deficits receive less attention compared to the loss of memory. In fact Subramanian et al (2008) have suggested that the elimination of the midbrain periaqueductal gray (PAG) control of brainstem respiratory networks may contribute to the animals (and humans) inability to express emotions as seen in very many neurodegenerative behavioural disorders. Thus, it could well be that breathing dysfunction may play a major role in cognitive, behavioural and emotional impairments
In fact dementia patients suffer from sleep-disordered breathing, with the common cascade of repeated transient apnoea and hypoxemia, disrupted sleep, day-time fatigue along with decreased cognitive abilities (Bliwise, 2002, 2004; Cooke et al., 2006). Possible links between sleep-disordered breathing and dementia are supported by the advent of mental impairment in persons suffering from sleep-disordered breathing and subsequent cognitive improvement with continuous positive airway pressure treatment (Cooke et al., 2009a, 2009b). The development of dementia in persons retaining a good respiratory function at midlife has been found to be low (Guo et al., 2007). The correlation between cardio-respiratory fitness and reduced brain atrophy in dementia patients has also been reported (Burns et al., 2008). These findings reveal that respiratory disorders may not be a secondary factor as commonly assumed in dementia.
More direct evidence for neurodegeneration of brainstem respiratory networks is provided by the finding of tauopathy in monoaminergic nuclei (Parvizi et al., 2001), raphe nuclei (Rüb et al., 2000; Grinberg et al., 2009), pontine parabrachial nuclei (Rüb et al., 2001) and medullary reticular formation (Attems et al., 2007). A recent study from our laboratories showed for the first time that tauopathy in the above mentioned brain-areas is also found in the transgenic mouse line Tau-P301L (Dutschmann et al., 2010). In this specific mouse line, tauopathy was most prevalent in the pontine respiratory group (e.g. parabrachial nuclei) and midbrain periaqueductal grey, which are involved in supra-medullary respiratory control and specifically in upper airway patency and upper airway related behaviours (Dutschmann et al., 2004; Gestreau et al., 2005; Subramanian et al., 2008). Dense tauopathy was also observed in the medullary raphe nuclei providing the main source of 5-HT mediated modulation of central breathing circuits, while the primary pattern and rhythm generating nuclei of the VRC were largely devoid of tauopathy (Dutschmann et al., 2010). This selective pattern of tauopathy is also reflected in the breathing phenotype of the animals. Upper airway dysfunction affects the laryngeal airflow control particularly during increased chemical drive in the respiratory network in response to hypercapnia (Dutschmann et al., 2010). The identified activation of the laryngeal adductors during the inspiratory phase may not only impair airflow and oxygen uptake but also other important upper airway behaviours such as vocalisation or swallowing which were not tested in the study. Upper airway disorders are common features in various forms of dementias. For instance, progressive aphasia is a frontotemporal dementia associated with downstream motor speech deficits (Hillert, 1999; Tolnay and Probst, 2002). In addition, laryngeal adductors are essential for laryngeal closure during swallowing preventing aspiration of food or fluids into the lungs. Dysphagia and aspiration pneumonia belong to the most serious and common complications in dementia patients (Kalia, 2003).
Amongst neurodegenerative diseases multiple systems atrophy (MSA) is most closely linked to cardio-respiratory function. MSA is linked to severe autonomic failure leading to sudden death. Hallmarks of MSA amongst endocrine and cardiovascular disturbances are OSA and laryngeal stridor (Benarroch, 2002; Benarroch, 2007). Autopsy of MSA victims show changes in the various catchelomaniergic cell groups and brainstem raphe nuclei (Benarroch et al., 2004; Benarroch et al., 2007). These changes in the serotonergic system could directly relate to the upper airway phenotype, since experimental evidence shows that serotonergic inputs from the medullary raphe facilitate activity of inspiratory laryngeal abductor motoneurons (Berkowitz et al., 2005; Sun et al., 2002). Thus, loss of serotonergic inputs to laryngeal motorneurones of nucleus ambiguus could explain the sleep related upper airway disorders not only in MSA but also dementia patients.
Disease related alteration of the 5-HT system may also contribute to behavioural symptoms (Rüb et al., 2000; Grinberg et al., 2009) in dementia. Autopsy of dementia patients reveal normal numbers of 5-HT neurons in the dorsal raphe nuclei (Hendricksen et al., 2004) but abnormal density of 5-HT1A and 5-HT2AR (Yeung et al., 2010) in pons and medulla. In the cortex of AD patients 5-HT1AR alteration was found in correlation with aggressive behaviours (Lai et al., 2003) and a reduced density of 5-HT4R expression connected to hyperphagia (Tsang et al., 2010). The reduced density of 5-HT2AR found post-mortem in patients was confirmed in the anterior cingulate cortex of a small sample of living patients with Alzheimer’s disease (AD), although 5-HT2AR binding was found normal (Santhosh et al., 2009). Also, a reduction of SERT binding was observed in the cortex of dementia patients by PET, consistently with a 5-HT dysfunction affecting higher centers of cognition and emotion (Ouchi et al., 2009).
Certain SERT inhibitors and drugs targeting 5-HTR have been tested in neurodegenerative diseases (Lauterbach et al., 2010; Neugroschl and Sano, 2010). However, no conclusive data on effective treatment of behavioural symptoms in dementia are published and potential effects of SERT inhibitors on upper airway dysfunction or sleep apnoea are not tested as yet.
