Abstract
Cholesterol homeostasis in the enterocyte is regulated by the interplay of multiple genes that ultimately determines the net amount of cholesterol reaching the circulation from the small intestine. The effect of deleting these genes, particularly acyl CoA:cholesterol acyl transferase 2 (ACAT2), on cholesterol absorption and fecal sterol excretion is well documented. We also know that the intestinal mRNA level for adenosine triphosphate-binding cassette transporter A1 (ABCA1) increases in Acat2−/− mice. However, none of these studies has specifically addressed how ACAT2 deficiency impacts the relative proportions of esterified and unesterified cholesterol (UC) in the enterocyte and whether the concurrent loss of ABCA1 might result in a marked buildup of UC. Therefore, the present studies measured the expression of numerous genes and related metabolic parameters in the intestine and liver of ACAT2-deficient mice fed diets containing either added cholesterol or ezetimibe, a selective sterol absorption inhibitor. Cholesterol feeding raised the concentration of UC in the small intestine, and this was accompanied by a significant reduction in the relative mRNA level for Niemann-Pick C1-like 1 (NPC1L1) and an increase in the mRNA level for both ABCA1 and ABCG5/8. All these changes were reversed by ezetimibe. When mice deficient in both ACAT2 and ABCA1 were fed a high-cholesterol diet, the increase in intestinal UC levels was no greater than it was in mice lacking only ACAT2. This resulted from a combination of compensatory mechanisms including diminished NPC1L1-mediated cholesterol uptake, increased cholesterol efflux via ABCG5/8, and possibly rapid cell turnover.
Keywords: cholesterol homeostasis, cholesterol absorption, cholesteryl ester, fecal neutral sterol excretion, intestinal mucosa
the small intestine, although representing only a very small proportion of total body mass, plays a profoundly important role in regulating whole body cholesterol balance and ultimately the plasma lipoprotein composition. Thus not only is it the site for the absorption of cholesterol from the diet, bile, and other sources, but it is also responsible for conservation of the bile acid pool and the production of multiple apolipoproteins, including apolipoprotein B-48 (apo B-48), apo A-IV, and apo C-II (14, 29, 36). The small bowel also actively synthesizes cholesterol and clears LDL-cholesterol from the circulation (8, 18, 32, 35).
The fact that in humans the cholesterol absorption pathway is responsible for delivering hundreds of milligrams of chylomicron cholesterol daily to the liver has long made it a target for pharmacological intervention in the drive for more effective management of hypercholesterolemia in the general population (2, 15, 24). Such endeavors have facilitated a great expansion of our knowledge of how each of the steps in the absorption pathway operates at a molecular level. Mouse models with selective deletions of proteins such as Niemann-Pick C1-like 1 (NPC1L1), adenosine triphosphate-binding cassette transporter G5/G8 (ABCG5/8), microsomal triglyceride transfer protein (MTP), and acyl CoA:cholesterol acyltransferase 2 (ACAT2), as well as the discovery and development of ezetimibe, a potent, novel, and selective inhibitor of sterol absorption, have together taken research on the regulation of intestinal cholesterol homeostasis to a new level (5, 7, 13, 23, 27, 39, 40).
Like almost all other organs in the body, the small intestine contains both unesterified and esterified cholesterol, with the latter normally representing only a small proportion of the total cholesterol content (26). The cholesterol in enterocytes is derived from several sources including de novo synthesis, uptake from the lumen via the NPC1L1-mediated pathway, and clearance of LDL from the plasma (8, 23, 27, 35). Cholesterol that is either internalized from the lumen, synthesized locally, or taken up in lipoproteins can potentially be esterified by ACAT2 (6, 19). Much of this esterified cholesterol is in turn incorporated into chylomicrons that are secreted into the lymph (14). Ordinarily, this constitutes the major route for the delivery of intestinal cholesterol into the circulation. Any manipulation that eliminates or diminishes ACAT2 activity can therefore potentially cause expansion of the pool of unesterified cholesterol in the cell. This can in turn lead to altered activity of two transcription factors, sterol regulatory element binding protein-2 (SREBP-2) and liver X receptor (LXR), which regulate the expression of a constellation of genes involved in the control of cellular cholesterol homeostasis (10, 21). Although the ACAT2-deficient mouse has been the subject of a series of studies, including several that have utilized models lacking ACAT2 and one other protein such as apolipoprotein E or LDL receptor (1, 21, 24, 28), none of these provide any data defining the magnitude of change in the concentration of unesterified cholesterol in the small intestine of animals maintained on a basal low-cholesterol chow diet.
Our initial investigations of cholesterol metabolism in ACAT2-deficient mice showed that except when they were fed a cholesterol-enriched diet, the absence of ACAT2 in the small intestine had little impact on fractional cholesterol absorption. There was a clearer reduction when cholesterol was added to the diet but the Acat2−/− mice still absorbed significant amounts of cholesterol. This is not unexpected given that chylomicrons ordinarily contain both esterified and unesterified cholesterol (20). It was also found that the relative level of expression of mRNA for ABCA1 in the small intestine was markedly elevated in the ACAT2-deficient mice and that cholesterol feeding magnified this increase. Such findings, although implying a possible role of ABCA1 in facilitating intestinal cholesterol delivery into the circulation independent of the chylomicron particle, also raise the more general question of the extent to which the level of expression of other key genes involved in regulating sterol movement across the enterocyte is affected by the chemical activity of unesterified cholesterol in the cell. Therefore, these experiments were designed to systematically investigate the association between unesterified and esterified cholesterol content and the expression level of multiple genes in the small intestine of Acat2−/− and Acat2+/+ mice, as well as in mice lacking both ACAT2 and ABCA1 under conditions of both low and high dietary cholesterol intake. The data show that multiple aspects of intestinal cholesterol metabolism change when one or both of these proteins are absent and that together these adaptations prevent a marked accumulation of cholesterol in the intestine when the intake of dietary cholesterol is greatly increased.
MATERIALS AND METHODS
Animals and diets.
Acat2-deficient mice were generated as described previously (21) and maintained on a mixed strain background (C57BL/6:129/SvJae), as were matching Acat2+/+ controls. Two sets of experiments were conducted in Acat2−/− and matching Acat2+/+ mice. The first of these compared the effects of feeding phytosterols, surfomer, and ezetimibe on intestinal cholesterol absorption and intestinal cholesterol concentration. Either female or male mice were used in these experiments. The second set of experiments investigated the effects of feeding cholesterol and ezetimibe, alone and in combination, on several parameters, in particular the absolute concentration of unesterified and esterified cholesterol in the whole small intestine and liver. Only female Acat2−/− and Acat2+/+ mice were used for these experiments.
