Abstract
Selye’s pioneer the concept of biological stress in 1936 culminating to the identification of the corticotropin releasing factor (CRF) signaling pathways by Vale’s group in the last two decades. The characterization of the 41 amino-acid CRF and other peptide members of the mammalian CRF family, urocortin 1, urocortin 2 and urocortin 3, the cloning of CRF1 and CRF2 receptors, which display distinct affinity for CRF ligands, combined with the development of selective CRF receptor antagonists enable to unravel the importance of CRF1 receptor in the stress-related endocrine (activation of pituitary-adrenal axis), behavioral (anxiety/depression, altered feeding), autonomic (activation of sympathetic nervous system) and immune responses. The activation of CRF1 receptors is also part of key mechanisms through which various stressors impact the gut to stimulate colonic propulsive motor function and to induce hypersensitivity to colorectal distension as shown by the efficacy of the CRF1 receptor antagonists in blunting these stress-related components. The importance of CRF1 signaling pathways in the visceral response to stress in experimental animals provided new therapeutic approaches for treatment of functional bowel disorder such as irritable bowel syndrome, a multifactor functional disorder characterized by altered bowel habits and visceral pain for which stress has been implicated in the pathophysiology and is associated with anxiety-depression in subset of patients.
Keywords: CRF, CRF receptor, CRF antagonists, colonic motor function, irritable bowel syndrome, stress
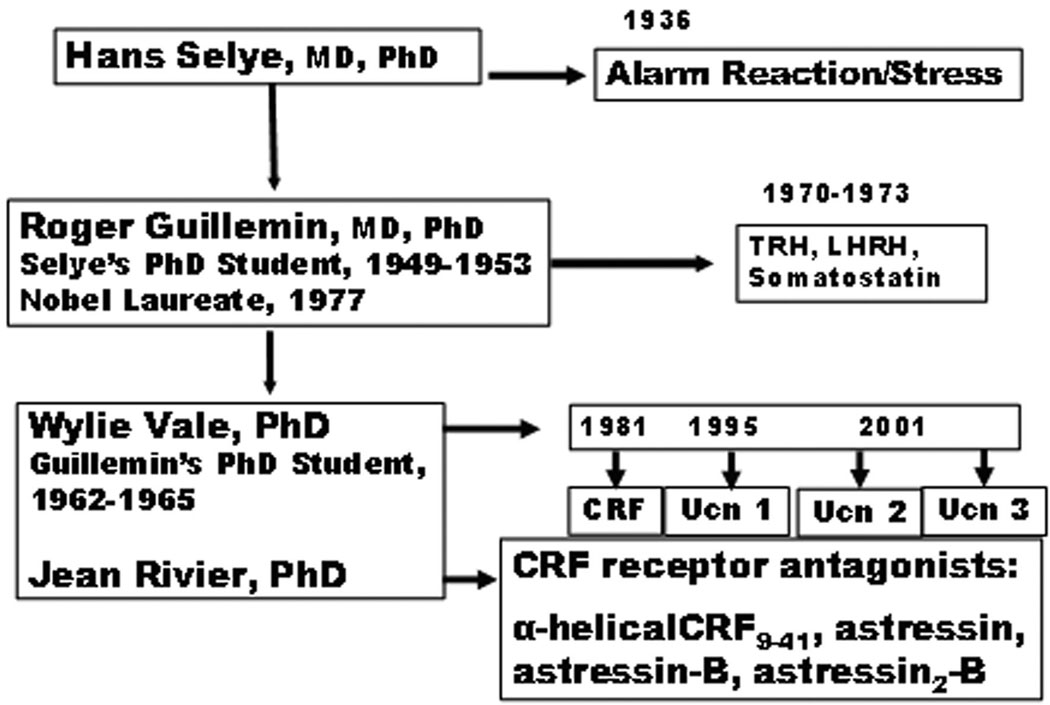
Hans Selye pioneered the concept of biological stress borrowing the word stress from the physic terminology that defines stress as the interaction between a deforming force and the resistance to it. His initial report in 1936 provided experimental evidence that the adrenal cortex, the immune system and the gut are commonly altered organs as shown by the hypertrophy of the adrenals, involution of the lymphatic nodes along with the occurrence gastric erosions in rats exposed to various nocuous chemical or physical stimuli.1 2 Subsequent contributions by Geoffrey Harris’ in the 1950’s established that stress-induced adrenocorticotropic hormone (ACTH) secretion involves “neural control via the hypothalamus and the hypophyseal portal vessels of the pituitary stalk”.3 Biochemical support for this pathway came fifteen years later when Guillemin, a former Selye’s Ph.D. student, and Schally’s group independently demonstrated the existence of hypothalamic factor(s) that elicited ACTH release from the rat pituitary.4,5 The name, corticotropin-releasing factor (CRF), was established in line with its ability to stimulate ACTH release and in keeping with the fact that its chemical structure was yet to be identified.4,5 Interestingly, although CRF was one of the first hypothalamic releasing factor to be named, its biochemical identification lingered for three subsequent decades. However, intense research to isolate CRF in the 1970’s led to the identification of other hypothalamic releasing factors composed of 3–10 amino acid (a.a.) including thyrotropin-releasing hormone (TRH) and luteinizing hormone-releasing hormone (LH-RH) for which Guillemin and Schally obtained the Nobel Price in 1977.6 However in 1981, Vale and his group contributed major mile-stones with the identification of the 41-a.a. peptide, CRF characterized from ovine hypothalami, and subsequently the cloning of CRF receptors and the development of specific CRF receptor antagonists (Fig. 1).7–10
Figure 1.
From Selye to unraveling the biochemical coding of stress response: the mentoring linkage.
