Abstract
Cdx2 is an intestine-specific transcription factor required for normal intestinal epithelium development. Cdx2 regulates the expression of intestine-specific genes and induces cell adhesion and columnar morphogenesis. Cdx2 also has tumor-suppressor properties, including the reduction of colon cancer cell proliferation and cell invasion, the latter due to its effects on cell adhesion. E-cadherin is a cell adhesion protein required for adherens junction formation and the establishment of intestinal cell polarity. The objective of this study was to elucidate the mechanism by which Cdx2 regulates E-cadherin function. Two colon cancer cell lines were identified in which Cdx2 expression was associated with increased cell-cell adhesion and diminished cell migration. In both cell lines, Cdx2 did not directly alter E-cadherin levels but increased its trafficking to the cell membrane compartment. Cdx2 enhanced this trafficking by altering receptor tyrosine kinase (RTK) activity. Cdx2 expression diminished phosphorylated Abl and phosphorylated Rac levels, which are downstream effectors of RTKs. Specific chemical inhibition or short interfering RNA (shRNA) knockdown of c-Abl kinase phenocopied Cdx2's cell-cell adhesion effects. In Colo 205 cells, Cdx2 reduced PDGF receptor and IGF-I receptor activation. This was mediated by caveolin-1, which was induced by Cdx2. Targeted shRNA knockdown of caveolin-1 restored PDGF receptor and reversed E-cadherin membrane trafficking, despite Cdx2 expression. We conclude that Cdx2 regulates E-cadherin function indirectly by disrupting RTK activity and enhancing E-cadherin trafficking to the cell membrane compartment. This novel mechanism advances Cdx2's prodifferentiation and antitumor properties and suggests that Cdx2 may broadly regulate RTK activity in normal intestinal epithelium by modulating membrane trafficking of proteins.
Keywords: adherens junction, platelet-derived growth factor receptor, insulin-like growth factor I receptor, c-Abl, caveolin-1
the homeodomain transcription factor Cdx2 is required for columnar morphogenesis and cell differentiation in the normal intestinal epithelium (19). Cdx2 regulates the intestine-specific expression of a number of genes (8, 12, 22, 23, 44). Cdx2 has also been suggested to be a tumor suppressor in the colon (4, 6) or to have colon tumor-inhibitory properties (16, 17, 19); however, the full extent of Cdx2's antitumor effects has yet to be elucidated. In previous studies, we demonstrated that Cdx2 expression in colon cancer cells limits cell proliferation and promotes the adoption of a columnar, polarized cell morphology with apical microvilli (17, 18, 25, 31, 43, 44). Cdx2 expression appears to modulate the activity of tight, adherens, and desmosomal junctions (11, 12, 25).
Published reports suggest that Cdx2 may regulate tight junction formation, in part by transcriptional regulation of the claudin-2 gene (33, 39). More recently, we demonstrated that the desmocollin-2 gene, a component of desmosomes, is also a transcriptional target for Cdx2 (12). Adherens junction regulation by Cdx2 is less well understood. Interest in Cdx2 regulation of E-cadherin has increased since Cdx2 was identified as an inhibitor of metastasis and epithelial-to-mesenchymal transitions (EMTs) in colorectal cancer (15), both of which are dependent on regulation of E-cadherin function. In our prior published studies, we found no evidence that Cdx2 regulated the expression of any components of the adherens junction (11, 25). We did observe that Cdx2 expression was associated with reduced β-catenin and p120-catenin tyrosine phosphorylation. However, the tyrosine kinase whose activity is modulated by Cdx2 was not identified. An important question left to resolve was the identity of these kinases and how Cdx2, a nuclear transcription factor, regulated their activity in the cell cytoplasm and membrane compartments. This problem is not unique to our work. Previously, it was demonstrated that Cdx2 haploinsufficiency was associated with increased intestinal polyposis due to increased mammalian target of rapamycin (mTOR) activity (4, 6). However, the mechanism by which Cdx2 haploinsufficiency led to increased mTOR activity was never fully resolved.