Mental disorders linked to disturbed breathing: A role for 5-HT
Amongst a variety of mental disorders the panic disorder is considered most directly to be linked to an aberration in the control of respiration (Klein, 1993, Nardi et al., 2009). Contrary to other anxiety related disorders panic disorder is often characterized by respiratory symptoms, such as hyperventilation and shortness of breath (Nardi et al., 2009). Moreover, patients with panic disorder were reported to show increased respiratory variability between panic attacks (Gorman et al., 1988; Martinez et al., 2001) even during sleep (Stein et al., 1995). Interestingly, patients also display hypersensitivity to CO2 (Papp et al., 1993). Whether the breathing abnormalities are causal to specific changes in neuronal circuits sensitive to CO2 (potentially including the medullary raphe) remains undetermined. Evidence that changes in the 5-HT system may be indirectly implicated in the breathing abnormality arise from the established finding that long-term treatment with 5-HT reuptake inhibitors can alleviate panic attacks. However, direct evidence of pathological changes in 5-HT system from autopsy is not yet available and to our knowledge an animal model for panic disorder is not currently established.
4. Therapeutic use of serotonergic drugs in central respiratory dysfunction
The targeted use of serotonergic drugs to treat centrally mediated respiratory disorders is sparse. The use of the 5-HT1AR agonist buspirone was successful in the treatment of severe apneusis occurring in a child after ponto-medullary surgery (Wilken et al., 1997). Further, it has been shown that breathing stabilizes in a RTT patient after buspirone administration (Andaku et al., 2005). The use of 5-HT2/3R antagonist significantly reduces the apnoea index in sleep apnoea patients but also reveals sedation and weight-gain as unwarranted side effects (Carley et al., 2007). A recent placebo controlled clinical trial completely failed to show effective treatment of respiratory abnormality in panic disorder (Coryell and Rickels, 2009).
Besides the largely unsatisfactory results in clinical trials, experimental evidence proposes interesting future avenues for the use of serotonergic drugs in central respiratory disturbance and failure. For example, 5-HT4R and 5-HT1AR agonists have been shown to compensate central respiratory disturbances including apneusis or apnoeic periods in the experimental settings (El-Khatib et al. 2003, Lalley et al. 1994a, Manzke et al. 2003, 2009, Shabizada et al., 2000, Stettner et al. 2008, Wilken et al. 1997, Yamauchi et al. 2008a, b, Guenther et al., 2009). Specifically 5-HT4R and 5-HT1AR agonists may have a broader applicability in the treatment of opioid mediated respiratory arrest. Opioids are currently the most powerful analgesics available to treat pain but their use is limited by side effects including the risk of fatal apnoea. Recent evidence provided by Diethelm Richter’s group suggest that 5-HT4R or 5-HT1AR mediated recovery of μ-opioid receptor evoked respiratory depression or even total arrest (Manzke et al. 2003, 2009). The use of a 5-HT1AR agonist has been shown to evoke breathing recovery following opioid induced respiratory depression in awake rodents (Dutschmann et al., 2009). However it needs to be mentioned that preliminary test in humans failed to produce similar beneficial effect on opioid evoked respiratory depression (Lötsch et al., 2005; Oertel et al., 2007).
In summary, the discrepancies between promising effect of serotonergic drugs on various central breathing instabilities as seen in animal models and the comparable results as seen in human trials remain. One reason for the unpredictability of serotonergic drug treatment is related to pronounced pre-synaptic autoinhibitory mechanism, which fine tunes afferent synaptic inputs to 5-HT neurons. For instance the block of 5-HT re-uptake can lead to increased activation of pre-synaptic 5HT1AR of the raphe nuclei by endogenous 5-HT. This in turn suppresses synaptic drive to 5HT neurons consequently reducing 5-HT release in central nervous system. Therefore a drug aiming to potentiate the action the 5-HT system does not produce the warranted net-effect in the brain. Future breakthroughs in drug design may help to tailor serotonergic drugs to specifically target respiratory disorder with minimal impact on other physiological systems.
5. Conclusion
Compelling experimental evidence shows that the 5-HT plays a crucial role in the central control of breathing. Disease-related alterations of 5-HT system (linked to genetic and/or environmental factors) induce severe breathing disorders occurring at different stages in life, ranging form the early post-natal period with SIDS, neurodevelopmental disorders and to neurodegenerative disease in the elderly and ageing. The wide range of neurologic diseases correlate to several differential mechanisms through which 5-HT may affect the respiratory system. There is much to be deciphered on the precise mechanism through which 5HT mediates respiratory function (and dysfunction) and the future is bright and forthcoming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abad VC, Guilleminault C. Pharmacological management of sleep apnoea. Expert Opin Pharmacother. 2007;7:11–23. doi: 10.1517/14656566.7.1.11. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Sprouse JS, Sheldon P, Rasmussen K. Electrophysiology of the central serotonin system: receptor subtypes and transducer mechanisms. Ann N Y Acad Sci. 1990;600:93–103. doi: 10.1111/j.1749-6632.1990.tb16875.x. [DOI] [PubMed] [Google Scholar]
- Akefeldt A, Ekman R, Gillberg C, Månsson JE. Cerebrospinal fluid monoamines in Prader-Willi syndrome. Biol Psychiatry. 1998;44:1321–1328. doi: 10.1016/s0006-3223(97)00519-2. [DOI] [PubMed] [Google Scholar]