A broad number of studies have documented the pathways through which CRF mediates the HPA axis limb of the stress response. The peptide is synthesized in a discrete population of hypophysiotrophic neurons in the parvocellular part of the paraventricular nucleus of the hypothalamus (pPVN) that also co-express arginine vasopressin (AVP). These peptides are released into the hypophyseal-portal blood vessels from axon terminals located in the Zona externa of the median eminence. Then CRF binds to specific CRF subtype 1 (CRF1) receptor located on membranes of anterior pituitary corticotrope cells to induce ACTH secretion, while AVP interacts with V1b pituitary receptors to potentiate the ACTH release.11,12
In keeping with the insightful concept suggested by Selye in the 50’s on the existence of a “first mediator” that integrates the adaptive bodily response to stress,13 the biological actions of CRF expanded quickly far beyond the neuroendocrine component of stress response. Consistent experimental reports showed that CRF injected into the brain recapitulated the overall behavioral (anxiety/depression, alterations of feeding), autonomic (sympathetic and sacral parasympathetic activation), immune, metabolic and visceral responses induced by various systemic or cognitive stressors.14–18 In particular, early observations in rats and dogs showed that exogenous administration of CRF into the brain or peripherally mimicked Walter Cannon’s early experimental findings that stress inhibited gastric acid secretion and emptying.19–22 In addition, blockade of CRF receptors by the injection of peptide CRF antagonist, α-helical CRF9–41, prevented gastric inhibition of acid and emptying induced by exposing rats to restraint or abdominal surgery.21,23 These data supported a physiological role of CRF signaling pathways in the alterations of gastric secretory and motor functions in rodents exposed to various stressors and paved the way to delineate the central and peripheral sites of CRF actions, the CRF receptor subtypes and autonomic effectors involved in mediating the alterations of gut function elicited by stress exposure.16 In addition, these observations provided new venues for pharmacological interventions in stress-related functional bowel disorders.24
This review will address briefly the state-of-knowledge on CRF signaling system including the expanding members of mammalian CRF-related peptides, their pharmacological characterization on cloned CRF1 and CRF2 receptors and the development of selective CRF receptor subtype antagonists. We will integrate these advances to the understanding of stress-induced alterations of gut function particularly the stimulation of colonic propagative motility and the development of hyperalgesia in experimental animals. Lastly, emergent clinical evidence supporting therapeutic use of CRF1 receptor antagonists to alleviate stress-responsive functional bowel diseases such as irritable bowel syndrome (IBS) often associated with anxiety and depression co-morbidity will be presented.
CRF SIGNALING PATHWAYS
Seminal contributions to the identification of the CRF signaling pathways opened a new era of research which expended greatly the understanding of the biochemical coding of stress-related processes.25
The Family of CRF Peptides
In mammals, CRF is a well-conserved 41-a.a. peptide with an identical primary structure in humans, primates, dogs, horses and rodents, while ovine CRF differs byseven a.a.26 In 1995, urocortin 1 (Ucn 1, also known as urocortin)27 was characterized from rat midbrain as a 40-a.a. peptide with 45% sequence identity with human/rat(r/h)CRF.28 Similarly to CRF, Ucn 1 structure is highly conserved across mammalian species since human shares 95% identity with rat, mice and sheep that are 100% homologous.28–30 Brain mapping studies revealed the existence of mismatches between the distribution CRF and Ucn 1 and that of CRF receptors in specific area.31 This triggered the search for additional endogenous CRF-related agonists resulting in the cloning of two novel putative CRF-related peptides named urocortin 2 (Ucn 2) and urocortin 3 (Ucn 3).27,32–34 The mouse Ucn 2 is a 38-a.a. peptide that displays 76% homology with the human Ucn 2 counterpart27,33,34 and more distant homology with r/hCRF (34%), and r/mUcn 1 (42%).33 Mouse urocortin 3 (mUcn 3) and human Ucn 3,32 also named human stresscopin, 34 are more distantly related to r/hCRF, and r/hUcn 1 with 18% and 21% homology respectively.32
CRF Receptors
CRF ligands interact with CRF1 and/or CRF2 receptors. The receptors were cloned from two distinct genes that have 70% identity at the a.a. level.14 Both CRF1 and CRF2 belong to the B1 subfamily of seven-transmembrane domain receptors.35 Radioreceptor and functional assays have demonstrated that CRF1 and CRF2 receptors differ considerably in their binding characteristics.27,36 CRF1 receptor displays high affinity to CRF and Ucn 1 but shows no appreciable binding affinity to Ucn 2 and Ucn 3. In contrast, CRF2 binds to Ucn 1, Ucn 2 and Ucn 3 with greater affinity than CRF making this receptor subtype highly selective for Ucns signaling.27,28 Both CRF1 and CRF2 receptors exist in a number of splice variants.37,38 In rodents, CRF2 receptor is expressed in two functional isoforms, α and β, that differ in their N-terminal domains.39 The CRF2α and CRF2β variants have similar pharmacological profiles, but distinct central vs. peripheral distribution, respectively in rats.37,40,41 In mice brain, the presence of a soluble (s)CRF2α splice variant that binds CRF1 ligands and inhibits the cellular response to CRF or Ucn 1 indicates a possible functional relevance to modulate CRF/Ucn 1 actions.42 While ovine CRF and to a lesser extent r/hCRF are considered as preferential CRF1 agonists, so far there is no selective endogenous CRF1 agonist.43 To selectively activate CRF1 receptor, CRF1 peptide agonists, cortagine and stressin1-A have been recently developed that display 100-fold greater affinity on CRF1 versus CRF2 receptors.44,45
The CRF ligand-CRF receptor interactions is primarily coupled to Gαs and adenyl cyclase activation, which leads to cAMP-dependent cascades including protein kinase A.36,37,46 Another sensitive and consistent intracellular signaling resulting from the activation of CRF receptors is the phosphorylation of extracellular–signal regulated kinases-1 and -2 (ERK1/2) that is cell type and ligand specific.38,47,48 Activation of ERK1/2 pathway is involved in memory, learning process, and stress related behaviors, especially in hippocampus, amygdala and cortex.49