A subsequent series of studies was designed to explore more fully the role that ABCA1 plays in regulating intestinal sterol metabolism. This involved generating mice of four separate genotypes; Acat2+/+/Abca1+/+, Acat2+/+/Abca1−/−, Acat2−/−/Abca1+/+ and Acat2−/−/Abca1−/−. In the first step, Abca1−/− mice were generated from heterozygous breeding stock derived from a colony at the R. W. Johnson Pharmaceutical Research Institute, San Diego, CA. This breeding stock was also of mixed background (primarily C57BL/6:129/Ola). Subsequently, Abca1−/− males and Acat2−/− females were used to generate mice that were Acat2+/−/Abca1+/−. In turn, the mating of these double heterozygotes yielded progeny of the four genotypes needed for this set of studies. One set of experiments with these mice used females and males fed only the control diet defined below, whereas subsequent experiments used only females fed a cholesterol-rich diet.
Until used in specific experiments, all mice were maintained on a cereal-based rodent diet (Wayne Lab Blox, no. 8604; Harlan Teklad, Madison, WI). This diet, defined here as the “control diet,” had an inherent cholesterol and total lipid content of ∼0.02% wt/wt and 5% wt/wt, respectively (25). The fatty acid composition of this diet is given elsewhere (31). Five separate experimental diets were prepared using the meal form of 8604. One contained added phytosterols (0.5% wt/wt). This was prepared by using a mixture of plant sterols (Sigma-Aldrich, St. Louis, MO, cat. no. S-5753). Our analysis of this product showed that it contained β-sitosterol (58%), campesterol/dihydrobrassicasterol (29%), and stigmasterol (13%). Surfomer (AOMA, a copolymer of maleic acid and an 18-carbon α-olefin) (Monsanto, St. Louis, MO), was added to the diet at a level of 2% wt/wt. A third diet contained only added cholesterol (0.5% wt/wt) (Byron Chemical, Long Island City, NY). For the fourth diet, ezetimibe ((-)-1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone) (provided by Schering-Plough Research Institute, Kenilworth, NJ) was mixed directly in the chow at a level of 0.00625% wt/wt. This provided a dose of ∼10 mg·day−1·kg body wt−1. A fifth diet was made to contain both ezetimibe (0.00625% wt/wt) and cholesterol (0.5% wt/wt). In all experiments the mice were fed their respective diets ad libitum for periods ranging from 8 to 10 or from 13 to 15 days, as specified. In essentially all studies the mice were maintained in groups of three or four per cage in plastic colony cages containing wood shavings. For experiments involving the measurement of cholesterol absorption and neutral sterol excretion, they were housed individually. All mice were kept in a room with 12-h periods of light and darkness. They were studied in the fed state toward the end of the dark phase. The age range of all mice at the time of study was 3–5 mo. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
Intestinal cholesterol absorption, fecal neutral sterol excretion, and plasma and tissue total cholesterol concentrations.
Fractional cholesterol absorption (%) was determined by a fecal dual-isotope ratio method (25). Fecal neutral sterol excretion was measured as described (25). Unless the concentrations of unesterified and esterified cholesterol were needed, aliquots of plasma and liver and the whole small intestine were taken directly for the measurement of total cholesterol concentration by a gas chromatographic method (25).
Concentration of unesterified and esterified cholesterol in small intestine and liver.
For these measurements the entire small intestine was rinsed with sodium chloride solution (0.9% wt/vol), blotted, weighed, cut into small sections, and added to 60 ml of chloroform-methanol (2:1 vol/vol), as was an aliquot of liver pieces (0.6–0.8 g). Each sample was extracted in the presence of two internal standards ([4-14C]cholesteryl oleate and [1,2-3H(N)]cholesterol; PerkinElmer Life Sciences, Boston, MA). The extracts were filtered into a 100-ml volumetric flask, and a 20-ml aliquot of this was dried under air. The lipids were then dissolved in 2 ml of hexane:tert-butyl methyl ether (100:1.5 vol/vol) (solvent 1) and placed on a silica column (Sep-Pak Vac RC, 500 mg) (Waters, Milford, MA) that had been prewashed with 2 ml of solvent 1. The column was then eluted with 18 ml of solvent 1 (esterified cholesterol) and then with 18 ml of tert-butyl methyl ether:glacial acetic acid (100:0.2 vol/vol) (solvent 2) to remove the unesterified cholesterol and other lipids. The esterified and unesterified cholesterol fractions were dried under air, saponified in alcoholic KOH, and extracted with petroleum ether. Aliquots of this extract were used to determine recovery of the radiolabeled standards and also to quantitate the mass of cholesterol by gas chromatography. The unesterified, esterified, and total cholesterol concentrations were calculated as mg/g wet weight of tissue.
Relative mRNA expression analysis.
Small intestines were removed, flushed with ice-cold PBS and then cut longitudinally. The intestinal mucosae were removed by gentle scraping, immediately frozen in liquid nitrogen, and stored at −85°C. mRNA levels were measured using a quantitative real-time PCR assay (34). Total RNA was treated with DNase 1 (RNase-free; Roche, Indianapolis, IN) and reverse transcribed with random hexamers by using SuperScript reverse transcriptase to generate cDNA. Primer Express Software (Perkin-Elmer Life Sciences, Wellesley, MA) was used to design the primers which were validated by analysis of template titration and dissociation curves (see Table 1 for primer sequences). PCR assays were performed on an Applied Biosystems Prism 7000 sequence-detection system. The PCR mixture contained (in a final volume of 20 μl) 50 ng of reverse-transcribed RNA, 150 nM of each primer, and 10 μl of 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). All analyses were determined by the comparative cycle number at threshold method (User Bulletin No. 2, Perkin-Elmer Life Sciences) with cyclophilin as the internal control. Relative mRNA levels in individual animals were determined by expressing the amount of mRNA found relative to that obtained for mice fed the basal diet alone (control diet), which in each case was arbitrarily set at 1.0.
Table 1.
Quantitative real-time PCR primer sequences used to measure RNA levels
| Gene | Gene Name | NCB1 Accession Number | Sequence of Primers (5′ to 3′) | Amplicon Nucleotide Numbers |
|---|---|---|---|---|
| Abca1 | ATP-binding cassette subfamily A member 1 | NM_013454 | F: CGTTTCCGGGAAGTGTCCTA | 6805–6883 |
| R: GCTAGAGATGACAAGGAGGATGGA | ||||
| Abcg5 | ATP-binding cassette subfamily G member 5 | NM_031884 | F: TGGATCCAACACCTCTATGCTAAA | 1817–1893 |
| R: GGCAGGTTTTCTCGATGAACTG | ||||
| Acat2 | Sterol O-acyltransferase 2 (SOAT2) | NM_146064 | F: GCCTTCGCCGAGATGCT | 1161–1238 |
| R: GTAGTTGGAGAAGGAAGTCGAGTTC | ||||
| ApoAI | Apolipoprotein A-I | NM_009692 | F: TCCTCCTTGGGCCAACA | 301–359 |
| R: GAACCCAGAGTGTCCCAGTTT | ||||
| ApoAIV | Apolipoprotein A-IV | NM_007468 | F: ACAGTTTCAGAAGACGGATGTCA | 451–523 |
| R: CGTACTAGCATCCCCAAGTTTG | ||||
| ApoB | Apolipoprotein B | NM_009693 | F: CGTGGGCTCCAGCATTCTA | 9072–9143 |
| R: TCACCAGTCATTTCTGCCTTTG | ||||
| Ldlr | Low density lipoprotein receptor | NM_010700 | F: GAGGAACTGGCGGCTGAA | 2620–2858 |
| R: GTGCTGGATGGGGAGGTCT | ||||
| Mtp | Microsomal triglyceride transfer protein (Mttp) | NM_008642 | F: CCTACCAGGCCCAACAAGAC | 554–617 |
| R: CGCTCAATTTTGCATGTATCC | ||||
| Npc1l1 | Niemann-Pick C1-like 1 | NM_207242 | F: TGGACTGGAAGGACCATTTCC | 1547–1650 |
| R: GACAGGTGCCCCGTAGTCA | ||||
| Sar1b | SAR1 gene homolog B | NM_025535 | F: TGCTGAAAGATGACAGGCTCG | 260–348 |
| R: AGTTGTAAACGTCATGCCAGCAA | ||||
| SrbI | Scavenger receptor class B, member 1 | NM_016741 | F: TCCCCATGAACTGTTCTGTGAA | 1331–1397 |
| R: TGCCCGATGCCCTTGA | ||||
| Srebp-1c | Sterol regulatory element binding factor1 | AL669954 | F: GGAGCCATGGATTGCACATT | 878–937 |
| R: GGCCCGGGAAGTCACTGT |
Analysis of data.