- Alenina N, Bashammakh S, Bader M. Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2006;2:5–10. doi: 10.1007/s12015-006-0002-2. [DOI] [PubMed] [Google Scholar]
- Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol. 2009;168:125–132. doi: 10.1016/j.resp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Andaku DK, Mercadante MT, Schwartzman JS. Buspirone in Rett syndrome respiratory dysfunction. Brain Dev. 2005;27:437–438. doi: 10.1016/j.braindev.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Attems J, Quass M, Jellinger KA. Tau and alpha-synuclein brainstem pathology in Alzheimer disease: relation with extrapyramidal signs. Acta Neuropathol. 2007;113:53–62. doi: 10.1007/s00401-006-0146-9. [DOI] [PubMed] [Google Scholar]
- Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science. 2008;321(5885):130–1333. doi: 10.1126/science.1157871. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. New findings on the neuropathology of multiple system atrophy. Auton Neurosci. 2002;96:59–62. doi: 10.1016/s1566-0702(01)00374-5. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, Schmeichel AM, Low PA, Parisi JE. Involvement of medullary serotonergic groups in multiple system atrophy. Ann Neurol. 2004;55:418–422. doi: 10.1002/ana.20021. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Brainstem respiratory control: substrates of respiratory failure of multiple system atrophy. Mov Disord. 2007a;22:155–161. doi: 10.1002/mds.21236. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, Schmeichel AM, Sandroni P, Parisi JE, Low PA. Rostral raphe involvement in Lewy body dementia and multiple system atrophy. Acta Neuropathol. 2007b;114:213–220. doi: 10.1007/s00401-007-0260-3. [DOI] [PubMed] [Google Scholar]
- Berkowitz RG, Sun QJ, Goodchild AK, Pilowsky PM. Serotonin inputs to laryngeal constrictor motoneurons in the rat. Laryngoscope. 2005;115:105–9. doi: 10.1097/01.mlg.0000150695.15883.a4. [DOI] [PubMed] [Google Scholar]
- Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphé. J Appl Physiol. 1996;80:108–115. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: patterning by medullary raphe, not ventrolateral medulla. J Physiol. 2000;524:279–292. doi: 10.1111/j.1469-7793.2000.t01-2-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL. Sleep apnea, APOE4 and Alzheimer’s disease 20 years and counting? J Psychosom Res. 2002;53:539–546. doi: 10.1016/s0022-3999(02)00436-1. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6(Suppl 1A):S16–28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci. 2000;20:4646–4656. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H, Gaytán SP, Portalier P, Zanella S, Pásaro R, Coulon P, Hilaire G. Prenatal activation of 5-HT2A receptor induces expression of 5-HT1B receptor in phrenic motoneurons and alters the organization of their premotor network in newborn mice. Eur J Neurosci. 2009;28(6):1097–1107. doi: 10.1111/j.1460-9568.2008.06407.x. [DOI] [PubMed] [Google Scholar]
- Burlhis TM, Aghajanian GK. Pacemaker potentials of serotonergic dorsal raphe neurons: contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1:582–588. doi: 10.1002/syn.890010611. [DOI] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carley DW, Olopade C, Ruigt GS, Radulovacki M. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep. 2007;30:35–41. doi: 10.1093/sleep/30.1.35. [DOI] [PubMed] [Google Scholar]
- Caroff J, Girin E, Alix D, Cann-Moisan C, Sizun J, Barthelemy L. Neurotransmission and sudden infant death. Study of cerebrospinal fluid. C R Acad Sci III. 1992;314(10):451–454. [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Müller U, Aguet M, Babinet C, Shih JC, Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Connely CA, Elllenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989;105:30–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Cooke JR, Liu L, Natarajan L, He F, Marler M, Loredo JS, Corey-Bloom J, Palmer BW, Greenfield D, Ancoli-Israel S. The effect of sleep-disordered breathing on stages of sleep in patients with Alzheimer’s disease. Behav Sleep Med. 2006;4:219–227. doi: 10.1207/s15402010bsm0404_2. [DOI] [PubMed] [Google Scholar]
- Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009a;5:305–309. [PMC free article] [PubMed] [Google Scholar]
- Cooke JR, Ancoli-Israel S, Liu L, Loredo JS, Natarajan L, Palmer BS, He F, Corey-Bloom J. Continuous positive airway pressure deepens sleep in patients with Alzheimer’s disease and obstructive sleep apnea. Sleep Med. 2009b;10(10):1101–1106. doi: 10.1016/j.sleep.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. Ox Uni Press; Oxford: 1991. [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Rickels H. Effects of escitalopram on anxiety and respiratory responses to carbon dioxide inhalation in subjects at high risk for panic disorder: a placebo-controlled, crossover study. J Clin Psychopharmacol. 2009;29:174–178. doi: 10.1097/JCP.0b013e31819a8d96. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;232:1–55. [PubMed] [Google Scholar]
- De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1996;268(5218):1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Olsen EB, Skatrud JB. In the Handbook of Physiology-The Respiratory System II. Chapter 7. APS; Washington DC: Hormones and neurochemicals in the regulation of breathing; pp. 181–221. [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Lindsay A, Feldman JL, Monteau R, Hilaire G. Serotonergic inhibition of phrenic motoneuron activity: an in vitro study in neonatal rat. Neurosci Lett. 1997;230:29–32. doi: 10.1016/s0304-3940(97)00469-2. [DOI] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LG. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol. 2007;103(5):1780–1788. doi: 10.1152/japplphysiol.00424.2007. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6(9):933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- Douglan WW, Toh CC. The respiratory stimulamnt action of 5-Hydroxytryptamine (serotonin) in the dog. J Physiol. 