CRF Receptor Antagonists
Key to the assessment of the role of endogenous CRF ligands and CRF receptors in the stress response was the development of specific CRF antagonists. Earlier studies relied on the use of non-selective CRF1/CRF2 peptide antagonists, mainly α-helical CRF9–41,9 D-Phe12CRF12–41,50 followed by the potent and long acting, astressin and astressin-B.51 Recently, selective peptide CRF2 receptor antagonists, namely antisauvagine-30 and the more potent, long acting analog, astressin2-B, were developed.10,52 As a whole, peptide antagonists generally have a poor penetrance into the brain. For instance, astressin injected intravenously (iv) at a dose blocking iv CRF-induced delayed gastric emptying, did not influence the inhibition of gastric transit induced by CRF-injected into the cerebrospinal fluid (CSF) at the level of the cisterna magna in rats.53 Due to the intense interest to target the CRF1 system in the context of various human pathologies including anxiety disorders,54,55 extensive pharmaceutical industry efforts resulted in the development of a variety of CRF1 antagonists.56–58 These compounds are small hydrophobic orally active molecules that cross the blood-brain barrier. 57,58 Their availability has been instrumental in enabling a wide range of preclinical studies that established the role of brain CRF-CRF1 signaling pathways in stress-related endocrine, anxiogenic behavior, autonomic and visceral responses.14,59,60 For instance the role of CRF-CRF1 receptor activation in the HPA induced by stress was demonstrated using CRF antibody along with various CRF1 receptor antagonists which inhibited the rise in circulating levels of ACTH and corticosterone in response to various psychological, physical and immune stressors while Ucn 1 antibody did not.11,61 With regard to the visceral response, growing evidence indicates that CRF1 signaling pathways contribute also to altered colonic function and hyperalgesia induced by stress independently from the HPA activation.60
CENTRAL CRF SIGNALING PATHWAYS: FUNCTIONAL ROLE IN STRESS-RELATED COLONIC STIMULATION AND VISCERAL HYPERALGESIA: PRECLINICAL EVIDENCE
Central Injection of CRF induces a CRF1 Receptor Mediated Stimulation of Colonic Motor Function
In rodents, several stressors as diverse as restraint, open field test, conditioned fear, loud sound, restraint, cold exposure, fear conditioning, water avoidance, inescapable foot or tail shocks, and central injection of interleukin-1 stimulate colonic motor function monitored by the shortening of colonic transit time, increased motility index and/or defecation.21,62–67 Primates exposed to a social stressor, manifested stress responses including urination and defecation.15 Likewise, in humans, various stressors, such as dichotomous listening, painful stimuli of intermittent hand immersion in cold water, fear, anxiety and stressful interviews, increased colonic motility in healthy subjects.68–72
CRF injected into the lateral brain ventricle (icv) mimicked stress-related colonic functional alterations as shown by the stimulation of colonic transit, defecation and at highest doses, the induction of diarrhea in experimental animals as reviewed recently. 24,73 Ucn 1 injected icv also stimulates colonic transit in mice.66 Consistent with an acceleration of propulsive colonic transit, icv CRF stimulates motor activity in the proximal and distal colon and induces the occurrence of colonic spike burst activity in rats.62,67,74–76 Pharmacological studies showed that the CRF1 receptors is the subtype involved in CRF action. This was supported by the rank order of potency of icv ovine CRF >r/hCRF and Ucn 1>Ucn 2>Ucn 3 to induce defecation in mice consistent with a CRF1 mediated effect.66 In addition, the icv injection of selective CRF1 antagonists, NBI-35965 and NBI-27914 blocked icv CRF- and Ucn 1-induced acceleration of colonic transit, and increased in the colonic motility index while icv injection of the selective CRF2 agonist, astressin2-B or anti-sauvagine had no effect in rats and mice.66,76,77
Brain nuclei responsive to CRF resulting in the stimulation of colonic motor function have been localized in specific hypothalamic (PVN) and pontine areas such as the noradrenergic, locus coeruleus (LC)/subLC and Barrington nucleus.78–81 These responsive sites are also those involved in CRF-induced anxiety and depression.82,83 Pharmacologic and surgical approaches established the pathway through which central CRF stimulates colonic motor function. It is not related to the concomitant stimulation of HPA but involves the activation of celiac vagal and sacral parasympathetic outflow to the pelvic organs.62,67,78,81,84 Effector mechanisms within the colon that activate colonic transit involve parasympathetic mediated activation of colonic serotonin (5-HT) acting on 5-HT3 and 5-HT4 receptors as shown by the blockade of colonic motor stimulation to icv CRF by atropine and by subcutaneous or intracolonic administration of 5-HT3 antagonists, granistron, ramosteron, ondansetron and azasetron, and 5-HT4 antagonist, SB-204070 while icv injection of these antagonists had no effects.67,76,85 This is also supported by the demonstration that icv injection of CRF increases the 5-HT content in the feces of the rat proximal colon.67
CRF1 Receptor Antagonists Alleviate Stress-Induced Stimulation of Colonic Motor Function
Substantial preclinical evidence has accumulated to support that stress-related stimulation of colonic motor function is primarily mediated by the activation of CRF1 signaling pathway. First, there is mimicry between the colonic response to stress and that induced by centrally administered CRF1 receptor agonists.60,73 Moreover, the CRF receptor antagonist, α-helical CRF9–41 injected icv abolishes wrap restraint- and partial body restraint-induced stimulation of colonic transit in female and male rats.21,86 α-Helical CRF9–41, D-Phe12CRF12–41, and astressin injected icv also antagonized the increased frequency of colonic spike-bursts induced by conditioned fear stress and reduces the defecatory response to wrap restraint in rats.21,62,87–89 Furthermore an array of selective CRF1 antagonists, namely CP-154,526, CRA 1000, NBI 27914, NBI 35965, antalarmin and JTC-017 injected either icv or peripherally alleviate various stressors (restraint, water avoidance stress, elevated plus maze, social intruder)-induced stimulation of colonic motor function.24,66,73 By contrast astressin2-B injected icv at doses that blocked CRF2 mediated inhibition of gastric emptying did not alter the stress-related defecation in mice.66 In an open field test, CRF1 knockout mice had significantly less defecation than the wild type.90 Taken together these data are consistent with the involvement of CRF1 signaling pathways in the colonic motor response to acute stress. Of particular interest for future clinical use of these compounds are convergent reports showing that CRF receptor antagonists did not impact on the basal and postprandial functioning of the colon in non-stress conditions in rodents.60