All data are reported as means ± SE for the specified number of individual animals. GraphPad Prism software (GraphPad, San Diego, CA) was used to perform all statistical analyses. Differences between means were tested for statistical significance (P < 0.05) by either one-way or two-way analysis of variance with genotype and diet as factors. Newman-Keuls multiple-comparison test for statistical significance was used for all one-way analyses of variance. Transformed data were used if unequal variance among the groups was evident by Bartlett's test.
RESULTS
Elevated cholesterol concentration in small intestine of Acat2−/− mice is reduced when cholesterol absorption is inhibited.
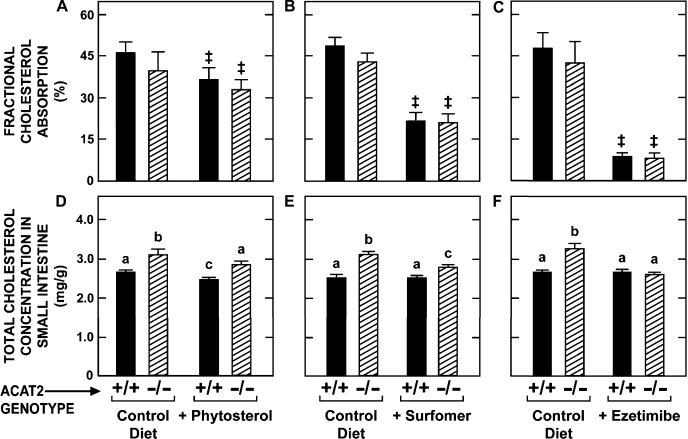
The objective of the first set of experiments was to establish whether inhibiting cholesterol absorption in Acat2−/− mice had any impact on the cholesterol concentration in the small intestine. As shown in Fig. 1, three classes of inhibitors were tested in Acat2−/− and Acat2+/+ mice, all of which were fed either the control diet alone or the same diet containing one of the inhibitors. These agents were either a phytosterol mixture (principally β-sitosterol); surfomer, a polymeric compound that makes the surface environment of the brush border membranes more hydrophilic (31); or ezetimibe, an inhibitor of NPC1L1, a transporter that facilitates the uptake of sterols across the brush border membrane (13). For mice of the same genotype, phytosterol feeding resulted in modest but statistically significant reductions in both fractional cholesterol absorption (∼20%) (Fig. 1A) and the total cholesterol concentration in the small intestine (∼8%) (Fig. 1D) in mice of both genotypes. Surfomer inhibited cholesterol absorption by ∼50% in both Acat2−/− and Acat2+/+ mice (Fig. 1B) and also significantly lowered the intestinal cholesterol concentration in the Acat2−/− mice from 3.11 ± 0.09 to 2.81 ± 0.06 mg/g (Fig. 1E). In the Acat2+/+ mice given surfomer, the intestinal cholesterol concentration (2.54 ± 0.06 mg/g) was not different from that in matching untreated controls (2.54 ± 0.04 mg/g) (Fig. 1E).
Fig. 1.
Fractional cholesterol absorption and total cholesterol concentration in the small intestine of Acat2+/+ and Acat2−/− mice fed diets containing various agents that alter the enterohepatic flux of cholesterol. Values are means ± SE of data from 7–12 mice in A, B, D and E, and from 5–7 mice in C and F. Two-way ANOVA revealed no interaction between genotype and diet (A–C). There was a statistically significant (P < 0.05) effect of diet (as denoted by ‡) but no effect of genotype. One-way ANOVA revealed significant effects of genotype or diet (D–F). Bars designated with different letters denote statistically different values, P < 0.05.
In contrast to phytosterols and surfomer, ezetimibe markedly inhibited cholesterol absorption (by ∼82% in mice of both genotypes) (Fig. 1C) and also lowered the total cholesterol concentration in the small intestine of the Acat2−/− mice from 3.25 ± 0.12 to 2.58 ± 0.06 mg/g (Fig. 1F). In the matching Acat2+/+ mice, the intestinal cholesterol concentration averaged 2.65 mg/g in both the control and ezetimibe-treated groups. Thus, in the Acat2−/− animals given ezetimibe, intestinal cholesterol concentrations were returned to normal levels.
Inhibiting cholesterol absorption by ezetimibe normalizes the proportions of cholesterol that are unesterified and esterified in the small intestine of Acat2−/− and Acat2+/+ mice fed a high-cholesterol diet.
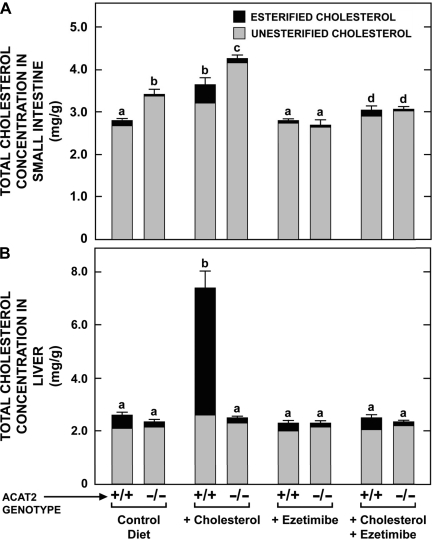
The next set of experiments used ezetimibe and cholesterol feeding, alone and in combination, to manipulate the relative proportions of unesterified and esterified cholesterol in the small intestines and livers of Acat2−/− and Acat2+/+ mice. Separate, matching groups of mice were used to determine also how these dietary treatments impacted the relative levels of expression of mRNA for several proteins involved in regulating cholesterol homeostasis in the absorptive cells of the intestine. In the Acat2−/− mice given only the control diet, the additional cholesterol contained in the small intestine was all unesterified (Fig. 2A). This fraction was significantly expanded in the Acat2−/− mice fed the high-cholesterol diet alone. In the matching Acat2+/+ mice fed cholesterol, the intestinal total cholesterol concentration also increased, but this reflected primarily an expansion of the esterified cholesterol fraction (Fig. 2A). Ezetimibe treatment had a striking impact on intestinal cholesterol concentrations. In the Acat2−/− mice given ezetimibe alone, the concentration of unesterified cholesterol in the small intestine was reduced to levels that were the same as those found in Acat2+/+ mice given the control diet alone. The potency of ezetimibe was such that even when cholesterol was included with this inhibitor in the diet, intestinal total cholesterol concentrations in the Acat2−/− mice remained at levels that only marginally exceeded those found in the Acat2+/+ mice on the control diet (Fig. 2A).