1953;120:311–318. doi: 10.1113/jphysiol.1953.sp004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kolliker-Fuse nucleus. Respir Physiol Neurobiol. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Waki H, Manzke T, Simms AE, Pickering AE, Richter DW, Paton JF. The potency of different serotonergic agonists in counteracting opioid evoked cardiorespiratory disturbances. Philos Trans R Soc Lond B Biol Sci. 2009;364:2611–23. doi: 10.1098/rstb.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Menuet C, Stettner GM, Gestreau C, Borghgraef P, Devijver H, Gielis L, Hilaire G, Van Leuven F. Upper airway dysfunction of Tau-P301L mice correlates with tauopathy in midbrain and ponto-medullary brainstem nuclei. J Neurosci. 2010;30:1810–1821. doi: 10.1523/JNEUROSCI.5261-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khatib MF, Kiwan RA, Jamaleddine GW. Buspirone treatment for apneustic breathing in brain stem infarct. Respir Care. 2003;48:956–958. [PubMed] [Google Scholar]
- Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007;159:85–101. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V, Vialli M. Ricerche sul secreto delle cellule enterocromaffini. Boll d Soc Med-chir Pavia. 1937;51:357–363. [Google Scholar]
- Falck B. Observations on the possibilities of the cellular localization of monoamines by a fluorescence method. Acta Physiol Scand. 1962;56(Suppl 197) [Google Scholar]
- Falck B, Hillarp NA, Thieme G, Torp A. Fluorescence of Catechol Amines and related compounds condensed with formaldehyde. J Histochem Cytochem. 1962;10:348–354. doi: 10.1016/0361-9230(82)90113-7. [DOI] [PubMed] [Google Scholar]
- Fallert M, Böhmer G, Dinse HR, Sommer TJ, Bittner A. Microelectrophoretic application of putative neurotransmitters onto various types of bulbar respiratory neurons. Arch Ital Biol. 1979;117:1–12. [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festen DA, de Weerd AW, van den Bossche RA, Joosten K, Hoeve H, Hokken-Koelega AC. Sleep-related breathing disorders in prepubertal children with Prader-Willi syndrome and effects of growth hormone treatment. J Clin Endocrinol Metab. 2006;91(12):4911–4915. doi: 10.1210/jc.2006-0765. [DOI] [PubMed] [Google Scholar]
- Fortin G, Thoby-Brisson M. Embryonic emergence of the respiratory rhythm generator. Respir Physiol Neurobiol. 2009;168:86–91. doi: 10.1016/j.resp.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Gaddum JH, Picarelli ZP. Two kinds of tryptamine receptors. Br J Pharmacol. 1957;12:323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gaspar P. Genetic models to understand how 5-HT acts during development. J Soc Biol. 2004;198:18–21. [PubMed] [Google Scholar]
- Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol. 2005;147:159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Ginzel KH, Kottegoda SR. The action of 5-Hydroxytryptamine and tryptamine on aortic and carotid sinus receptors in the cat. J Physiol. 1954;123:277–288. doi: 10.1113/jphysiol.1954.sp005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone AP. Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab. 2004;15:12–20. doi: 10.1016/j.tem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, Kinney J, Klein DF. Ventilatory physiology of patients with panic disorder. Arch Gen Psychiatry. 1988;45:31–39. doi: 10.1001/archpsyc.1988.01800250035006. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Rüb U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H Brazilian Brain Bank Study Group. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol. 2009;35:406–416. doi: 10.1111/j.1365-2990.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- Guenther U, Manzke T, Wrigge H, Dutschmann M, Zinserling J, Putensen C, Hoeft A. The counteraction of opioid-induced ventilatory depression by the serotonin 1A-agonist 8-OH-DPAT does not antagonize antinociception in rats in situ and in vivo. Anesth Analg. 2009;108:1169–1176. doi: 10.1213/ane.0b013e318198f828. [DOI] [PubMed] [Google Scholar]
- Guo X, Waern M, Sjögren K, Lissner L, Bengtsson C, Björkelund C, Ostling S, Gustafson D, Skoog I. Midlife respiratory function and Incidence of Alzheimer’s disease: a 29-year longitudinal study in women. Neurobiol Aging. 2007;28:343–350. doi: 10.1016/j.neurobiolaging.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner T, Lundborg P, Engel J. Effect of hypoxia on monoamine synthesis in brains of developing rats. III. Various O2 levels. Biol Neonate. 1978;34:55–60. doi: 10.1159/000241105. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19(23):10348–56. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hendricksen M, Thomas AJ, Ferrier IN, Ince P, O’Brien JT. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am J Psychiatry. 2004;161(6):1096–1102. doi: 10.1176/appi.ajp.161.6.1096. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Forebrain projections of the rostral nucleus raphe magnus shown by iontophoretic application of choleratoxin b in rats. Neurosci Lett. 1996;216:151–154. doi: 10.1016/0304-3940(96)13013-5. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Morin D, Lajard AM, Monteau R. Changes in serotonin metabolism may elicit obstructive apnoea in the newborn rat. J Physiol. 1993;466:367–381. [PMC free article] [PubMed] [Google Scholar]
- Hilaire G. Endogenous noradrenaline affects the maturation and function of the respiratory network: possible implication for SIDS. Auton Neurosci. 2006;126–127:320–331. doi: 10.1016/j.autneu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Hillert D. On processing lexical meanings in aphasia and Alzheimer’s disease: some (re)considerations. Brain Lang. 1999;69:95–118. doi: 10.1006/brln.1999.2053. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol. 2008;164:350–357. doi: 10.1016/j.resp.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28(10):2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary 5-HT (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010;108(5):1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR, Jr, Dick TE, Berjer AJ. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of raphe obscurus. J Neurosci. 1986;6:1185–1193. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR, Jr, Dick TE, Berjer AJ. Serotonin mediated excitation of recurrent laryngeal and phrenic motoneurons evoked by stimulation of raphe obscurus. Brain Res. 1987;417:12–20. doi: 10.1016/0006-8993(87)90174-0. [DOI] [PubMed] [Google Scholar]