CRF1 Receptors Mediate Stress-Induced Colonic Hyperalgesia
Gué et al. provided the first evidence in rats that icv injection of CRF mimicked stress-induced colonic hyperalgesia to colorectal distention (CRD) and that icv injection of α-helical CRF9–41 blocked icv CRF and restraint-induced colonic sensitization to CRD.91 Thereafter the stress-related visceral hyperalgesia was characterized to involve central CRF1 receptors and expanded to a number of experimental models of CRD-induced hypersensitivity using several selective CRF1 antagonists as recently reviewed.24 For instance, icv CRF-induced colonic hypersensitivity to a tonic CRD is no longer observed in rats pretreated with antalarmin.92 In the anxiety prone Wistar Kyoto rats, intracolonic instillation of acetic acid-induced colonic hyperalgesia to a 2nd set of tonic CRD is inhibited by antalarmin.92 In a model of neonatal maternal separation, the colonic hypersensitivity in adult rats exposed acutely to water avoidance stress and phasic CRD is blocked by oral pretreatment with NBI 35965.93 Likewise, acute water avoidance-induced a delayed colonic hypersensitivity to phasic CRD is abolished by CP-154,526.94 and colonic hypersensitivity induced by two sets of tonic CRD in female rats is alleviated by antalarmin.95 Lastly, microinjection of α-helical CRF9–41 into the hippocampus or peripheral injection of the CRF1 antagonist, JTC-017 results in the reduction of visceral pain induced by noxious tonic CRD along with the anxiety response to CRD in rats.96
The central and peripheral mechanisms through which activation of CRF1 receptor and their blockade influence the development of visceral hyperalgesia are still to be defined. CRF1 receptor antagonists may act by dampening CRD-induced activation of brain noradrenergic pathways. Recent electrophysiological studies in anesthetized rats showed that [DPhe12]CRF12–41, administered icv or microinfused into the LC, astressin injected into the cisterna magna and selective CRF1 antagonist, NBI 35965 given iv prevented LC neuronal activation in response to central injection of CRF and CRD at submaximal distention (40 mmHg).97,98 In addition, peripheral injection of CRF1 antagonists JTC-017 reduced the rise in noradrenaline levels in the hippocampus induced by CRD. Bursting activity in the LC is associated with the release of noradrenaline in the cortical and limbic rostral efferent projections of the LC leading to arousal and anxiogenic response.99 Still to be delineated is how blockade of CRF1 receptors influence the neural pain pathways in the brain and spinal cord involved in the development of hyperalgesia. In the periphery, central CRF may contribute to colonic hypersensitivity by activating colonic mast cells. The icv injection of CRF induced a rapid increase in the release of rat mast cell protease II, prostaglandin E2 and histamine levels in the colon.100,101 α-Helical CRF9–41 injected icv prevents both icv CRF and stress-induced enhancement of colonic mast cell content of histamine.101 In addition, mast cell stabilizer doxantrazole prevents icv CRF and stress-induced colonic hypersensitivity to a 2nd set of CRD.91
CRF1 SIGNALING PATHWAYS AS A NEW THERAPEUTIC TARGET FOR IRRITABLE BOWEL SYNDROME
IBS, Stress, Co-Morbidity with Anxiety/Depression
According to ROME-III, IBS is classified as a functional bowel disorder (category C-1), associated with recurrent changes in bowel habits, increased sensitivity to CRD, and abdominal discomfort/pain/bloating that are occurring in the absence of detectable organic disorders in routine examination.102 IBS subgroups have been based on the predominance of symptoms: diarrhea, constipation, alternating constipation and diarrhea, and abdominal pain.103 In the US, 10–15% of the population suffers from this condition with women seeking healthcare services 2 or 3 times more frequently than men.104 IBS patients also complain of additional symptoms not being included into the general diagnostic criteria of IBS leading to the concept of co-morbidity which is to be found in 29% to 92% of IBS patients.105 In particular, IBS patients have a high prevalence of co-existent psychiatric disorders, predominantly anxiety/depression and it has also been associated with fibromyalgia.105–108 Moreover, stressful life events, including history of major traumatic events in childhood are important risk factors for IBS and influence the onset and severity of symptoms.109,110 Prospective studies established that there is a 4%–31% incidence of post infectious IBS following bacterial gastroenteritis and stressful life events increase the risk to develop such post-infection IBS.111,112
CRF Signaling System and IBS
The convergent preclinical data pointing to the role of central and peripheral CRF1 signaling pathways in stress-related processes including those related to altered colonic function and visceral hypersensitivity increased interest to target CRF receptors as a new promising therapeutic intervention for IBS diarrhea predominant symptoms (Table 1).24,73,113 The role of CRF signaling system at central and/or peripheral levels or a combination of both is gaining clinical recognition as part of the neurobiological common denominator of IBS symptoms susceptible to stress and anxiety/depression.114,115 For instance, there is evidence for elevated levels of CRF in the LC in patients with major depression.116 Elevated concentrations of CRF in the CSF is also present in patients with anxiety and vulnerability to stress as well as those suffering from obsessive compulsive disorders, posttraumatic stress disorders or childhood trauma.117–119 Carpenter et al. showed in a controlled study with depressed and healthy subjects that CRF in the CSF is a predictor of perceived aversive early life experiences.120 In patients suffering from fibromyalgia, CSF levels of CRF are associated with both pain symptoms and autonomic dysfunction but not with fatigue.121 Investigations in IBS patients indicate also that there is an overactivity of the HPA and enhanced plasma CRF response to mental stress.122,123
Table 1.