Fig. 2.
Concentrations of total, unesterified, and esterified cholesterol in the intestine (A) and liver (B) of Acat2+/+ and Acat2−/− mice fed diets containing cholesterol or ezetimibe, alone or in combination. The height of each bar defines the total cholesterol concentration, which is presented as the means ± SE of data from 6–8 mice in each group. For both organs the portion of each bar with dark shading represents the absolute fraction of the total cholesterol that was esterified. The lighter shaded portion represents the unesterified cholesterol fraction. The respective SE for the mean values for the esterified and unesterified fractions are not shown. Bars designated with different letters denote statistically different values, P < 0.05.
As shown by the data in Fig. 2B, both the total cholesterol concentration and the proportions of the total that were unesterified and esterified in the livers of the Acat2−/− mice remained essentially constant, irrespective of which diet they were given. However, in the case of the matching Acat2+/+ mice, cholesterol feeding caused a dramatic increase in the total cholesterol concentration in the liver, with essentially all of this increase being in the esterified fraction. When ezetimibe was included in the high-cholesterol diet, these changes did not occur. Hence, the total cholesterol concentration, and the proportion of it that was esterified in the Acat2+/+ mice given cholesterol and ezetimibe together, were identical to the values found for the Acat2+/+ mice fed the control diet alone.
Changes in expression level of mRNA for ABCA1 and other proteins in the small intestine of Acat2−/− and Acat2+/+ mice fed high-cholesterol diet without and with ezetimibe closely reflect shift in the proportion of cholesterol in the tissue that was unesterified.
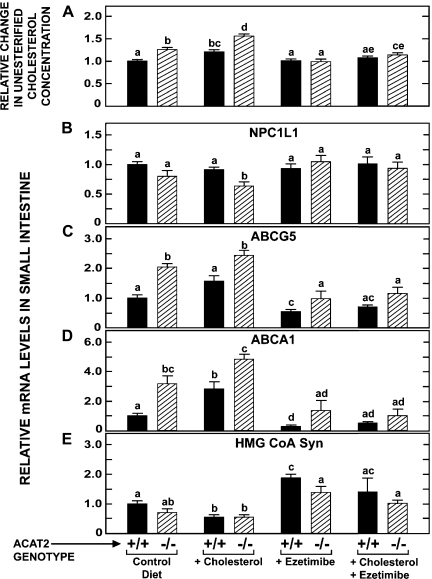
The data in Fig. 3A show the relative change in the unesterified cholesterol concentration in the small intestine of the same groups of mice for which absolute concentration data are given in Fig. 2A. The value for the absolute concentration in the small intestine of the Acat2+/+ mice fed the control diet (2.67 ± 0.02 mg/g) was arbitrarily set at 1.0. Each of the corresponding absolute concentration values for the remaining seven groups of mice was then expressed as a ratio of the value for the Acat2+/+ mice on the control diet. As illustrated in Fig. 3A, there was generally little change in the relative level of unesterified cholesterol in the small intestine of the Acat2+/+ mice across all treatments. In contrast, there were marked shifts in the relative unesterified cholesterol content in the intestine of the Acat2−/− mice, depending on their dietary cholesterol intake and whether or not ezetimibe was added to the diet. In the Acat2−/− mice on the control diet, there was 26% more unesterified cholesterol in the intestine than was the case in their matching Acat2+/+ controls. This value increased to 56% in the Acat2−/− group fed cholesterol, whereas there was no increase in the Acat2−/− mice given ezetimibe alone. In the Acat2−/− mice given the high-cholesterol diet and ezetimibe, the amount of unesterified cholesterol in the intestine was only 14% more than it was in Acat2+/+ mice on the control diet.
Fig. 3.
Relative change in unesterified cholesterol concentration and relative levels of expression of mRNA for various proteins in the small intestine of Acat2+/+ and Acat2−/− mice fed diets containing cholesterol or ezetimibe, alone and in combination. Matching groups of female Acat2+/+ and Acat2−/− mice that were fed the same diets as described in the legend to Fig. 2 were used for the measurement of the relative level of expression of mRNA for multiple proteins by real-time PCR. The values in A were calculated by using the absolute concentration data given in Fig. 2A. The concentration of unesterified cholesterol in the small intestine of the Acat2+/+ fed the control diet was arbitrarily set at 1.0, and the change in the unesterified cholesterol concentration in each of the other groups of mice was expressed relative to that for the Acat2+/+ control diet group (A). Relative mRNA levels are expressed relative to that obtained for Acat2+/+ mice fed the control diet. All values are means ± SE of data from 6–8 mice in each group. Two-way ANOVA revealed interaction between diet and genotype (A–E). One-way ANOVA revealed significant effects of genotype or diet. Bars designated with different letters denote statistically different values, P < 0.05.
When these changes in the relative unesterified cholesterol content are compared directly to those seen in the relative mRNA levels for NPC1L1 (Fig. 3B), ABCG5 (Fig. 3C), ABCA1 (Fig. 3D) and 3-hydroxy-3-methylglutaryl (HMG) CoA synthase (Fig. 3E), several findings warrant emphasis. It is clearly evident that the modest changes in relative unesterified cholesterol levels were accompanied by much more marked changes in the relative mRNA levels for at least three of these proteins. Compared with the other genes investigated, NPC1L1 mRNA levels changed the least across all treatment groups (Fig. 3B). The only significant change found was for the Acat2−/− mice fed cholesterol, in which case the relative mRNA level for NPC1L1 was 36% lower compared with the level for Acat2+/+ mice on the control diet. In the case of ABCG5 (Fig. 3C), there were marked changes in the relative mRNA level for mice of both genotypes, but in every treatment group the value for the Acat2−/− mice always exceeded that for their matching Acat2+/+ controls. Under those conditions where there was an expanded cellular content of unesterified cholesterol, the mRNA level for ABCG5 was elevated. The converse was true when unesterified cholesterol levels contracted in response to the inhibition of cholesterol absorption by ezetimibe.
