Abstract
Introduction
Pancreatic cancer has a poor prognosis with <5% alive at 5 years, despite active surgical treatment. The study aim was to review patients undergoing pancreatic resection and assess the effect of clinical and pathological parameters on survival.
Patients and methods
All patients who had undergone radical pancreatic surgery, January 1996 to December 2008, were identified from the unit database. Additional information was retrieved from the patient records. The demographic, clinical, and pathological records were recorded using Microsoft Excel. Survival was assessed using Kaplan-Meier and predictors of survival determined by multinominal logistic regression and log rank test.
Results
126 patients were identified from the database. The majority (106) had a Whipple's procedure, 14 had a distal pancreatectomy and 6 had local periampullary excision. The average age of the Whipple's group of patients was 61.7 years (± 11.7) with most procedures performed for malignancy (n=100). Survival was worse with adenocarcinoma compared to all other pathologies (p=0.013), while periampullary tumours had a better prognosis compared to other locations (p=0.019). Survival decreased with poorer differentiation (p=0.001), increasing pT (p<0.001) and pN stage (p<0.001). Survival was worse with perineural (p=0.04) or lymphovascular invasion (p=0.05). A microscopic postive resection margin (R1) was associated with a worse survival (p=0.007). Tumour differentiation (p=0.001) and positive nodal status (p<0.001) were found to be independent predictors of mortality.
Conclusion
Tumour differentiation and nodal status are important predictors of outcome. A positive resection margin is associated with a poorer survival.
Keywords: Whipple's procedure, pancreatic cancer, survival
Introduction
Pancreatic cancer was the eleventh most common cancer in Northern Ireland in 2001, with 160-180 new cases per year in Northern Ireland and 6000 in the UK. Overall it accounts for approximately 2% of all cancer, with an average age of presentation of 69 years old and UK incidence of 10 per 100,000. The male to female ratio in Northern Ireland of pancreatic cancer diagnosis is 1.3:1. It carries a bleak prognosis, with less than 3% surviving three years following diagnosis.1 The Campbell report “Cancer services – investing for the future”, published in 2001, sought to address the provision of treatment for cancer and made several recommendations.2 Although centralisation of pancreatic surgical services in Northern Ireland has not been fully implemented, there has been an establishment of a multidisciplinary approach to cancer treatment, with clear pathways to incorporate multimodal treatment including palliative care when indicated. This is particularly important for pancreatic cancer, with an increased array of treatment interventions available and adjuvant chemotherapy standard since 2001. The involvement of oncologists, gastroenterologists, pancreatic surgeons, interventional radiologists, palliative care physicians and the primary care team in multidisciplinary discussion has resulted in improved management options and outcome, rendering the former nihilistic attitude to pancreatic cancer outdated.
Although pancreatic cancer is the main focus of this study, four anatomical locations, where cancer can arise to give similar symptoms, have been included, namely the duodenum, common bile duct, ampulla of Vater and the head of pancreas. The purpose of this study was to review patients undergoing pancreatic resection for cancer in any of these locations and assess the effect of clinical and pathological parameters on survival.
Anatomy
The pancreas gland lies transversely in the posterior portion of the epigastric and left hypochrondrial areas. The broad right lateral portion is called the head, which is separated from the body by a constriction known as the neck. The tapering left lateral portion is the tail, while the uncinate process emerges from the head at the angle between its lower and left lateral borders.
The head of the pancreas lies within the duodenal curve, with the upper, lower and right lateral borders lying intimately to the duodenum (Figure 1). The ascending portion of the duodenum lies in front of the left lateral border of the head. The anterior aspect is largely covered by the transverse colon, with the superior mesenteric artery crossing the uncinate process. The corresponding vein travels up behind the neck to form the portal vein. Posterior to the head of pancreas lies the inferior vena cava, the common bile duct, the renal veins, the aorta and right crus of the diaphragm.
Fig 1.
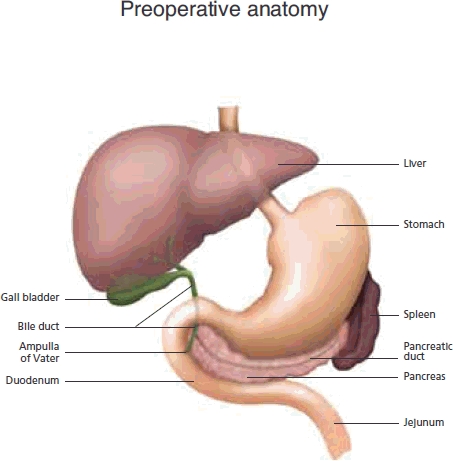
Normal peri-pancreatic anatomy
The body is covered anteriorly by the stomach, with the aorta, left kidney and vessels and the origin of the superior mesenteric artery lying behind. The duodenojejenal flexure lies inferiorly. The pancreatic tail continues to the left lateral aspect to finish at the lower part of the spleen. All these anatomical relationships are very important, as the degree of local invasion will often determine operative resectability.