Preclinical evidence to target CRF1 receptors in IBS-diarrhea predominant symptoms
| IBS-diarrhea: predominant features: Exacerbated by stress | CRF1 antagonists: block stress-related: |
|---|---|
| Anxiety/depression comorbidity | Anxiety/depression |
| Increased colonic motility | Stimulation of colonic motility |
| Ion transport dysfunction | Diarrhea |
| Change in mast cells | Activation of mast cells |
| Increase barrier permeability | Increase barrier permeability |
| Lower pain threshold to colorectal distention | Hypersensibility to colorectal distention |
PhaseI clinical studies indicate that CRF administration reproduced features of IBS symptoms in healthy volunteers consistent with experimental animals and also enhanced those in IBS patients.73,113,124 Reports indicate that systemic administration of CRF increases colonic motility and the response is exaggerated in IBS compared with healthy subjects.124 In particular, CRF induces the occurrence of clustered contractions in the descending and sigmoid colon along with abdominal pain and discomfort in IBS patients that are not observed in healthy controls.124 Other studies showed that in healthy human subjects, the administration of CRF decreased the visceral pain threshold to repetitive rectal distensions, and enhanced the intensity of discomfort sensation to CRD. 125,126 Recently, CRF was found to activate subepithelial mast cells and stimulate transcellular uptake of protein antigens in the mucosa in colonic biopsies of healthy subjects127, as previously reported in experimental animals.113,128 Since increased uptake of antigen-sized macromolecules is associated with inflammation, a role of CRF in this process will be consistent with increasing evidence that IBS patients display a low graded colonic inflammation, including plasmatic cytokines (interleukin-6), intraepithelial lymphocytes, mast cell degranulation and increased permeability. 122,129
In support of CRF signaling pathways in the pathophysiology of IBS, Fukudo’s group reported that the peripheral injection of α-helical CRF9–41 prevents rectal electrical stimulation-induced enhanced sigmoid colonic motility, visceral perception and anxiety in IBS patients compared to healthy controls without altering the HPA axis.130 In addition the CRF antagonists was recently reported to almost normalize the altered EEG activities in IBS patients under basal and in response to CRD. 131
CONCLUSIONS
As we are entering Selye’s centennial anniversary (1907–1982), and as former Ph.D. Selye student (YT), it is gratifying to see how much has been accomplished since his pioneer description of the stress concept emphasing the activation of the HPA as key component. Through the relentless efforts of several groups, the biochemical coding of the CRF/Ucns-CRF1/CRF2 receptor signaling pathways has been identified and characterized to coordinate the various facets of the response to stress. In particular, the activation of CRF1 receptors plays a pivotal role in the HPA stimulation and anxiogenic response to various stressors. In addition, consistent preclinical reports established that the activation of CRF1 receptors by CRF also recapitulates key symptomes of IBS-diarrhea predominent patients as it relates to stimulation colonic motility, watery diarrhea, mucus secretion, mast cell activation, visceral hyperalgesia, and anxiogenic/hypervigilance that are alleviated by various selective CRF1 receptors antagonists (Table 1). Preliminary clinical studies also support a role of the CRF signaling system in the induction of IBS-like symptoms in healthy subjects and highten sensivity in IBS patients that are alleviated by a peptide CRF antagonist.115 Targetting CRF1 receptor may provide a new therapeutic venue in the treatment of IBS.24
ACKNOWLEDGEMENTS
The authors’ work was supported by the National Institute of Arthritis, Metabolism and Digestive Diseases, Grants R01 DK-33061, R01 DK-57236, DK-41301 (Animal Core), P50 AR-049550 and VA Merit and Senior Scientist Awards. The authors thank Drs. J. Rivier (Salk Institute, La Jolla, CA), D. Grigoriadis (Neurocrine Biosciences Inc., La Jolla, CA) and E.D. Pagani (Center Research Division, Pfizer Inc., Croton, CT) for the generous supply of different CRF agonists and antagonists used in the studies. Dr S. Brunnhuber is in support by the University of Wuerzburg/Germany and the European Academy of Science and Arts (F. Unger) that is greatly acknowledged.
REFERENCES
- 1.Selye H. Syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 2.Selye H, Collip JB. Fundamental factors in the interpretation of stimuli infuencing endocrine glands. Endocrinology. 1936;20:667–672. [Google Scholar]
- 3.Harris GW. The hypothalamus and endocrine glands. Br Med Bull. 1950;6:345–350. doi: 10.1093/oxfordjournals.bmb.a073628. [DOI] [PubMed] [Google Scholar]
- 4.Guillemin R, Rosenberg B. Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 1955;57:599–607. doi: 10.1210/endo-57-5-599. [DOI] [PubMed] [Google Scholar]
- 5.Saffran M, Schally AV, Benfey BG. Stimulation of the release of corticotropin from the adenohypophysis by a neurohypophysial factor. Endocrinology. 1955;57:439–444. doi: 10.1210/endo-57-4-439. [DOI] [PubMed] [Google Scholar]
- 6.Guillemin R. Hypothalamic hormones a.k.a. hypothalamic releasing factors. J Endocrinol. 2005;184:11–28. doi: 10.1677/joe.1.05883. [DOI] [PubMed] [Google Scholar]
- 7.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 8.Rivier J, Gulyas J, Corrigan A, Martinez V, Craig AG, Taché Y, Vale W, Rivier C. Astressin analogues (corticotropin-releasing factor antagonists) with extended duration of action in the rat. J Med Chem. 1998;41:5012–5019. doi: 10.1021/jm980426c. [DOI] [PubMed] [Google Scholar]
- 9.Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin-releasing factor: effect on ACTH secretion in the rat. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- 10.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, Digruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 11.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu TA, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selye H. Stress in Health and Disease. AnonymousBoston-London: Butterworths; 1976. Theories; pp. 928–1148. [Google Scholar]