The changes in relative ABCA1 mRNA levels (Fig. 3D) paralleled those for ABCG5 but were greater in magnitude. This was equally true for mice of both genotypes across all treatments. In the case of the Acat2−/− mice given the high-cholesterol diet alone, the relative level of ABCA1 mRNA was 4.8-fold greater than it was in Acat2+/+ mice on the control diet. This increase was completely erased by the inclusion of ezetimibe in the cholesterol-rich regimen. In parallel with this change, there was a fall in the relative unesterified cholesterol content of the tissue to near baseline values. Another striking change in relative ABCA1 mRNA levels was seen in the Acat2+/+ mice given ezetimibe only. Here the relative ABCA1 mRNA level fell to only 29% of the baseline value for Acat2+/+ mice on the control diet. This occurred even though there was essentially no change in the relative unesterified cholesterol content in the intestine of Acat2+/+ mice given ezetimibe only (Fig. 3A).
The data in Fig. 3E show that the relative expression level of mRNA for HMG CoA synthase varied appreciably with both genotype and dietary treatment, thus indicating quite marked adaptive changes in the rate of cholesterol synthesis in the small bowel. In the Acat2+/+ mice, the HMG CoA synthase mRNA expression level was reduced ∼50% with cholesterol feeding and increased nearly twofold with ezetimibe treatment. Although the relative mRNA level for the enzyme at baseline was significantly less in the Acat2−/− mice than in their matching Acat2+/+ controls, overall the effect of cholesterol and ezetimibe feeding in the mice lacking ACAT2 was qualitatively about the same as it was in matching Acat2+/+ mice.
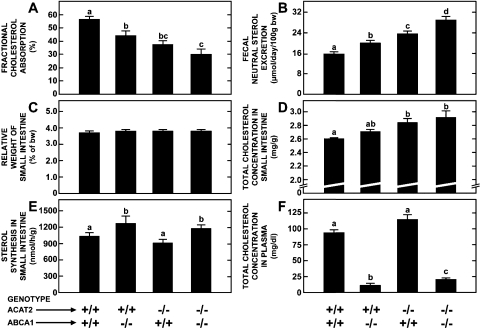
Deletion of ABCA1 in Acat2−/− mice markedly lowers cholesterol absorption and drives fecal sterol excretion but has no additional impact on cellular cholesterol content in the small intestine, even when dietary cholesterol intake is raised.
The findings of these initial studies prompted us to investigate how the deletion of ABCA1 from ACAT2-deficient mice might impact their intestinal cholesterol metabolism. The first experiment was carried out in matching groups of mice fed only the basal control diet and involved the measurement of multiple parameters as detailed in Fig. 4 (A–F). The data in Fig. 4, A and B are for females whereas those in 4, C–F are for males. The deletion of either ABCA1 or ACAT2 resulted in a modest but significant reduction in fractional cholesterol absorption (Fig. 4A) and a corresponding increase in fecal neutral sterol excretion (Fig. 4B). These changes were greater in mice that were lacking both ABCA1 and ACAT2. None of the genetic manipulations affected the relative weight of the small intestine (Fig. 4C), but the loss of ACAT2 did result in a significant rise in the intestinal total cholesterol concentration (Fig. 4D). The magnitude of this rise was about the same in mice lacking both ACAT2 and ABCA1. The rate of intestinal cholesterol synthesis was unchanged in the mice lacking only ACAT2 whereas in those deficient in ABCA1, or in both of these genes, there was a modest increase (Fig. 4E). Plasma total cholesterol concentrations were markedly reduced in the mice deficient in ABCA1 with or without ACAT2 (Fig. 4F).
Fig. 4.
Fractional cholesterol absorption, fecal neutral sterol excretion, total cholesterol concentration, and rate of sterol synthesis in the small intestine of mice deficient in both ABCA1 and ACAT2. The data in A and B are for females and those in C–F are for males. Values are means ± SE of data for a minimum of 9 mice per group except in E where there were 5–8 animals per group. Bars designated with different letters denote statistically significant values, P < 0.05.
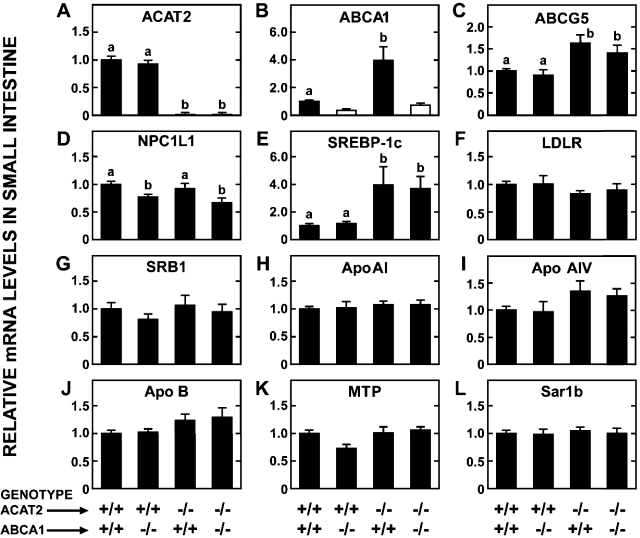
A parallel study was conducted in matching groups of mice to measure the relative level of mRNA expression for a constellation of proteins in the small intestine. The data for ACAT2 (Fig. 5A) and ABCA1 (Fig. 5B) not only confirm the respective genotypes of the mice but again show that the loss of ACAT2 alone results in a marked increase in the relative mRNA level for ABCA1 (Fig. 5B). The elevation in ABCG5 expression that was associated with loss of ACAT2 was unaffected by the concurrent deletion of ABCA1 (Fig. 5C). One of the key proteins in these analyses was NPC1L1, which was of particular interest given the changes in fractional cholesterol absorption described in Fig. 4A. As shown in Fig. 5D, the loss of ABCA1, but not of ACAT2, resulted in lower mRNA expression levels for NPC1L1. In the mice lacking just ABCA1, the reduction in the relative mRNA level for NPC1L1 compared with Acat2+/+/Abca1+/+ controls was 23%, whereas in those mice deficient in both ABCA1 and ACAT2 it was 33%. Another gene of particular importance was SREBP-1c, an LXR target gene, that is affected by unesterified cholesterol content (22). As shown in Fig. 5E, there was a marked elevation in the mRNA level for SREBP-1c in mice that were deficient in either ACAT2 alone or both ACAT2 and ABCA1. In contrast to the genes shown in Fig. 5, A–E, no significant genotype-related differences were found in the relative mRNA for LDL receptor (Fig. 5F), SRBI (Fig. 5G), ApoAI (Fig. 5H), ApoAIV (Fig. 5I), ApoB (Fig. 5J), MTP (Fig. 5K), or Sar1b (Fig. 5L) (see Table 1). Although the data are not shown, there were also no significant changes in the mRNA levels for ACAT1, CD13, or CD36 among the four groups.
Fig. 5.