The common bile duct (CBD) is formed by the left and right hepatic duct at the hilar confluence. It travels down as described above to drain into the second part of the duodenum, through the ampulla of Vater, beside the pancreatic duct. Periampullary tumours are usually defined as those in the last centrimetre of the CBD, where it traverses the ampullary papilla and duodenal wall.
Presentation and risk factors
Pancreatic cancer often progresses asymptomatically and then presents late, resulting in only a minority being suitable for resection. Symptoms can be quite non-specific and include nausea, anoxeria, jaundice, weight loss and abdominal pain. Pain, when present, is in the upper abdomen radiating through to the back and is often more associated with the body or tail of the pancreas. Alternatively, tumours of the head of the pancreas are more likely to present with painless jaundice and possibly steatorrhoea. Early cachexia is often seen in patients diagnosed with a tumour in the head of the pancreas, with weight loss of at least 10% of total body weight. Other less common symptoms include diabetes, diarrhoea and depression. Tumours arising from the ampulla of Vater are likely to present initially with jaundice and thus at an earlier stage, thus increasing their resection potential. An obstructive pattern to the liver function tests is likely to exist, while it may be helpful to check the associated tumour marker Carbohydrate Antigen 19-9 (CA 19-9), although this lacks sensitivity and specificity, particularly in the presence of obstructive jaundice. Endocrine tumours of the pancreas are less common and presenting symptoms relate to the hormone being secreted.
Similar to many other cancers, smoking makes a wellrecognised contribution to the risk of pancreatic cancer. A close relative with the condition doubles the risk, while the synergistic effect of these two gives an eight-fold increase to the risk. Other factors, including dietary, environmental and genetic aberrations have only weak associations.3
Pathology
Carcinoma of the pancreas usually arises from the ductal epithelial cells of the exocrine part of the gland. Neoplasms derived from the endocrine component of the galnd, so-called pancreatic endocrine neoplasms, are not uncommon, while acinar cell carcinomas, arising from the enzyme-producing pancreatic cells, are rare. Histologically the vast majority of malignant pancreatic cancers are adenocarcinoma, which can have varying degrees of mucin production. Subtypes of ductal carcinoma include adenosquamous, mucinous, signet ring cell and medullary.4 Some may arise within intraductal papillary mucinous neoplasms.
The anatomy described above is important to the pathological specimen evaluation tumour staging and assessment of resection margins. Despite guidelines from the pathology professional bodies of the UK and USA, there is as yet no agreed consensus on what constitutes a positive resection margin (R1).5–7 There are three main surfaces to a typical pancreaticoduodenectomy specimen, namely the anterior, posterior and superior mesenteric vein (SMV) groove surfaces. At pathological evaluation, the specimen is usually serially sliced in a horizontal plane perpendicular to the duodenal axis. A R1 (incomplete) resection is usually defined as tumour within 1mm of a margin microscopically.7 This may be primary tumour, or tumour within lymph nodes or lymphovascular channels.
Treatment
Investigations of a suspected malignancy in this area include ultrasound, computerised tomography, endoscopic and intraoperative ultrasound. The two important categories of management are chemotherapy and surgery. The latter alone offers potential cure, but the late presentation of disease renders many patients unresectable. The role of chemotherapy can be either palliative or as an adjunct to surgery.
The surgical procedure is a pancreaticoduodenectomy, or Whipple's procedure (Figure 2). This is named after Allen Oldfather Whipple (1881–1963), who pioneered the resection, although it was first described in 1898 by Codivilla. It involves removal of the distal half of the stomach, gallbladder, distal portion of the common bile duct, as well as the head of the pancreas, duodenum, proximal jejenum and lymph nodes. Reconstruction requires three anastomoses, namely pancreaticojejunostomy, choledochojejunostomy and gastrojejunostomy. Total pancreatectomy is not usually recommended and often leads to brittle diabetes. An important modification of the Whipple's procedure is the pylorus-sparing pancreaticoduodenectomy.8
Fig 2.