- 14.Bale TL, Vale WW. CRF and CRF receptor: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 15.Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, Mccann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci U S A. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- 17.De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 18.Dunn AJ, Berridge CW. Physiological and behavioral response to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 19.Cannon WB. Bodily changes in pain, hunger, fear and rage. Branford, CT: Boston; 1953. pp. 1–404. [Google Scholar]
- 20.Taché Y, Goto Y, Gunion MW, Vale W, Rivier J, Brown M. Inhibition of gastric acid secretion in rats by intracerebral injection of corticotropin-releasing factor. Science. 1983;222:935–937. doi: 10.1126/science.6415815. [DOI] [PubMed] [Google Scholar]
- 21.Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253:G582–G586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- 22.Taché Y, Goto Y, Gunion M, Rivier J, Debas H. Inhibition of gastric acid secretion in rats and in dogs by corticotropin-releasing factor. Gastroenterology. 1984;86:281–286. [PubMed] [Google Scholar]
- 23.Stephens RL, Yang H, Rivier J, Taché Y. Intracisternal injection of CRF antagonist blocks surgical stress-induced inhibition of gastric secretion in the rat. Peptides. 1988;9:1067–1070. doi: 10.1016/0196-9781(88)90090-3. [DOI] [PubMed] [Google Scholar]
- 24.Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:1–18. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- 25.Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- 27.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Donaldson CJ, Smith GW, Vale WW. The structures of the mouse and human urocortin genes (Ucn and UCN) Genomics. 1998;50:23–33. doi: 10.1006/geno.1998.5292. [DOI] [PubMed] [Google Scholar]
- 30.Cepoi D, Sutton S, Arias C, Sawchenko P, Vale WW. Ovine genomic urocortin: cloning, pharmacologic characterization, and distribution of central mRNA. Brain Res Mol Brain Res. 1999;68:109–118. doi: 10.1016/s0169-328x(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 31.Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- 32.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 35.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 36.Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- 37.Wu SV, Yuan P-Q, Wang L, Peng YL, Chen C-Y, Taché Y. Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology. 2007;148:1675–1687. doi: 10.1210/en.2006-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 39.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ardati A, Goetschy V, Gottowick J, Henriot S, Valdenaire O, Deuschle U, Kilpatrick GJ. Human CRF2 α and β splice variants: pharmacological characterization using radioligand binding and a luciferase gene expression assay. Neuropharmacology. 1999;38:441–448. doi: 10.1016/s0028-3908(98)00201-9. [DOI] [PubMed] [Google Scholar]
- 41.Suman-Chauhan N, Carnell P, Franks R, Webdale L, Gee NS, Mcnulty S, Rossant CJ, Van Leeuwen D, Mackenzie R, Hall MD. Expression and characterisation of human and rat CRF2alpha receptors. Eur J Pharmacol. 1999;379:219–227. doi: 10.1016/s0014-2999(99)00505-1. [DOI] [PubMed] [Google Scholar]
- 42.Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. A soluble mouse brain splice variant of type 2{alpha} corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci U S A. 2005;102:2620–2625. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotropin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- 44.Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci U S A. 2004;101:9468–9473. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivier J, Gulyas J, Kunitake K, Digruccio M, Cantle J, Perrin MH, Donaldson C, Vaughan J, Million M, Gourcerol G, Adelson D, Rivier C, Taché Y, Vale W. Stressin1-A, a potent corticotropin releasing factor receptor 1 (CRF1)-selective peptide agonist. J Med Chem. 2007;50:1668–1674. doi: 10.1021/jm0613875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traver S, Marien M, Martin E, Hirsch EC, Michel PP. The phenotypic differentiation of locus ceruleus noradrenergic neurons mediated by brain-derived neurotrophic factor is enhanced by corticotropin releasing factor through the activation of a cAMP-dependent signaling pathway. Mol Pharmacol. 2006;70:30–40. doi: 10.1124/mol.106.022715. [DOI] [PubMed] [Google Scholar]
- 47.Refojo D, Echenique C, Muller MB, Reul JM, Deussing JM, Wurst W, Sillaber I, Paez-Pereda M, Holsboer F, Arzt E. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc Natl Acad Sci U S A. 2005;102:6183–6188. doi: 10.1073/pnas.0502070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grammatopoulos DK, Randeva HS, Levine MA, Katsanou ES, Hillhouse EW. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1alpha and R2β CRH receptor subtypes and stimulation of Gq-proteins. Mol Endocrinol. 2000;14:2076–2091. doi: 10.1210/mend.14.12.0574. [DOI] [PubMed] [Google Scholar]
- 49.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 50.Hernandez JF, Kornreich W, Rivier C, Miranda A, Yamamoto G, Andrews J, Taché Y, Vale W, Rivier J. Synthesis and relative potency of new constrained CRF antagonists. J Med Chem. 1993;36:2860–2867. doi: 10.1021/jm00072a004. [DOI] [PubMed] [Google Scholar]
- 51.Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL. Constrained corticotropin releasing factor antagonists (astressin analogues) with long duration of action in the rat. J Med Chem. 1999;42:3175–3182. doi: 10.1021/jm9902133. [DOI] [PubMed] [Google Scholar]
- 52.Ruhmann A, Bonk I, Lin CR, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin- releasing factor receptor (CRFR): development of CRFR2β selective antisauvagine-30. Proc Natl Acad Sci U S A. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez V, Rivier J, Taché Y. Peripheral injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks peripheral CRF-and abdominal surgery-induced delayed gastric emptying in rats. J Pharmacol Exp Ther. 1999;290:629–634. [PubMed] [Google Scholar]
- 54.Kehne J, De Lombaert S. Non-peptidic CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr Drug Target CNS Neurol Disord. 2002;1:467–493. doi: 10.2174/1568007023339049. [DOI] [PubMed] [Google Scholar]
- 55.Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- 56.Gilligan PJ, Robertson DW, Zaczek R. Corticotropin releasing factor (CRF) receptor modulators: progress and opportunities for new therapeutic agents. J Med Chem. 2000;43:1641–1660. doi: 10.1021/jm990590f. [DOI] [PubMed] [Google Scholar]