- Holtman JR., Jr Immunohistochemical localization of serotonin and substance P containing fibers around respiratory muscle motoneurons in the nucleus ambiguss of the cat. Neuroscience. 1988;26:169–178. doi: 10.1016/0306-4522(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Harting PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII International union of pharmacology classification of receptors for 5-hydroxytryptamine. Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hunt PN, McCabe AK, Bosma MM. Midline serotonergic neurones contribute to widespread synchronized activity in embryonic mouse hindbrain. J Physiol. 2005;566:807–19. doi: 10.1113/jphysiol.2005.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kalia M. Dysphagia and aspiration pneumonia in patients with Alzheimer’s disease. Metabolism. 2003;52(Suppl 2):36–38. doi: 10.1016/s0026-0495(03)00300-7. [DOI] [PubMed] [Google Scholar]
- Kanamaru M, Homma I. Dorsomedial medullary 5-HT2 receptors mediate immediate onset of initial hyperventilation, airway dilation, and ventilatory decline during hypoxia in mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R34–41. doi: 10.1152/ajpregu.90802.2008. [DOI] [PubMed] [Google Scholar]
- Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol. 2009;168:101–108. doi: 10.1016/j.resp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater-Boidin J, Rose D, Duron B. Central effects of 5-HT on activity of respiratory and hypoglossally innervated muscles in newborn kittens. J Physiol. 1996;495:255–265. doi: 10.1113/jphysiol.1996.sp021590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Belliveau RA, Trachtenberg FL, Rava LA, Paterson DS. The development of the medullary serotonergic system in early human life. Auton Neurosci. 2007;132:81–102. doi: 10.1016/j.autneu.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE., Jr Inactivation of brain tryptophan hydroxylase by nitric oxide. J Neurochem. 1996;67(3):1072–1077. doi: 10.1046/j.1471-4159.1996.67031072.x. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE., Jr Inactivation of tryptophan hydroxylase by nitric oxide: enhancement by tetrahydrobiopterin. J Neurochem. 1997;68(4):1495–502. doi: 10.1046/j.1471-4159.1997.68041495.x. [DOI] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Francis PT, Esiri MM, Keene J, Hope T, Chen CP. Reduced 5-HT 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003;974:82–87. doi: 10.1016/s0006-8993(03)02554-x. [DOI] [PubMed] [Google Scholar]
- Lajard AM, Bou C, Monteau R, Hilaire G. Serotonin levels are abnormally elevated in the fetus of the monoamine oxidase-A-deficient transgenic mouse. Neurosci Lett. 1999;261:41–44. doi: 10.1016/s0304-3940(98)01012-x. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol. 1994a;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW. Serotonin 1A-receptor activation suppresses respiratory apneusis in the cat. Neurosci Lett. 1994b;172:59–62. doi: 10.1016/0304-3940(94)90662-9. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW. 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. J Physiol. 1995;487:653–661. doi: 10.1113/jphysiol.1995.sp020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Benacka R, Bischoff AM, Richter DW. Nucleus raphe obscurus evokes 5-HT-1A receptor-mediated modulation of respiratory neurons. Brain Res. 1997;747:156–159. doi: 10.1016/s0006-8993(96)01233-4. [DOI] [PubMed] [Google Scholar]
- Lanoir J, Hilaire G, Seif I. Reduced density of functional 5-HT1A receptors in the brain, medulla and spinal cord of monoamine oxidase-A knockout mouse neonates. J Comp Neurol. 2006;495(5):607–623. doi: 10.1002/cne.20916. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC, Victoroff J, Coburn KL, Shillcutt SD, Doonan SM, Mendez MF. Psychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical data. J Neuropsychiatry Clin Neurosci. 2010;22:8–18. doi: 10.1176/jnp.2010.22.1.8. [DOI] [PubMed] [Google Scholar]
- Lavie P, Lavie L. Cardiovascular morbidity and mortality in obstructive sleep apnea. Curr Pharm Des. 2008;14(32):3466–3473. doi: 10.2174/138161208786549317. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of 5-HT in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Lee S, Walker CL, Wevrick R. Prader-Willi syndrome transcripts are expressed in phenotypically significant regions of the developing mouse brain. Gene Expr Patterns. 2003;3(5):599–609. doi: 10.1016/s1567-133x(03)00113-3. [DOI] [PubMed] [Google Scholar]
- Léger L, Wiklund L, Descarries L, Persson M. Description of an indolaminergic cell component in the cat locus coeruleus: a fluorescence histochemical and radioautographic study. Brain Res. 1979;168:43–56. doi: 10.1016/0006-8993(79)90127-6. [DOI] [PubMed] [Google Scholar]
- Leger L, Charnay Y, Hof PR, Bouras C, Cespuglio R. Anatomical distribution of serotonin-containing neurons and axons in the central nervous system of the cat. J Comp Neurol. 2001;433:157–182. [PubMed] [Google Scholar]