Relative levels of expression of mRNA for various proteins in the small intestine of mice deficient in both ABCA1 and ACAT2. All the mice were females fed a basal rodent chow diet. Relative mRNA levels were determined by expressing the amount of mRNA found relative to that for the Acat2+/+/Abca1+/+ mice that in each case was arbitrarily set at 1.0. In B the open bars for the 2 groups lacking ABCA1 reveal that the PCR primers utilized for this assay detect an aberrant transcript that does not produce functional ABCA1 protein. In all panels values are means ± SE of data from a minimum of 5–7 mice per group. One-way ANOVA revealed significant differences among groups (A–E) and bars designated with different letters denote statistically different values, P < 0.05.
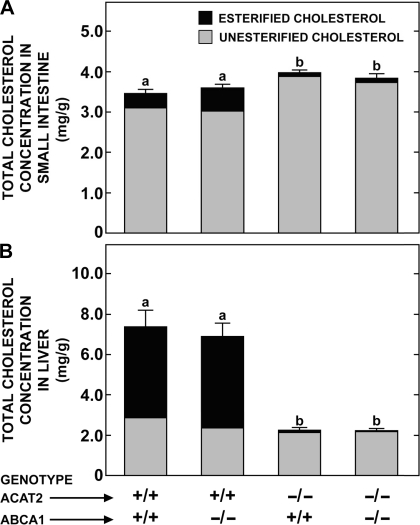
The final experiment specifically examined the impact of feeding a high-cholesterol diet (0.5% wt/wt) on the concentrations of unesterified and esterified cholesterol in the small intestine and liver of mice of the same four genotypes as those described in Figs. 4 and 5. These data, shown in Fig. 6, reveal several findings, the most striking of which is that the concentrations of total cholesterol, and of the unesterified and esterified fractions, in the Acat2−/−/Abca1−/− mice were not different from those in matching Acat2−/−/Abca1+/+ mice (Fig. 6A). In both of these groups the total cholesterol concentration in the intestine was, however, significantly higher than it was in the Acat2+/+/Abca1−/− mice, and in the Acat2+/+/Abca1+/+ controls. In these latter two groups, the proportion of the total cholesterol in the intestine that was esterified was notably greater than it was in the matching mice lacking either just ACAT2, or both ACAT2 and ABCA1. This same trend was much more evident in the livers of these mice (Fig. 6B). This finding is fully consistent with the data from the earlier experiment that tested the effects of cholesterol and ezetimibe feeding in mice deficient in only ACAT2 (Fig. 2B). In the small intestine of the mice lacking only ABCA1, the amount of esterified cholesterol was about twice that in the Acat2+/+/Abca1+/+ controls but remained only a small proportion of the total cholesterol content (Fig. 6A). This increment in the esterified fraction was, however, not seen in mice that lacked both ABCA1 and ACAT2.
Fig. 6.
Effect of cholesterol feeding on the concentration of total, unesterified, and esterified cholesterol in the small intestine and liver of mice deficient in both ABCA1 and ACAT2. All mice were females fed a basal diet containing added cholesterol at a level of 0.5% wt/wt for 8–10 days. The height of each bar defines the total cholesterol concentration, which is presented as means ± SE of data from 12–17 animals in all groups. For both organs, the portion of each bar with dark shading represents the absolute fraction of the total cholesterol that was esterified. The respective SE for the mean values for the esterified and unesterified fractions are not shown. One-way ANOVA revealed significant effects of genotype on total cholesterol concentrations. Bars designated with different letters denote statistically different values, P < 0.05.
DISCUSSION
The present studies not only significantly extend earlier findings from this and other laboratories on the respective roles of ACAT2 and ABCA1 in the maintenance of cholesterol homeostasis in the small intestine but also provide clearer insight into the likely adaptive mechanisms that operate in enterocytes lacking both of these proteins to prevent marked expansion of the intracellular pool of unesterified cholesterol. Broadly, three sets of conclusions can be drawn from this work. One set relates to the impact of ACAT2 deficiency on the handling of cholesterol by the enterocyte. The present studies confirm and extend previous findings in Acat2−/− mice fed a low-cholesterol diet or one containing added cholesterol (5, 21, 28). In the case of the groups fed the basal diet only, mice lacking ACAT2, compared with their matching Acat2+/+ controls, had marginally lower fractional cholesterol absorption values (Fig. 1A–C, Fig. 4A), higher rates of fecal neutral sterol excretion (Fig. 4B), and a modestly higher concentration of total cholesterol in the small intestine (Fig. 1, D–F, Fig. 4D), with all of this increase being in the unesterified fraction (Fig. 2A). Although this increase was only 26% (Fig. 3A), there were nevertheless corresponding marked elevations in the relative level of expression of mRNA for several proteins including ABCG5 (Figs. 3C and 5C), and particularly ABCA1 (Figs. 3D and 5B). Such data imply that one of the adaptive responses that enterocytes make in the face of a buildup of unesterified cholesterol caused by ACAT2 deficiency is to increase the efflux of cholesterol via the luminal surface (ABCG5) and the basal side of the cells (ABCA1). There were also marginal declines in the relative mRNA level for NPC1L1 (Fig. 3B) and HMG CoA synthase (Fig. 3E), as well as in the rate of sterol synthesis measured (Fig. 4E). Although such relative data cannot be used to ascertain the quantitative importance that reduced cellular uptake, suppressed local synthesis, and accelerated efflux of cholesterol each play in minimizing the accumulation of unesterified cholesterol in the enterocyte when ACAT2 is absent, these data together suggest that there exist multiple defense mechanisms.
Indeed, the corresponding data for the ACAT2-deficient mice fed the diets containing either added cholesterol or ezetimibe alone, or in combination, support this conclusion. Thus, with cholesterol feeding, the relative concentration of unesterified cholesterol in the intestine increased from 26 to 56% (Fig. 3A), and the magnitude of change in the relative mRNA levels for NPC1L1, ABCG5, ABCA1, and HMG CoA synthase was comparatively greater than seen in ACAT2-deficient mice given only the basal diet. Other investigators have similarly found that, in the small intestine of Acat2−/− mice maintained on a diet enriched with cholesterol and palm oil, the expression level of mRNA for NPC1L1 was significantly reduced compared with matching Acat2+/+ mice (28). The present studies further show that, in the converse situation, when the Acat2−/− mice were given ezetimibe alone, the buildup of unesterified cholesterol in the intestine was prevented and the relative mRNA expression levels for NPC1L1, ABCG5, ABCG1, and HMG CoA synthase all remained at values not different than those seen for Acat2+/+ mice on the basal diet. This was still the case when Acat2−/− mice were given the cholesterol-rich diet containing ezetimibe. This is a key observation because it suggests that high dietary cholesterol intake will have virtually no impact on cholesterol handling in the enterocyte lacking ACAT2 if a concurrent block on the NPC1L1 facilitated uptake of cholesterol from the lumen is applied. In the model used in the present studies it is clear that this can be achieved with ezetimibe, but this may not be the case for other cholesterol absorption inhibitors such as surfomer that act intraluminally and are comparatively much less potent than ezetimibe, despite being used at far higher doses (Fig. 1).