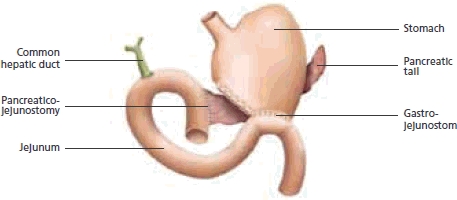
Anatomy and anastomoses after a Whipple's procedure
The aim of this retrospective study was to determine the survival patterns and pathological predictors of survival in patients undergoing pancreatic resection in one unit in Northern Ireland.
Patients and methods
Patient details
A retrospective review of the Hepatopancreaticobiliary unit database was performed and all patients who had major pancreatic surgery over a twelve-year period were included. Patient demographics were recoded, as well as operation and pathology results. The full pathological report of resected specimens was obtained from the department of pathology. The date of death, where appropriate was obtained from the hospital medical records, and subsequently verified with the patient's General Practitioner. All details were recorded on Microsoft Excel (Microsoft Corporation, USA) and analysed on SPSS (Version 13, SPSS Inc, Chicago, Il, USA).
Statistical analysis
Although some patients with non-neoplastic conditions were identified as having undergone pancreatic surgery, only those with a malignant process were included in the survival analysis and the determination of survival predictors. Survival analysis was performed using Kaplan-Meier for location of tumour, pT and pN stage, type of tumour and resection margin status. In this analysis, only patients who had undergone Whipple's procedure for malignancy were included. Each variable was further investigated using analysis of variance (ANOVA) to quantify the difference. Independent predictors of survival were determined using multinominal regression analysis. A p value <0.05 was considered significant in all tests.
>Results
Patient selection
One hundred and seventy-four patients, underwent a pancreatic-related procedure, between January 1996 and December 2008. After exclusion of necrosectomy, pseudocyst drainage and palliative bypass procedures, 126 patients were included in the study cohort.
Spectrum of operations performed
Six patients were managed with local excision of a malignant polyp in the periampullary region of the duodenum. The average age was 75.2 years (± 11.1). There was one perioperative death and two others have died, at follow-up periods of 3 and 7 months. The remaining three patients were alive 8, 10 and 13 months follow-up post-operatively.
Fifteen patients underwent distal pancreatectomy for either adenocarcinoma (n=4), endocrine neoplasm (n=8) or non-neoplastic conditions (n=3). The average age was 48.3 years (± 19.2). Three of the patients diagnosed with adenocarcinoma died, at a follow-up periods of 8, 8 and 47 months, with one still alive after 18 months.
During the study period 106 Whipple's procedures were performed by three consultant surgeons. The average age of the patients was 61.7 years (± 11.7). The operation was performed for a variety of diagnoses, including chronic pancreatitis (n=3), locally invasive hepatic flexure colonic adenocarcinoma (n=1), inflammatory abscess (n=1), primary sclerosing cholangitis (n=1) and a primary tumour (n=100). There were seven peri-operative deaths (6.6%) following a Whipple's procedure, with a overall median survival of 24 months. The number of Whipple's procedures performed annually has increased during the study period, with a decreasing trend of mortality, with no deaths in the last 3 years of the study period out of 52 operations.
Whipple's patients and survival trends
Histologically 92 of the 100 tumours proved to be ductal adenocarcinoma. The remaining comprised duodenal gastrointestinal stromal tumour (n=1), and well-differentiated endocrine tumours (n=6) and anaplastic carcinoma (n=1). Patients diagnosed with adenocarcinoma had a significantly poorer survival rate than patients diagnosed with the other tumours grouped together (p=0.013; Figure 3).
Fig 3.
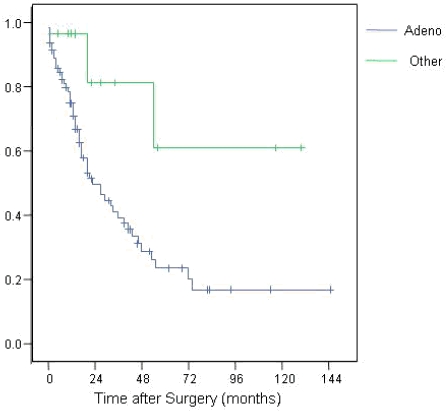
Survival trends of adenocarcinoma compared to other cancers
The 92 patients with adenocarcinoma had disease arising from four areas, namely the ampulla of Vater (n=31), duodenum (n=4), head of pancreas (n=45) and common bile duct (n=12). The survival trends according to the four different locations is illustrated in Figure 4 (p=0.019). Tumour differentiation varied from well-differentiated (n=13) to moderate (n=55) and poor (n=21), with survival affected accordingly (p=0.001; Figure 5). The differentiation of 3 tumours was not reported.