- Li A, Nattie EE. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol. 2008;586:2321–2329. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993;461:213–33. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in tryptophan hydroxylase and serotonin transporter immunoreactivity in multiple brainstem nuclei of the rat: implications for a sensitive period. J Comp Neurol. 2010;518:1082–1097. doi: 10.1002/cne.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Skarke C, Schneider A, Hummel T, Geisslinger G. The 5-hydroxytryptamine 4 receptor agonist mosapride does not antagonize morphine-induced respiratory depression. Clin Pharmacol Ther. 2005;78:278–287. doi: 10.1016/j.clpt.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotonergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–9. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Manzke T, Bidon O, Dutschmann M, Richter DW. Mecp2-deficiency causes developmental changes in the serotonergic system. Acta Physiol. 2008;192:PT03–PT05. [Google Scholar]
- Manzke T, Dutschmann M, Schlaf G, Morschel M, Koch UR, Ponimaskin E, Bidon O, Lalley PM, Richter DW. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc Lond B Biol Sci. 2009;364:2589–602. doi: 10.1098/rstb.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JM, Kent JM, Coplan JD, Browne ST, Papp LA, Sullivan GM, Kleber M, Perepletchikova F, Fyer AJ, Klein DF, Gorman JM. Respiratory variability in panic disorder. Depress Anxiety. 2001;14:232–237. doi: 10.1002/da.1072. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Tanaka M, Ootsuka Y, McKinley MJ. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol. 2010;109:27–33. doi: 10.1007/s00421-009-1295-z. [DOI] [PubMed] [Google Scholar]
- McCann UD, Sgambati FP, Schwartz AR, Ricaurte GA. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73(23):2011–2017. doi: 10.1212/WNL.0b013e3181c51a62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Lally PM. Inhibition of respiratory neural discharges by clonidine and 5-Hydroxytryptamine. J Pharmacol Exp Ther. 1982;2222:771–777. [PubMed] [Google Scholar]
- Michelson AL, Hollander W, Lowell FC. The effect of 5-Hydroxytryptamine on the respiration of non-asthmatic and asthmatic subjects. J Lab Clin Med. 1958;51:57–62. [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Minson J, Chalmers J, Drolet G, Kapoor V, Llewellyn-Smith I, Mills E, Morris M, Pilowsky P. Central serotonergic mechanisms in cardiovascular regulation. Cardiovasc Drugs Ther Suppl. 1990;1:27–32. doi: 10.1007/BF00053423. [DOI] [PubMed] [Google Scholar]
- Monteau R, Hillaire G. Spinal respiratory motoneurons. Prog Neurobiol. 1991;37:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Morin D, Hennequin S, Monteau R, Hilaire G. Serotonergic influences on central respiratory activity: an in vitro study in the newborn rat. Brain Res. 1990;535:281–287. doi: 10.1016/0006-8993(90)91611-j. [DOI] [PubMed] [Google Scholar]
- Morin D, Monteau R, Hilaire G. 5-Hydroxytryptamine modulates central respiratory activity in the newborn rat: an in vitro study. Eur J Pharmacol. 1991;192(1):89–95. doi: 10.1016/0014-2999(91)90073-y. [DOI] [PubMed] [Google Scholar]
- Morin D, Monteau R, Hilaire G. Compared effects of serotonin on cervical and hypoglossal inspiratory activities: an in vitro study in the newborn rat. J Physiol. 1992;451:605–629. doi: 10.1113/jphysiol.1992.sp019181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley ME, Rand CM, Berry-Kravis EM, Zhou L, Fan W, Weese-Mayer DE. Genetic variation in the HTR1A gene and sudden infant death syndrome. Am J Med Genet A. 2008;146(7):930–933. doi: 10.1002/ajmg.a.32112. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93(7):773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic Neurons Activate Chemosensitive Retrotrapezoid Nucleus Neurons by a pH-Independent Mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hasegawa H. Developmental role of tryptophan hydroxylase in the nervous system. Mol Neurobiol. 2007;35:45–54. doi: 10.1007/BF02700623. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Sgoifo A. Central 5-HT receptors in cardiovascular control during stress. Neurosci Biobehav Rev. 2009;33:95–106. doi: 10.1016/j.neubiorev.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Freire RC, Zin WA. Panic disorder and control of breathing. Respir Physiol Neurobiol. 2009;167:133–143. doi: 10.1016/j.resp.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Narita N, Narita M, Takashima S, Nakayama M, Nagai T, Okado N. Serotonin transporter gene variation is a risk factor for sudden infant death syndrome in the Japanese population. Pediatrics. 2001;107(4):690–692. doi: 10.1542/peds.107.4.690. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90(4):1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson GB, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugroschl J, Sano M. Current treatment and recent clinical research in Alzheimer’s disease. Mt Sinai J Med. 77:3–16. doi: 10.1002/msj.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon GM, Brouillette RT. Sleep and breathing in Prader-Willi syndrome. Pediatr Pulmonol. 2002;34:209–217. doi: 10.1002/ppul.10152. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Schneider A, Rohrbacher M, Schmidt H, Tegeder I, Geisslinger G, Lötsch J. The partial 5-hydroxytryptamine1A receptor agonist buspirone does not antagonize morphine-induced respiratory depression in humans. Clin Pharmacol Ther. 2007;81:59–68. doi: 10.1038/sj.clpt.6100018. [DOI] [PubMed] [Google Scholar]
- Olsen EB, Dempsey JA, McCrimmon DR. Serotonin and the control of ventilation in awake rats. J Clin Invest. 1979;64:689–693. doi: 10.1172/JCI109510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:13–18. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Futatsubashi M, Yagi S, Ueki T, Nakamura K. Altered brain 5-HT transporter and associated glucose metabolism in Alzheimer disease. J Nucl Med. 2009;50(8):1260–1266. doi: 10.2967/jnumed.109.063008. [DOI] [PubMed] [Google Scholar]
- Page IH. The vascular action of natural serotonin 5 and 7-hydroxytryptamine and tryptamine. J Pharmacol. 1952;105:58–73. [PubMed] [Google Scholar]
- Page IH, McCubbin JW. The variable arterial pressure responses to serotonin and laboratory animals and man. Circulation Res. 1953;1:354–362. doi: 10.1161/01.res.1.4.354. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Waeber C, Hoyer D, Mengod D. Distribution of serotonin receptors. Ann NY Acad Sci. 1990;600:36–52. doi: 10.1111/j.1749-6632.1990.tb16871.x. [DOI] [PubMed] [Google Scholar]
- Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry. 1993;150:1149–1157. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- Parks VJ, Sandison AG, Skinner SL, Whelan RF. The stimulation of respiration by 5-hydroxytryptamine in man. J Physiol. 1960;151:342–51. doi: 10.1113/jphysiol.1960.sp006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann Neurol. 2001;49:53–66. doi: 10.1002/1531-8249(200101)49:1<53::aid-ana30>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296(17):2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Hilaire G, Weese-Mayer DE. Medullary serotonin defects and respiratory dysfunction in sudden infant death syndrome. Respir Physiol Neurobiol. 2009;168:133–143. doi: 10.1016/j.resp.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Vallstedt A, Dias JM, Samad OA, Krumlauf R, Rijli FM, Brunet JF, Ericson J. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003;17(6):729–737. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky PM, de Castro D, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreacive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990;10:1091–1098. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncet L, Denoroy L, Dalmaz Y, Pequignot JM. Activity of tryptophan hydroxylase and content of indolamines in discrete brain regions after a long-term hypoxic exposure in the rat. Brain Res. 1997;765:122–128. doi: 10.1016/s0006-8993(97)00520-9. [DOI] [PubMed] [Google Scholar]
- Poon CS. Optimal interaction of respiratory and thermal regulation at rest and during exercise: role of a 5-HT-gated spinoparabrachial thermoafferent pathway. Respir Physiol Neurobiol. 2009;169:234–242. doi: 10.1016/j.resp.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Léna C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M, Adrien J. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25(49):11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Yuan G, Kumar GK. Reactive oxygen species facilitate oxygen sensing. Novartis Found Symp. 2006;272:95–99. [PubMed] [Google Scholar]
- Rahman MS, Thomas P. Molecular cloning, characterization and expression of two tryptophan hydroxylase (TPH-1 and TPH-2) genes in the hypothalamus of Atlantic croaker: down-regulation after chronic exposure to hypoxia. Neuroscience. 2009;158:751–765. doi: 10.1016/j.neuroscience.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Villalón CM. 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci. 2008;29(9):472–81. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Rand CM, Weese-Mayer DE, Zhou L, Maher BS, Cooper ME, Marazita ML, Berry-Kravis EM. Sudden infant death syndrome: Case-control frequency differences in paired like homeobox Phox2B gene. Am J Med Genet A. 2006;140(15):1687–1691. doi: 10.1002/ajmg.a.31336. [DOI] [PubMed] [Google Scholar]
- Rand CM, Berry-Kravis EM, Fan W, Weese-Mayer DE. HTR2A variation and sudden infant death syndrome: a case-control analysis. Acta Paediatr. 2009;98:58–61. doi: 10.1111/j.1651-2227.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. Crystalline Serotonin. Science. 1948;108:329–330. doi: 10.1126/science.108.2804.329. [DOI] [PubMed] [Google Scholar]
- Real C, Popa D, Seif I, Callebert J, Launay JM, Adrien J, Escourrou P. Sleep apneas are increased in mice lacking monoamine oxidase A. Sleep. 2007;30(10):1295–1302. doi: 10.1093/sleep/30.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real C, Seif I, Adrien J, Escourrou P. Ondansetron and fluoxetine reduce sleep apnea in mice lacking monoamine oxidase. Respir Physiol Neurobiol. 2009;168:230–238. doi: 10.1016/j.resp.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Reid G, Rand M. Physiological actions of the partially purified neurons serum vasoconstrictor (serotonin) Aust J Exp Biol Med Sci. 1951;29:401–415. doi: 10.1038/icb.1951.46. [DOI] [PubMed] [Google Scholar]
- Ren J, Lee S, Pagliardini S, Gérard M, Stewart CL, Greer JJ, Wevrick R. Absence of Ndn, encoding the Prader-Willi syndrome-deleted gene necdin, results in congenital deficiency of central respiratory drive in neonatal mice. J Neurosci. 2003;23(5):1569–1573. doi: 10.1523/JNEUROSCI.23-05-01569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5(6):449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill W, Kirkman JL, Bosma MM. Spontaneous activity in the developing mouse midbrain driven by an external pacemaker. Dev Neurobiol. 2009;69:689–704. doi: 10.1002/dneu.20725. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Rüb U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol Appl Neurobiol. 2000;26(6):553–567. doi: 10.1046/j.0305-1846.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- Rüb U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The autonomic higher order processing nuclei of the lower brain stem are among the early targets of the Alzheimer’s disease-related cytoskeletal pathology. Acta Neuropathol. 2001;101(6):555–564. doi: 10.1007/s004010000320. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, Gillis RA. Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors. J Pharmacol Exp Ther. 2000;292:704–713. [PubMed] [Google Scholar]
- Santhosh L, Estok KM, Vogel RS, Tamagnan GD, Baldwin RM, Mitsis EM, Macavoy MG, Staley JK, van Dyck CH. Regional distribution and behavioral correlates of 5-HT(2A) receptors in Alzheimer’s disease with [(18)F]deuteroaltanserin and PET. Psychiatry Res. 2009;173(3):212–217. doi: 10.1016/j.pscychresns.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Yonkman FF. Species differences in the respiratory cardiovascular response to serotonin (5-Hydroxytryptamine) J Pharmacol. 1954;111:84–98. [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellengerger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Stein MB, Millar TW, Larsen DK, Kryger H. Irregular breathing during sleep in patients with panic disorder. Am J Psychiatry. 1995;152:1168–1173. doi: 10.1176/ajp.152.8.1168. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Mulder AH. Serotonin immunoreactive neurons and their projections in the CNS. In: Bjorklund A, Hokferl, Kuhar M, editors. Handbook of Chemical Neuroanatomy. Classical Transmitters and Transmitter Receptors in the CNS. Part II. Vol. 3. Elsevier Science; New York: 1984. [Google Scholar]