In the present study, we identify a novel mechanism by which Cdx2 modulates E-cadherin activity. We utilize two complementary colon cancer cell lines in which Cdx2 expression is absent or present and in which Cdx2 expression is associated with cell-cell adhesion and E-cadherin function. In both cell lines, when Cdx2 is absent, E-cadherin accumulates in the cytoplasm, and the cells acquire a more invasive and migratory phenotype. This is reversed by restoration of Cdx2 expression. Utilizing short interfering RNA (shRNA) targeting vectors and kinase inhibitors, we establish a mechanistic pathway by which the nucleus-localized transcription factor regulates these cytoplasmic- and cell membrane-localized processes. These novel findings have important implications beyond the regulation of E-cadherin function, as they suggest that Cdx2 may regulate cellular metabolism, proliferation, and stem cell function, as well as cell-cell adhesion and colon cancer cell metastasis, by regulating protein trafficking to and from the cell surface membrane compartment.
MATERIALS AND METHODS
Cell culture and transfections.
Colo 205 and LoVo colon cancer cells were obtained from the American Type Culture Collection or the Cell Center (University of Pennsylvania) and maintained as recommended by their supplier. MIGR1- and MIGR-Cdx2-infected Colo 205 cells were generated from Colo 205 cells as described elsewhere (11, 25). Colo-MSCV-Cav1 cells were produced as described elsewhere (11, 25), except 400 μg/ml geneticin (G418, Invitrogen) was used for selection. LoVo Cdx2 knockout cells were derived as described elsewhere (9). Colo 205 cells that were infected with shRNA lentiviruses targeting expression of c-Abl or CrkL were maintained in culture medium supplemented with 2 μg/ml puromycin. MIGR1 Cdx2-Colo 205 cells infected with a retrovirus targeting caveolin-1 were maintained in DMEM supplemented with 400 μg/ml geneticin.
Migration and invasion assays were carried out as described elsewhere (11). LoVo control and LoVo Cdx2 knockout cells were serum-starved overnight in F12K medium with only 0.5% FCS (Hyclone Laboratories, Logan, UT). A single-cell suspension of 1 × 105 cells was placed into the upper FluorBlok chamber (Falcon) with 8.0-μm pore size in RPMI medium with only 0.5% FCS. RPMI medium with 20% FCS was placed in the lower chamber, and cells were incubated at 37°C. Fresh medium was added daily. At 48 h, the cells were visualized by 4 μg/ml calcein-AM (C-3100, Molecular Probes) and counted. For invasion assays, cells and media were prepared as described above. A tumor invasion system (catalog no. 354165, BD Bioscience) coated with Matrigel was utilized, and inserts were rehydrated as directed by the manufacturer. Then 5 × 104 cells were placed in the upper chamber, and the cells were incubated and counted as described above. Means and SDs were calculated and compared using Student's t-test.
Reagents and antibodies.
Antibodies directed against α-tubulin, N-cadherin, Src and phosphorylated (Y416) Src, CrkL and phosphorylated (Y221/207) CrkL, PDGF receptor-α (PDGFRα) and phosphorylated (Y754) PDGFRα, c-Abl and phosphorylated (Y412 and Y245) c-Abl, caveolin-1 and phosphorylated (Y14) caveolin-1, and IGF I receptor (IGF-IR) and phosphorylated (Y1135/1136) IGF-IR were purchased from Cell Signaling Technology (Danvers, MA); phosphorylated (Y489) β-catenin from ECM Bioscience (Versailles, KY); β-catenin, c-Abl (K12; for immunoprecipitation), E-cadherin, and YY-1 from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies to phosphorylated Src (GD11), EGF receptor (EGFR) (Y1173), HER-2/ErbB2, and phosphotyrosine (4G10) from Upstate Biotechnology (Lake Placid, NY); and antibodies to EGFR and c-Abl (8E9; for Western blotting) from BD Biosciences (Chicago, IL). β-Catenin, E-cadherin, and p120-catenin antibodies (610154, 610182, 610134, and 610194, BD Transduction Laboratories) and Cdx2-88 (BioGenex Laboratories, San Ramon, CA) were used for Western blotting. Cdx-1 (CPSP) polyclonal antibody was previously described (41). For a Western blot loading control, we used anti-α-tubulin (Sigma-Aldrich, St. Louis, MO). Secondary antibodies included anti-rabbit or anti-mouse immunoglobulin (Amersham Pharmacia).