Fig 4.
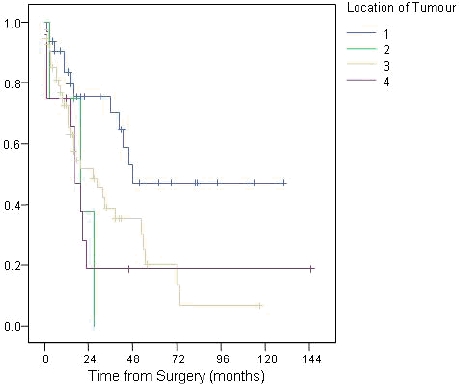
Survival trends after Whipple's according to location of tumour (1=ampulla of Vater, 2=duodenum, 3=head of pancreas, 4=common bile duct)
Fig 5.
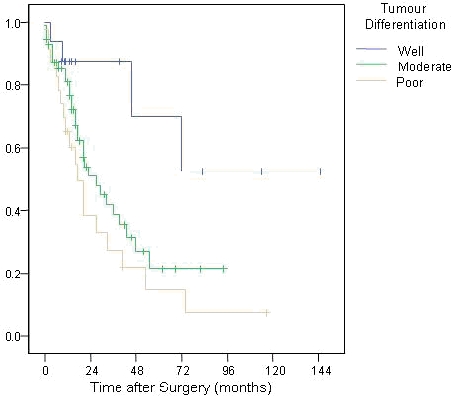
Survival trends according to tumour differentiation
Pathological (pT) tumour stages were pT1 (n=4), pT2 (n=20), pT3 (n=63) and pT4 (n=5), and this reflected outcome (p<0.001; Figure 6). The nodal status was positive (pN1) in 58 patients with an associated mortality (p<0.001; Figure 7).
Fig 6.
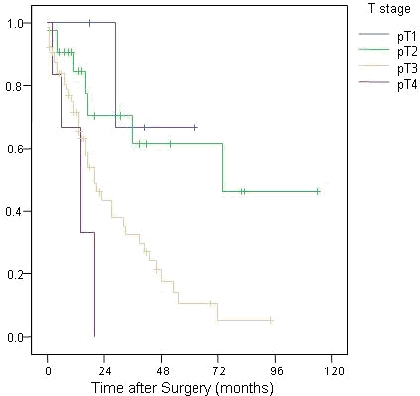
Survival trends according to T stage of tumour
Fig 7.
Survival trends according to N stage of tumour
The presence of perineurnal (p=0.044) or lymphovascular invasion (p=0.052) were also found to be poor prognostic indicators. Clear resection margins achieved microscopically in 56 operations improved outcome (p=0.007).
The mean size of tumour was 29mm (±12). Multinominial logistic regression, which included all pathological factors, demonstrated tumour differentiation (p=0.001) and nodal status (p<0.001) were the only significant predictors of mortality. Log-rank test revealed perineural invasion (p=0.012) and lymphovascular invasion (p=0.005) were significant predictors of survival in the months following surgery. In regard to these particular parameters, the relative risk of death was calculated for perineural invasion (1.76), lymphovascular invasion (1.97), positive resection margin (2.01) and positive nodal status (2.79).
Discussion
Pancreaticoduodenectomy is a major operation, carrying significant risk of morbidity and mortality. Complications include delayed gastric emptying (20%), wound infection (10%) and intra-abdominal collections or fistulae (15%). The latter is often secondary to anastomotic breakdown, particularly the pancreatico-jejunal anastomosis. Most eventualities can be treated conservatively and mortality in high-volume institutions is about 1%.11, 12 Therefore, patient selection is vital, to optimise the surgical curative rate.
The results of this study indicate that the location of the tumour can influence the survival pattern, where patients with periampullary tumours have a better prognosis. This reflects their earlier presentation and as a result the surgical resection margin was only involved in 3 (9.6%) patients of this sub-group. Consequently, while it was not the practice in these patients, some authors advocate the less radical pylorus-preserving resection to reduce morbidity, without compromise of oncological clearance.13, 14 Transduodenal local excision was performed on a small number of patients in this study, but carries significant risks, including pancreatitis and duodenal or pancreatic fistula formation. Pre and intra-operative determination of the site of the tumour is often difficult. However, only 75% of periampullary tumours are truly of pancreatic origin, of which at least 12% are of a more favourable variety than ductal adenocarcinoma of the pancreas.15 Thus, such patients may have a better than anticipated prognosis.