- Steinbusch HW, Verhofstad AA, Penke B, Varga J, Joosten HW. Immunohistochemical characterization of monoamine-containing neurons in the central nervous system by antibodies to serotonin and noradrenalin. A study in the rat and the lamprey (lampetra fluviatilis) Acta Histochem Suppl. 1981;24:107–122. [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Huppke P, Gärtner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008a;160:21–27. doi: 10.1016/j.resp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Hilaire G, Dutschmann M. 8-OH-DPAT suppresses spontaneous central apneas in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008b;161:10–15. doi: 10.1016/j.resp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci. 2008;28(47):12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QJ, Berkowitz RG, Goodchild AK, Pilowsky PM. Serotonin inputs to inspiratory laryngeal motoneurons in the rat. J Comp Neurol. 2002;451:91–8. doi: 10.1002/cne.10329. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlén M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBötzinger complex. Nat Neurosci. 2009;12(8):1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Tork I, Hornung JP. Raphe nuclei and the serotonergic system. In: Paxinos, editor. The Human Nervous System. Academic Press; San Diego. CA: 1990. pp. 1001–1022. [Google Scholar]
- Tsang SW, Keene J, Hope T, Spence I, Francis PT, Wong PT, Chen CP, Lai MK. A 5-HTergic basis for hyperphagic eating changes in Alzheimer’s disease. J Neurol Sci. 2010;288:151–155. doi: 10.1016/j.jns.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Twarog BM, Page IH. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953;175:157–161. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- Twarog BM. Responses of a molluscan smooth muscle to acetylcholine and 5-hydroxytryptamine. J Cell Comp Physiol. 1954;44:141–163. doi: 10.1002/jcp.1030440112. [DOI] [PubMed] [Google Scholar]
- Upton AL, Salichon N, Lebrand C, Ravary A, Blakely R, Seif I, Gaspar P. Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. J Neurosci. 1999;19(16):7007–7024. doi: 10.1523/JNEUROSCI.19-16-07007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25(50):11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir Physiol Neurobiol. 2009;168:109–118. doi: 10.1016/j.resp.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Lajard AM, Dutschmann M, Hilaire G. Early abnormalities of post-sigh breathing in a mouse model of Rett syndrome. Respir Physiol Neurobiol. 2010a;170:173–182. doi: 10.1016/j.resp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Physiological definition of upper airway obstructions in mouse model for Rett Syndrome. Respir Physiol Neurobiol. 2010b doi: 10.1016/j.resp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Voss MD, de Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Maher BS, Silvestri JM, Curran ME, Marazita ML. Sudden infant death syndrome: association with a promoter polymorphism of the serotonin transporter gene. Am J Med Genet. 2003a;117A:268–274. doi: 10.1002/ajmg.a.20005. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Zhou L, Berry-Kravis EM, Maher BS, Silvestri JM, Marazita ML. Association of the serotonin transporter gene with sudden infant death syndrome: a haplotype analysis. Am J Med Genet. 2003b;122A:238–245. doi: 10.1002/ajmg.a.20427. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, Ramirez JM. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60(4):443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez JM. Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr Pulmonol. 2008;43(11):1045–1060. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- Wilken B, Lalley PM, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr. 1997;130:89–94. doi: 10.1016/s0022-3476(97)70315-9. [DOI] [PubMed] [Google Scholar]
- Woolley DW. The Biochemical Bases of Psychoses or the Serotonin Hypothesis about Mental Illness. John Wiley and Sons, Inc; New York, NY: 1963. [Google Scholar]
- Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol. 2008;162:117–25. doi: 10.1016/j.resp.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung LY, Kung HF, Yew DT. Localization of 5-HT1A and 5-HT2A positive cells in the brainstems of control age-matched and Alzheimer individuals. Age (Dordr) 2010 doi: 10.1007/s11357-010-9152-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Liu H, Zhang J, Zhang X, Wang X, Liu T, Liu P, Hao W. Association study of 5-HT transporter gene polymorphisms with obstructive sleep apnea syndrome in Chinese Han population. Sleep. 2008;31(11):1535–1541. doi: 10.1093/sleep/31.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S, Watrin F, Mebarek S, Marly F, Roussel M, Gire C, Diene G, Tauber M, Muscatelli F, Hilaire G. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J Neurosci. 2008;28(7):1745–1755. doi: 10.1523/JNEUROSCI.4334-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S, Tauber M, Muscatelli F. Breathing deficits of the Prader-Willi syndrome. Respir Physiol Neurobiol. 2009;168:119–124. doi: 10.1016/j.resp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Ellenberger HH, Feldman JL. Monoaminergic and GABAergic terminations in the phrenic nucleus of rat identified by immunohistochemical labelling. Neuroscience. 1989;31:105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, 4th, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26(49):12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]