- 57.Seymour PA, Schmidt AW, Schulz DW. The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor: a review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 59.Kehne JH. The CRF1 receptor, a novel target for the treatment of depression, anxiety, and stress-related disorders. CNS Neurol Disord Drug Targets. 2007;6:163–182. doi: 10.2174/187152707780619344. [DOI] [PubMed] [Google Scholar]
- 60.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine response to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- 62.Gué M, Junien JL, Buéno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991;100:964–970. doi: 10.1016/0016-5085(91)90270-u. [DOI] [PubMed] [Google Scholar]
- 63.Enck P, Holtmann G. Stress and gastrointestinal motility in animals: a review of the literature. J Gastrointest Motil. 1992;1:83–90. [Google Scholar]
- 64.Yamamoto O, Niida H, Tajima K, Shirouchi Y, Masui Y, Ueda F, Kise M, Kimura K. Inhibition of stress-stimulated colonic propulsion by alpha 2-adrenoceptor antagonists in rats. Neurogastroenterol Motil. 1998;10:523–532. doi: 10.1046/j.1365-2982.1998.00127.x. [DOI] [PubMed] [Google Scholar]
- 65.Morrow NS, Garrick T. Effects of intermittent tail shock or water avoidance on proximal colonic motor contractility in rats. Physiol Behav. 1997;62:233–239. doi: 10.1016/s0031-9384(97)00108-x. [DOI] [PubMed] [Google Scholar]
- 66.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556.1:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1037–G1044. doi: 10.1152/ajpgi.00419.2006. [DOI] [PubMed] [Google Scholar]
- 68.Rao SS, Hatfield RA, Suls JM, Chamberlain MJ. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93:985–990. doi: 10.1111/j.1572-0241.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 69.Fukudo S, Nomura T, Muranaka M, Taguchi F. Brain-gut response to stress and cholinergic stimulation in irritable bowel syndrome. J Clin Gastroenterol. 1993;17:133–141. doi: 10.1097/00004836-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Narducci F, Snape WJ, Battle WM, JR, London RL, Cohen S. Increased colonic motility during exposure to a stressful situation. Dig Dis Sci. 1985;30:40–44. doi: 10.1007/BF01318369. [DOI] [PubMed] [Google Scholar]
- 71.Welgan P, Meshkinpour H, Hoehler F. The effect of stress on colon motor and electrical activity in irritable bowel syndrome. Psychosom Med. 1985;47:139–149. doi: 10.1097/00006842-198503000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Welgan P, Meshkinpour H, Beeler M. Effect of anger on colon motor and myoelectric activity in irritable bowel syndrome. Gastroenterology. 1988;94:1150–1156. doi: 10.1016/0016-5085(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 73.Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gué M, Tekamp A, Tabis N, Junien JL, Buéno L. Cholecystokinin blockade of emotional stress- and CRF-induced colonic motor alterations in rats: role of the amygdala. Brain Res. 1994;658:232–238. doi: 10.1016/s0006-8993(09)90030-0. [DOI] [PubMed] [Google Scholar]
- 75.Jiménez M, Buéno L. Inhibitory effects of neuropeptide Y (NPY) on CRF and stress-induced cecal motor response in rats. Life Sci. 1990;47:205–211. doi: 10.1016/0024-3205(90)90321-h. [DOI] [PubMed] [Google Scholar]
- 76.Ataka K, Kuge T, Fujino K, Takahashi T, Fujimiya M. Wood creosote prevents CRF-induced motility via 5-HT3 receptors in proximal and 5-HT4 receptors in distal colon in rats. Auton Neurosci. 2007;133:136–145. doi: 10.1016/j.autneu.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Martinez V, Taché Y. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 2001;893:29–35. doi: 10.1016/s0006-8993(00)03277-7. [DOI] [PubMed] [Google Scholar]
- 78.Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716–723. doi: 10.1016/0016-5085(93)91006-4. [DOI] [PubMed] [Google Scholar]
- 79.Mönnikes H, Raybould HE, Schmidt B, Taché Y. CRF in the paraventricular nucleus of the hypothalamus stimulates colonic motor activity in fasted rats. Peptides. 1993;14:743–747. doi: 10.1016/0196-9781(93)90107-r. [DOI] [PubMed] [Google Scholar]
- 80.Mönnikes H, Schmidt BG, Tebbe J, Bauer C, Taché Y. Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus stimulates colonic motor function in rats. Brain Res. 1994;644:101–108. doi: 10.1016/0006-8993(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 81.Mönnikes H, Schmidt BG, Raybould HE, Taché Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol. 1992;262:G137–G143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- 82.Mönnikes H, Heymann-monnikes I, Taché Y. CRF in the paraventricular nucleus of the hypothalamus induces dose-related behavioral profile in rats. Brain Res. 1992;574:70–76. doi: 10.1016/0006-8993(92)90801-f. [DOI] [PubMed] [Google Scholar]
- 83.Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, Nemeroff CB. Depression and anxiety: role of the locus coeruleus and corticotropin-releasing factor. Brain Res Bull. 1994;35:561–572. doi: 10.1016/0361-9230(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 84.Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology. 1988;94:598–602. doi: 10.1016/0016-5085(88)90229-6. [DOI] [PubMed] [Google Scholar]
- 85.Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am J Physiol. 1998;274:G827–G831. doi: 10.1152/ajpgi.1998.274.5.G827. [DOI] [PubMed] [Google Scholar]
- 86.Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology. 1988;95:1510–1517. doi: 10.1016/s0016-5085(88)80070-2. [DOI] [PubMed] [Google Scholar]
- 87.Bonaz B, Taché Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- 88.Million M, Wang L, Martinez V, Taché Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res. 2000;877:345–353. doi: 10.1016/s0006-8993(00)02719-0. [DOI] [PubMed] [Google Scholar]
- 89.Martinez V, Rivier J, Wang L, Taché Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther. 1997;280:754–760. [PubMed] [Google Scholar]
- 90.Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gué M, Del rio-lacheze C, Eutamene H, Theodorou V, Fioramonti J, Buéno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 92.Cochrane SW, Gibson MS, Myers DA, Schulkin J, Rice KC, Gold PW, Greenwood-Vanmeerveld B. Role of corticotropin-releasing factor-1 (CRF1)-receptor mediated mechanisms in neural pathways modulating colonic hypersensitivity. Gastroenterology. 2001;120 suppl 1 A-7. (Abstract) [Google Scholar]