Imatinib (STI571) and dasatinib were obtained from LC Laboratories (Woburn, MA); the Src kinase inhibitor (SU6656), EGFR (PD153035), FGF receptor/VEGF receptor (PD173074), and EGFR/HER-2 (PD158780) kinase inhibitors from Calbiochem (La Jolla, CA); the PDGFR inhibitor V (sc-204174) from Santa Cruz Biotechnology; and the c-Met kinase inhibitor (SU11274), Rac1 inhibitor (NSC23766), Rho kinase inhibitor (Y 27632), staurosporine, N-benzoyl (CGP 41 251), Src kinase inhibitor I [4-(4′-phenoxyanilino)-6,7-dimethoxyquinazoline], IGF-IR inhibitor [picropodophyllin (PPP)], and PP2 (AG 1879), as well as the pLKO.1 vector of shRNA targeting human c-Abl, human CrkL, and control nontargeting shRNA from Sigma-Aldrich.
Receptor phosphotyrosine array.
Whole cell lysates were prepared as described by the manufacturer (R & D Systems, Minneapolis, MN). Cells were lysed in an NP-40 lysis buffer [1% NP-40, 20 mM Tris·HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM sodium orthovanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin]. Lysates were diluted to 500 μg/ml in array buffer. Arrays were blocked in array buffer for 1 h, then the buffer was removed, and the arrays were incubated with 1.5 ml of diluted cell lysates overnight at 4°C with rocking. Arrays were then washed, probed with the detection antibody, and exposed to chemiluminescence reagents as described in the protocol provided by the supplier. The array was then exposed to X-ray film, and the signal was quantified by densitometry.
Transfection, virus production, and infection.
Retrovirus production and infection were carried out as described previously using ϕ-NX cells (11, 25). For lentivirus production, 293T cells were utilized. Cells were transfected using a Lipofectamine 2000 transfection kit (Invitrogen) with lentiviral and packaging vectors (Sigma-Aldrich). Transfected cells were grown at 37°C for 48 h. Virus-containing medium was removed, filtered through a 0.45-μm filter, and supplemented with polybrene (8 μg/ml) prior to infection of target cells.
Immunofluorescence and Western blots.
Cells plated on glass coverslips were fixed and stained as described elsewhere (46). Whole cell extracts were prepared using the mammalian protein extraction reagent (M-PER) kit (Thermo Scientific). Cells were lysed in three volumes of M-PER containing protease inhibitor cocktail and phosphatase inhibitor cocktails I and II (Sigma, St. Louis, MO). Membrane (Mem-PER), nuclear (NE-PER), and cytoplasmic protein extraction reagent kits (Thermo Scientific) were used for isolating membrane, nuclear, and cytoplasmic fractions of protein, respectively. Proteins were visualized using an enhanced chemiluminescence (ECL) or ECL Plus kit (GE Healthcare). (See Supplemental Material, available online at the Journal website for a complete list of antibodies and other reagents.)
Quantitative real-time PCR.
Total RNA was isolated using RNeasy (Qiagen). The first-strand cDNA synthesis kit (Invitrogen) was used for cDNA synthesis. For the RT-PCR, cDNA and primers were mixed with SYBR Green PCR Master Mix (Applied Biosystems) and then assayed in an Prism 7000 sequence detection system (Applied Biosystems) as directed by the manufacturer. A ribosomal phosphoprotein, 36B4, was used as the normalization control. ΔCt values were calculated after duplicate PCRs for each sample as described elsewhere (11, 25), and statistical analysis was performed (ANOVA and Tukey's rank mean). ΔΔCt values were then calculated and used to determine fold change in expression.
RESULTS
Loss of Cdx2 expression is associated with disruption of colon cancer cell-cell adhesion.
Previously, we demonstrated that Cdx2 expression in human colon cancer Colo 205 cells induced a cell-cell adhesive phenotype associated with increased E-cadherin activity (11, 25). To better understand this, we explored cell-cell adhesion and E-cadherin function in a complementary cell culture model where Cdx2 function is disrupted, LoVo and LoVoCdx2−/− human colon cancer cells.