Together these initial studies showed that the accumulation of unesterified cholesterol in the enterocyte associated with loss of ACAT2 activity does not happen if a potent enough block on cholesterol entry into the cell at its luminal surface is applied. Without this, a significant rise in cellular levels of unesterified cholesterol is seen, especially if dietary cholesterol intake is high. However, as discussed later, a number of compensatory mechanisms come in to play to lessen the magnitude of the expansion of the unesterified cholesterol pool in the enterocyte.
The second set of conclusions from this work relate to the role of ABCA1 in the maintenance of intestinal cholesterol homeostasis in the presence and absence of ACAT2. Previous studies using different animal models, including mice with either global or intestine-specific deletion of ABCA1, and also a chicken model with inherent deficiency of this protein (4, 9, 11, 17, 38), demonstrate that ABCA1-mediated efflux of unesterified cholesterol from the basolateral surface of the enterocyte is important for the lipidation of nascent HDL. The movement of unesterified cholesterol across the basolateral membrane of enterocytes might, however, also occur by simple diffusion (12). Irrespective of the mechanisms involved, it is apparent from the present data and earlier studies (9, 17, 21, 28, 38) that the delivery of cholesterol from the intestinal lumen into the circulation is partially ABCA1 dependent. Thus in mice lacking ABCA1 there was a 22% decrease in fractional cholesterol absorption (Fig. 4A), a 27% increase in fecal neutral sterol excretion (Fig. 4B), and a marginal increase in intestinal cholesterol synthesis (Fig. 4E). However, in contrast to the mice deficient in only ACAT2, those lacking only ABCA1 did not show a significant change in either intestinal total cholesterol concentration (Fig. 4D) or the relative mRNA expression ABCG5 (Fig. 5C). The loss of ABCA1 alone was, however, associated with a rise in the fraction of esterified cholesterol in the small intestine (Fig. 6A), which is consistent with previous findings in mice with intestine-specific ABCA1 deletion (4). The cause(s) of this increased cholesteryl ester concentration is unknown, but it might partly reflect infiltration of lipid-laden macrophages (38). Although these various data for mice lacking ABCA1 do not provide a measure of the comparative quantitative importance of this protein, compared with ACAT2, in delivery of cholesterol from the intestine into the circulation, they do consolidate earlier work showing that the importance of ABCA1 in this process increases when ACAT2 is absent. This raised the intriguing question of what might happen to the concentration of unesterified cholesterol in the small intestine of mice lacking both of these proteins, especially if dietary cholesterol intake were markedly increased.
The third set of conclusions, then, center around the finding that the deletion of ABCA1 in ACAT2-deficient animals failed to cause additional unesterified cholesterol accumulation in the small intestine when the dietary cholesterol intake of these mice was increased (Fig. 6A). These mice, like those lacking ACAT2 alone, also showed no cholesterol accumulation in their livers in the face of a ∼25-fold increase in cholesterol intake (Fig. 6B). In the mice deficient in both proteins, fractional cholesterol absorption was lower than in the other three matching experimental groups (Fig. 4A) but still averaged ∼30%. Hence the amount of chylomicron cholesterol reaching the liver in these mice was still far greater than would be the case in mice fed the low-cholesterol diet. The fact that in both the double-knockout mice and in those lacking only ACAT2 hepatic concentrations of both unesterified and esterified cholesterol were virtually identical to those seen in Acat2+/+/Abca1+/+ mice fed only the basal diet (Fig. 2B) is likely the result of a combination of compensatory mechanisms including downregulation of cholesterol synthesis, upregulation of bile acid synthesis, and accelerated loss of cholesterol into the bile via ABCG5/8 (21, 30, 35, 40).
The finding that intestinal unesterified cholesterol levels in the Acat2−/−/Abca1−/− mice did not increase any more than they did in matching Acat2−/− mice with cholesterol feeding might be an indication that the pathway for effluxing cholesterol across the basolateral surface is of minor quantitative importance compared with the one operating on the luminal side of the enterocyte via ABCG5/8. Although the increase in mRNA for this transporter seen in the intestine of the double-knockout mice, as well as in those lacking only ACAT2, clearly indicates that this was one component of enterocyte's ability to reduce its content of unesterified cholesterol, the mRNA data for NPC1L1 in the mice missing both ACAT2 and ABCA1 (Fig. 5D) suggest that diminished cholesterol uptake from the lumen might also have played a defensive role. Although such a mechanism is teleologically attractive, the question of the extent to which the NPC1L1-mediated pathway in the intestine is regulated remains unclear. There are, nevertheless, data from other mouse models either fed a diet containing high levels of cholesterol and cholic acid or treated with high doses of fenofibrate that show marked reductions in the protein and/or relative mRNA levels for NPC1L1 in the proximal small intestine (7, 33).
Another possible explanation for the less than expected increase in the unesterified cholesterol content of the small bowel in the Acat2 −/−/Abca1−/− mice has to do with the high turnover rate of intestinal absorptive cells. The life span of these cells in mice is only 2–3 days (16). The continual sloughing of these cells could potentially be a major reason why the unesterified cholesterol content of the small intestine as a whole does not increase as markedly as one might expect in a mouse lacking both ACAT2 and ABCA1 and fed a high-cholesterol diet. A parallel situation occurs in the small intestine of mice deficient in the lysosomal sterol transport protein NPC1. In this model the accumulation of unesterified cholesterol in nearly all the organs continues throughout the short life span of these mice (3, 37). One exception is the small intestine, where the cholesterol content of the whole small intestine has at most increased twofold at the time of death. Another feature of the Npc1−/− mutant mouse relevant to the present studies is that the concentration of unesterified cholesterol in the small intestine of these mice reaches values higher than those seen in mice lacking either ACAT2 only or both ACAT2 and ABCA1 fed cholesterol, and yet there is no change in the relative mRNA level for ABCA1 in the intestine. In 49-day-old NPC1-deficient mice fed a low-cholesterol basal diet the unesterified cholesterol concentration in the small intestine was 5.81 mg/g compared with 2.86 mg/g in Npc1+/+ controls but the intestinal relative mRNA level for ABCA1 in the Npc1−/− mice was only about half of that in matching wild-type controls (C. M. Ramirez, M. A. Valasek, J. J. Repa, and J. M. Dietschy, unpublished observations). Together these findings show that if the buildup of unesterified cholesterol in the intestine occurs within a compartment such as the late endosome/lysosome, and not in the cytosol, then the adaptive mechanisms seen in intestinal cells lacking ACAT2 do not come into play.
In conclusion, this work, together with the earlier studies of Temel et al. (28), not only identifies the likely mechanisms that operate in the enterocyte to prevent unbridled expansion of its content of unesterified cholesterol when both ACAT2 and ABCA1 function are lost concurrently but also shows clearly the powerful downstream effects that changes in cholesterol handling by the small intestine have on hepatic cholesterol content. The data generated by these studies will therefore be of key importance in the development of any strategies that are designed to improve the plasma lipoprotein composition by simultaneous manipulation of both ACAT2 and ABCA1 in the small intestine.