- 93.Million M, Grigoriadis DE, Sullivan S, Crowe PD, Mcroberts JE, Zhou CY, Saunders PR, Maillot C, Mayer AE, Taché Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res. 2003;985:32–42. doi: 10.1016/s0006-8993(03)03027-0. [DOI] [PubMed] [Google Scholar]
- 94.Schwetz I, Bradesi S, Mcroberts JA, Sablad M, Miller JC, Zhou H, Ohning G, Mayer EA. Delayed stress-induced colonic hypoersensitivity in male Wistar rats: role of neurokinin-1 and corticotropin releasing factor-1 receptors. Am J Physiol Gastrointest Liver Physiol. 2004;286:G683–G691. doi: 10.1152/ajpgi.00358.2003. [DOI] [PubMed] [Google Scholar]
- 95.Million M, Maillot C, Adelson DW, Nozu T, Gauthier A, Rivier C, Chrousos GP, Bayati A, Mattsson H, Taché Y. Peripheral injection of sauvagine prevents repeated colorectal distention-induced visceral pain in female rats. Peptides. 2005;26:1188–1195. doi: 10.1016/j.peptides.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distention in rats. Gastroenterology. 2005;129:1533–1543. doi: 10.1053/j.gastro.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 97.Lechner SM, Curtis AL, Brons R, Valentino RJ. Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res. 1997;756:114–124. doi: 10.1016/s0006-8993(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 98.Kosoyan H, Grigoriadis D, Taché Y. The CRF-1 antagonist, NBI-35965 abolished the activtation of locus coeruleus by colorectal distention and intracisternal CRF in rats. Brain Res. 2004;1056:85–96. doi: 10.1016/j.brainres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 99.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 100.Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol. 1996;271:G884–G892. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- 101.Eutamene H, Theodorou V, Fioramonti J, Buéno L. Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotropin-releasing factor release in rats. J Physiol. 2003;553:959–966. doi: 10.1113/jphysiol.2003.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talley NJ. Irritable bowel syndrome: disease definition and symptom description. Eur J Surg Suppl. 1998:24–28. doi: 10.1080/11024159850191193. [DOI] [PubMed] [Google Scholar]
- 103.Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652–668. doi: 10.1053/gast.2001.21908. [DOI] [PubMed] [Google Scholar]
- 104.Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5:56–65. doi: 10.1177/1099800403005001006. [DOI] [PubMed] [Google Scholar]
- 105.Kurland JE, Coyle WJ, Winkler A, Zable E. Prevalence of irritable bowel syndrome and depression in fibromyalgia. Dig Dis Sci. 2006;51:454–460. doi: 10.1007/s10620-006-3154-7. [DOI] [PubMed] [Google Scholar]
- 106.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 107.North CS, Hong BA, Alpers DH. Relationship of functional gastrointestinal disorders and psychiatric disorders: Implications for treatment. World J Gastroenterol. 2007;13:2020–2027. doi: 10.3748/wjg.v13.i14.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang L. The association of functional gastrointestinal disorders and fibromyalgia. Eur J Surg Suppl. 1998:32–36. doi: 10.1080/11024159850191210. [DOI] [PubMed] [Google Scholar]
- 109.Mayer EA, Naliboff BD, Chang L, Coutinho SV. Stress and the gastrointestinal tract. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 110.Halpert A, Drossman D. Biopsychosocial issues in irritable bowel syndrome. J Clin Gastroenterol. 2005;39:665–669. doi: 10.1097/01.mcg.0000174024.81096.44. [DOI] [PubMed] [Google Scholar]
- 111.Gwee KA, Graham JC, Mckendrick MW, Collins SM, Marshall JS, Walters SJ, Read NW. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 112.Spiller RC. Role of infection in irritable bowel syndrome. J Gastroenterol. 2007;42 Suppl 17:41–47. doi: 10.1007/s00535-006-1925-8. [DOI] [PubMed] [Google Scholar]
- 113.Taché Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Mot. 2004;16 Suppl. 1:1–6. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 114.Taché Y. Corticotropin releasing factor receptor antagonists: potential future therapy in gastroenterology? Gut. 2004;53:919–921. doi: 10.1136/gut.2003.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42 Suppl 17:48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- 116.Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- 117.Lee R, Geracioti TD, Jr, Kasckow JW, Coccaro EF. Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. Am J Psychiatry. 2005;162:995–997. doi: 10.1176/appi.ajp.162.5.995. [DOI] [PubMed] [Google Scholar]
- 118.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 120.Carpenter LL, Tyrka AR, Mcdougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- 121.Mclean SA, Williams DA, Stein PK, Harris RE, Lyden AK, Whalen G, Park KM, Liberzon I, Sen A, Gracely RH, Baraniuk JN, Clauw DJ. Cerebrospinal fluid corticotropin-releasing factor concentration is associated with pain but not fatigue symptoms in patients with fibromyalgia. Neuropsychopharmacology. 2006;31:2776–2782. doi: 10.1038/sj.npp.1301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dinan TG, Quigley EM, Ahmed SM, Scully P, O'brien S, O'mahony L, O'mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 123.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lembo T, Plourde V, Shui Z, Fullerton S, Mertz H, Taché Y, Sytnik B, Munakata J, Mayer E. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol Motil. 1996;8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 126.Nozu T, Kudaira M. Corticotropin-releasing factor induces rectal hypersensitivity after repetitive painful rectal distention in healthy humans. J Gastroenterol. 2006;41:740–744. doi: 10.1007/s00535-006-1848-4. [DOI] [PubMed] [Google Scholar]
- 127.Wallon C, Yang P, Keita AV, Ericson AC, Mckay DM, Sherman PM, Perdue MH, Soderholm JD. Corticotropin releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2007 doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 128.Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Buéno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580:347–356. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 130.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tayama J, Sagami Y, Shimada Y, Hongo M, Fukudo S. Effect of alpha-helical CRH on quantitative electroencephalogram in patients with irritable bowel syndrome. Neurogastroenterol Mot. 2007;19:471–483. doi: 10.1111/j.1365-2982.2007.00903.x. [DOI] [PubMed] [Google Scholar]