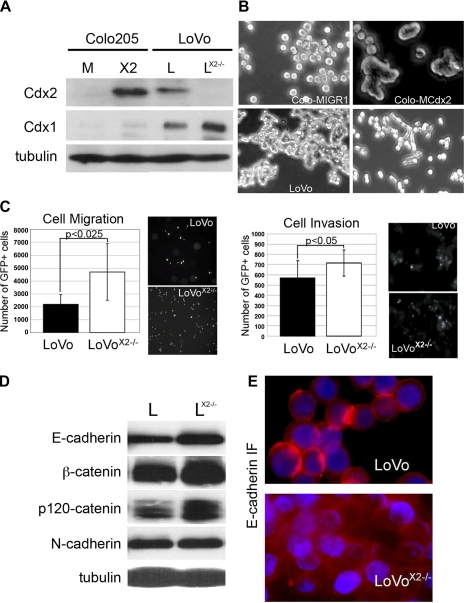
LoVo cells are near diploid in DNA content. Previously we disrupted both alleles of the Cdx2 gene by targeted homologous recombination (Fig. 1A) (9). Cdx2 is not detected in the LoVoCdx2−/− cells, but there was an increase in the homolog Cdx1 protein levels. We found that these LoVoCdx2−/− cells were more proliferative than the parental cells but were also less tumorigenic (9). Additionally, we noted that these cells are less adherent to adjacent cells (9) (Fig. 1B). These effects are similar to those we observed in Colo 205 cells, where restoration of Cdx2 expression reduced cell proliferation and induced cell-cell adhesion (Fig. 1A) (11, 25). Boyden chamber assays established that these LoVoCdx2−/− cells were significantly more migratory and invasive than the parental cells (Fig. 1C). However, loss of Cdx2 expression in the LoVo cells was not associated with loss of E-cadherin or any of the adherens junction proteins (Fig. 1D). This is identical to our observation in the Colo 205 cells. Immunofluorescence studies of E-cadherin demonstrated loss of E-cadherin protein accumulation at cell-cell junctions in the LoVoCdx2−/− cells, while cytoplasmic E-cadherin levels seemed unchanged (Fig. 1D).
Fig. 1.
Loss of Cdx2 expression in LoVo colon cancer cells is associated with diminished cell-cell adhesion and increased cell migration and invasion in in vitro assays. A: Western blots for Cdx1 and Cdx2 expression in Colo 205 cells receiving the control MIGR1 (M) or MIGR-Cdx2 retrovirus [Colo-MIGR-Cdx2 (X2)], LoVo (L), and LoVoCdx2−/− (LX2−/−) cells. α-Tubulin served as loading control. B: phase-contrast images of cells in culture. C: cell migration and invasion assays for LoVo and LoVoCdx2−/− cells in assays carried out in Boyden chambers using FluoroBlok inserts (Falcon). GFP, green fluorescent protein. n = 6. Data from 2 separate experiments were combined for analysis. D: Western blot analysis of whole cell lysates for adherens junction proteins in LoVo and LoVoCdx2−/− cells. α-Tubulin served as loading control. E: immunofluorescence (IF) shows E-cadherin in LoVo and LoVoCdx2−/− cells.
The significant loss of E-cadherin at the cell surface was not consistent with our previous observation in our Colo 205 cell studies (11, 25). To evaluate this finding more specifically, we isolated nuclear, cytoplasmic, and cell membrane fractions from LoVoCdx2−/− and control cells. YY1 and α-tubulin served as loading controls (nuclear and cytoplasmic fractions, respectively) and to illustrate the quality of the separated fractions. No reliable loading control is available to evaluate the membrane fraction in a similar manner. We found that targeted deletion of Cdx2 in LoVo cells led to a significant reduction in E-cadherin and β-catenin localized to the membrane fraction, even while N-cadherin levels remained unchanged (Fig. 2A). Cytoplasmic and nuclear levels of E-cadherin, N-cadherin, or β- and p120-catenin were also unchanged. The role of E-cadherin in the nucleus is unclear, but it has been reported to localize to the nucleus in other cancers as well (10, 40). Using the same technique, we then examined our Colo 205 cells and found that restoration of Cdx2 expression was associated with a very significant increase in E-cadherin and β- and p120-catenin protein in the membrane fraction (Fig. 2B). This suggests that, in LoVo and Colo 205 cells, Cdx2 expression induces the stable localization of E-cadherin, β-catenin, and p120-catenin to the cell membrane compartment. We conclude that Cdx2 promotes cell-cell adhesion by inducing E-cadherin localization to the cell membrane.