GRANTS
This research was supported by National Institutes of Health Grants HL09610 (J. M. Dietschy, S. D. Turley), GM07062 (M. A. Valasek), and DK078592 (J. J. Repa); the Moss Heart Fund (J. M. Dietschy); and a Beginning Grant-in-Aid from the American Heart Association, Texas Affiliate (No 0465115Y) (J. J. Repa).
DISCLOSURES
From time to time, Dr. Dietschy has served as a consultant for Merck/Schering-Plough Pharmaceuticals. Dr. Turley has served as a member of the Speaker's Bureau for Merck & Schering-Plough, and Merck/Schering-Plough Pharmaceuticals.
ACKNOWLEDGMENTS
We thank Heather Waddell, Sean Campbell, Mario Saucedo, Thien Tran, and Brian Griffith for excellent technical assistance and Annemarie Kelsey for preparation of the manuscript. The ezetimibe used in these studies was kindly provided by Dr. Harry R. Davis at the Schering-Plough Research Institute, Kenilworth, NJ. Surfomer was a gift of Monsanto, St. Louis, MO. Dr. Trudy Christiansen-Weber at the R. W. Johnson Pharmaceutical Research Institute, San Diego, CA, kindly supplied us with breeding stock for the generation of Abca1−/− mice.
REFERENCES
- 1.Alger HM, Brown JM, Sawyer JK, Kelley KL, Shah R, Wilson MD, Willingham MC, Rudel LL. Inhibition of acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol-associated steatosis by enhancing hepatic triglyceride mobilization. J Biol Chem 285: 14267–14274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bays HE, Neff D, Tomassini JE, Tershakovec AM. Ezetimibe: cholesterol lowering and beyond. Expert Rev Cardiovasc Ther 6: 447–470, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Beltroy EP, Richardson JA, Horton JD, Turley SD, Dietschy JM. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology 42: 886–893, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest 116: 1052–1062, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley SD, Farese RV., Jr Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med 6: 1341–1347, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RV., Jr ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem 273: 26755–26764, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SPN, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem 279: 33586–33592, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res 34: 1637–1659, 1993 [PubMed] [Google Scholar]
- 9.Drobnik W, Lindenthal B, Lieser B, Ritter M, Christiansen Weber T, Liebisch G, Giesa U, Igel M, Borsukova H, Büchler C, Fung-Leung WP, von Bergmann K, Schmitz G. ATP-binding cassette transporter A1 (ABCA1) affects total body sterol metabolism. Gastroenterology 120: 1203–1211, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Field FJ, Born E, Murthy S, Mathur SN. Regulation of sterol regulatory element-binding proteins in hamster intestine by changes in cholesterol flux. J Biol Chem 276: 17576–17583, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Field FJ, Watt K, Mathur SN. Origins of intestinal ABCA1-mediated HDL-cholesterol. J Lipid Res 49: 2605–2619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fielding CJ, Fielding PE. Cellular cholesterol efflux. Biochim Biophys Acta 1533: 175–189, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, MacIntyre DE, Ogawa A, O'Neill KA, Iyer SPN, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-like 1 (NPC1L1). Proc Natl Acad Sci USA 102: 8132–8137, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy SM. Absorption and metabolism of dietary cholesterol. Annu Rev Nutr 3: 71–96, 1983 [DOI] [PubMed] [Google Scholar]
- 15.Homan R, Krause BR. Established and emerging strategies for inhibition of cholesterol absorption. Curr Pharm Des 3: 29–44, 1997 [Google Scholar]
- 16.Lipkin M. Proliferation and differentiation of gastrointestinal cells in normal and disease states. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1981, p. 145–168 [Google Scholar]
- 17.Mulligan JD, Flowers MT, Tebon A, Bitgood JJ, Wellington C, Hayden MR, Attie AD. ABCA1 is essential for efficient basolateral cholesterol efflux during the absorption of dietary cholesterol in chickens. J Biol Chem 278: 13356–13366, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Osono Y, Woollett LA, Herz J, Dietschy JM. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J Clin Invest 95: 1124–1132, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parini P, Davis MA, Lada AT, Erickson SK, Wright TL, Gustafsson U, Sahlin S, Einarsson C, Eriksson M, Angelin B, Tomoda H, Omura S, Willingham MC, Rudel LL. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 110: 2017–2023, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Redgrave TG, Dunne KB. Chylomicron formation and composition in unanaesthetised rabbits. Atherosclerosis 22: 389–400, 1975 [DOI] [PubMed] [Google Scholar]
- 21.Repa JJ, Buhman KK, Farese RV, Jr, Dietschy JM, Turley SD. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology 40: 1088–1097, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JMA, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRa and LXRb. Genes Dev 14: 2819–2830, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Repa JJ, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J Lipid Res 46: 779–789, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Rudel LL, Lee RG, Parini P. ACAT2 is a target for treatment of coronary heart disease associated with hypercholesterolemia. Arterioscler Thromb Vasc Biol 25: 1112–1118, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7a-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J Lipid Res 39: 1833–1843, 1998 [PubMed] [Google Scholar]
- 26.Stange EF, Dietschy JM. Cholesterol absorption and metabolism by the intestinal epithelium. In: Sterols and Bile Acids, edited by Danielsson H, Sjövall J. Amsterdam: Elsevier, 1985, p. 121–149 [Google Scholar]
- 27.Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PHR, Huff MW. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J Lipid Res 48: 699–708, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J Lipid Res 46: 2423–2431, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Tso P. Intestinal lipid absorption. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1994, p. 1867–1907 [Google Scholar]
- 30.Turley SD, Dietschy JM. The metabolism and excretion of cholesterol by the liver. In: The Liver: Biology and Pathobiology, edited by Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA.New York: Raven, 1988, p. 617–641 [Google Scholar]
- 31.Turley SD, Herndon MW, Dietschy JM. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J Lipid Res 35: 328–339, 1994 [PubMed] [Google Scholar]
- 32.Turley SD, Spady DK, Dietschy JM. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J Lipid Res 36: 67–79, 1995 [PubMed] [Google Scholar]
- 33.Valasek MA, Clarke SL, Repa JJ. Fenofibrate reduces intestinal cholesterol absorption via PPARalpha-dependent modulation of NPC1L1 expression in mouse. J Lipid Res 48: 2725–2735, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Valasek MA, Repa JJ. The power of real-time PCR. Adv Physiol Educ 29: 151–159, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Valasek MA, Repa JJ, Quan G, Dietschy JM, Turley SD. Inhibiting intestinal NCP1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am J Physiol Gastrointest Liver Physiol 295: G813–G822, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang DQH. Regulation of intestinal cholesterol absorption. Annu Rev Physiol 69: 221–248, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Xie C, Burns DK, Turley SD, Dietschy JM. Cholesterol is sequestered in the brains of mice with Niemann-Pick type C disease but turnover is increased. J Neuropathol Exp Neurol 59: 1106–1117, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Xie C, Turley SD, Dietschy JM. ABCA1 plays no role in the centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse. J Lipid Res 50: 1316–1329, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem 281: 4075–4086, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Yu L, Hammer RE, Li-Hawkins J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA 99: 16237–16242, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]