The involvement of the resection margin, known as R1 resection, is an important factor in prognosis following pancreatic resection.9, 14, 15 This varied according to tumour location, where 3 (9.6%) periampullary, 1 (25%) duodenual, 30 (54.5%) head of pancreas and 2 (16.7%) CBD tumour patients had a R1 resection. A positive surgical margin is generally accepted as a poor prognostic factor, so it is surprising that in a study of 360 patients, Raut et al found no statistical significance in its effect on survival.16 They attributed this fact to the variable reporting patterns of histology and the lack of differentiating between micro and macroscopically involved margins in other studies. However, evidence from the ESPAC-1 trial indicates that R1 tumours represent a biologically more aggressive cancer.17 In addition to a poorer response to surgery, the magnitude of benefit from chemotherapy is decreased in patients with R1 margins.
Histological evidence of tumour deposition in lymph nodes removed with the specimen has been shown to be an independent prognostic factor. This is in-keeping with the findings of other researchers and reflects the first step of tumour spread from the primary site.17, 18, More recent evidence reveals that the ratio of metastatically involved to retrieved lymph nodes is also an important prognostic indicator.18–20
Perineural invasion also emerged as influential in survival. Perineural growth is generally defined as cancer cells growing in close apposition to the nerves. The importance of this is seen in other cancers, such as gastric and breast, as well as those considered in this study.21–23 It is thought that the tissue plane around the nerve provides a path of least resistance to tumour advancement and dissemination, possibly further stimulated by nerve derived growth factors.24 It also results in a macroscopically clear margin to be declared involved when identified histologically.
Tumour differentiation is key to understanding the biological aggressiveness of the disease. This was an important parameter in this study and others.9, 13, 25 This fact is also seen in colorectal cancer, where poorer differentiation is associated with more widely disseminated disease, higher recurrence rates and poorer overall prognosis.26
A non-biological factor that is now thought to influence the outcome following pancreatic resection, is institutional experience.2 A Department of Health document suggested that the population of a pancreatic service catchment area should be at least 2 million.27 The population of Northern Ireland is projected to increase from 1.70 million in 2008 to 1.84 million in 2031, but it has been acknowledged that geographical constraints made this difficult in the province. Nevertheless, over recent years, centralisation of such surgery has been encouraged.28 As a consequence of this and of an increase in surgeon numbers, there is a greater proportion of pancreaticoduodenectomies in this study performed in the latter years. A target of 20 resections per institution has been proposed to optimise patient outcome. This is because outcome is influenced, not just by surgical technique, but also by the holistic post-operative care.28, 29 The requirement of a two million population springs from an incident rate of 1 per 100,000 per year, with a resultant resection rate of 10%. Although hospital case volume and outcome are associated, evidence to support these exact figures is lacking. However, the results of the present study show that with an increased number of Whipple's procedures performed annually for the last 3 years, there have been improved mortality.
In conclusion, pancreatic cancer prognosis improved with better tumour differentiation and a lack of lymph node involvement.
The authors have no conflict of interest.
REFERENCES
- Kinnear H, Gavin A, Mole D, Ranaghan L. Cancer Services Audit 2001, Pancreas (also includes cholangiocarcinomas and ampulla of Vater carcinomas) Belfast: N Ireland Cancer Registry; 2001. Available online from: http://www.qub.ac.uk/research-centres/nicr/FileStore/Filetoupload,23734,en.pdf Last accessed April 2010. [Google Scholar]
- Campbell H. Investing for the future: report of the cancer working group. (Campbell Report) Belfast: Department of Health and Social Services; 1996. [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzesse JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91(11):1410–27. doi: 10.1002/bjs.4794. [DOI] [PubMed] [Google Scholar]
- Albores-Saavedra J, Heffess C, Hruban RH, Klimstra D, Longnecker D. Recommendations for the reporting of pancreatic specimens containing malignant tumors. The Association of Directors of Anatomic and Surgical Pathology. Am J Clin Pathol. 1999;111(3):304–7. doi: 10.1093/ajcp/111.3.304. [DOI] [PubMed] [Google Scholar]
- The Royal College of Pathologists, editor. Minimum dataset for the histopathological reporting of pancreatic, ampulla of Vater and bile duct carcinoma. London: The Royal College of Pathologists; 2002. Standards and minimum datasets for reporting cancers. [Google Scholar]
- Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93(10):1232–7. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- Michalski CW, Weitz J, Buchler MW. Surgery insight: surgical management of pancreatic cancer. Nat Clin Pract Oncol. 2007;4(9):526–35. doi: 10.1038/ncponc0925. [DOI] [PubMed] [Google Scholar]
- Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talmini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications and outcomes. Ann Surg. 1997;226(3):248–60. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301(25):1364–9. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- Grace PA, Pitt HA, Tompkins RK, DenBesten L, Longmire WP., Jr Decreased morbidity and mortality after pancreaticoduodenectomy. Am J Surg. 1986;151(1):141–9. doi: 10.1016/0002-9610(86)90024-3. [DOI] [PubMed] [Google Scholar]
- Braasch JW, Deziel DJ, Rossi RL, Watkins E, Jr, Winter PF. Pyloric and gastric preserving pancreatic resection. Experience with 87 patients. Ann Surg. 1986;204(4):411–8. doi: 10.1097/00000658-198610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet M, Gamagami RA, Gilpin EA, Romeo O, Sasson A, Easter DW, et al. Factors influencing survival after resection for periampullary neoplasms. Am J Surg. 2000;180(1):13–7. doi: 10.1016/s0002-9610(00)00405-0. [DOI] [PubMed] [Google Scholar]
- Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, Mazzeo F. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73(4):212–8. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Allema JH, Reinders ME, van Gulik TM, Koelemay MJ, van Leeuwen DJ, de Wit LT, et al. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer. 1995;75(8):2069–76. doi: 10.1002/1097-0142(19950415)75:8<2069::aid-cncr2820750807>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, Bassi C, dervenis C, Fernandez-Cruz L, Lacaine F, Buckels J, deakkin M, Adab FA, Sutton R, Imrie C, Ihse I, Tihanyi T, Olah A, Pedrazzoli S, Spooner D, Kerr DJ, Friess H, Buchler MW. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234(6):758–68. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea G, Dennison AR, Ong SL, Pattenden CJ, Neal CP, Sutton CD, et al. Tumour characteristics predictive of survival following resection for ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2007;33(7):892–7. doi: 10.1016/j.ejso.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/ examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreactic adenocarcinoma. Am Surg. 2004;70(3):235–40. [PubMed] [Google Scholar]
- Sierzega M, Popiela T, Kulig J, Nowak K. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic cancer. Pancreas. 2006;33(3):240–5. doi: 10.1097/01.mpa.0000235306.96486.2a. [DOI] [PubMed] [Google Scholar]
- Scartozzi M, Galizia E, Verdecchia L, Berardi R, Graziano F, Catalano V, et al. Lymphatic, blood vessel and perineural invasion identifies early-stage high-risk radically resected gastric cancer patients. Br J Cancer. 2006;95(4):445–449. doi: 10.1038/sj.bjc.6603286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetintas SK, Kurt M, Ozkan L, Engin K, Gokgoz S, Tasdelen I. Factors influencing axillary node metastasis in breast cancer. Tumori. 2006;92(5):416–422. doi: 10.1177/030089160609200509. [DOI] [PubMed] [Google Scholar]
- Van Roest MHG, Gouw ASH, Peeters PMJG, Porte RJ, Slooff MJH, Fidler V, de Jong KP. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: perineural growth more important prognostic factor than tumour localization. Ann Surg. 2008;248(1):97–103. doi: 10.1097/SLA.0b013e31817b6609. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Buchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107(1):219–30. doi: 10.1016/0016-5085(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJW, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma: actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40(4):549–58. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- McDermott FT, Hughes ES, Pihl EA, Milne BJ, Price AB. Influence of tumour differentiation on survival after resection for rectal cancer in a series of 1296 patients. Aust N Z J Surg. 1984;54(1):53–8. doi: 10.1111/j.1445-2197.1984.tb06685.x. [DOI] [PubMed] [Google Scholar]
- NHS Executive, editor. Guidance on commissioning cancer service: improving outcomes in upper gastro-intestinal cancers: the manual. London: Department of Health; 2001. Available online from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4010025 Last accessed April 2010. [Google Scholar]
- Birkmeyer JD, Dimick JB. Understanding and reducing variation in surgical mortality. Annu Rev Med. 2009;60:405–15. doi: 10.1146/annurev.med.60.062107.101214. [DOI] [PubMed] [Google Scholar]
- Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245(5):777–83. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]