Fig. 2.
Cdx2 expression induces stable localization of E-cadherin, β-catenin, and p120-catenin to the cell membrane compartment in LoVo and Colo 205 cells. Fractionated protein lysates from cell nuclei, cytoplasm, and cell membrane compartments were obtained from LoVo, LoVoCdx2−/−, Colo 205 wild-type (wt), and Colo 205 cells receiving the MIGR-Cdx2 or control MIGR1 retrovirus. Lysates were subjected to PAGE and Western blotting for the indicated proteins. YY1 and α-tubulin were loading controls for nuclear and cytoplasmic fractions, respectively. A: studies with LoVo and LoVoCdx2−/− fractionated lysates. One of several blots is shown. B: studies with Colo 205, Colo-MIGR1, and Colo-MIGR-Cdx2 fractionated lysates.
Restoration of E-cadherin localization to the cell membrane and induction of cell-cell adhesion by protein kinase inhibitors.
We confirmed a previously published observation (5) that treatment of Colo 205 cells with staurosporine, a broad-spectrum kinase inhibitor, induces a cell-cell adhesion phenotype that is indistinguishable from Cdx2 (Fig. 3A). Staurosporine also induced a localization of E-cadherin and β-catenin to the membrane fraction, just as Cdx2 did. However, p120-catenin was not also localized to the membrane fraction by this treatment. N-cadherin levels were unchanged throughout these treatments. Staurosporine had a similar effect on LoVo and LoVoCdx2−/− cells (Fig. 3B). These observations suggest that Cdx2 induces cell-cell adhesion and E-cadherin function in colon cancer cells by inhibiting the activity of cellular kinases.
Fig. 3.
Several protein kinase inhibitors can phenocopy Cdx2's effects on E-cadherin and cell-cell adhesion in Colo 205 cells. A: phase-contrast images of Colo-MIGR1 cells treated with vehicle or 10 μM staurosporine for 24 h. Cells appear to cluster only when treated with the inhibitor. Fractionated cell protein lysates were prepared from Colo-MIGR1 and Colo-MIGR-Cdx2 cells treated with 10 μM staurosporine for 24 h. Lysates were then examined by Western blotting for the presence of adherens junction proteins. α-Tubulin in the cytoplasmic fraction is included as a control for protein quality and a relative loading control. B: phase-contrast images of LoVoCdx2−/− cells treated with vehicle or 10 μM staurosporine for 24 h. Cells appear to cluster only when treated with the inhibitor. Fractionated cell protein lysates were prepared from LoVo and LoVoCdx2−/− cells treated with 10 μM staurosporine for 24 h. Lysates were then examined by Western blotting for the presence of adherens junction proteins. Tubulin in the cytoplasmic fraction is included as a control for protein quality and a relative loading control.
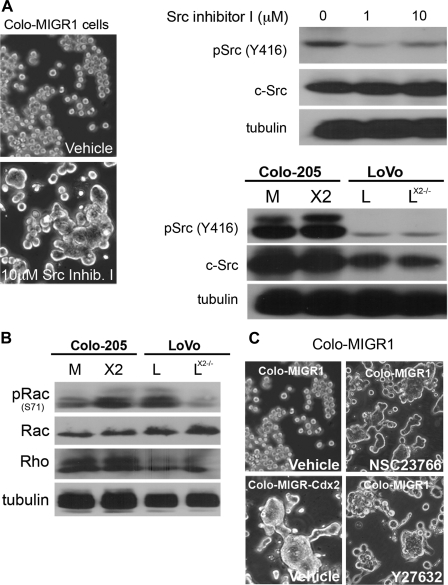
Staurosporine is reported to inhibit Src family kinases, receptor tyrosine kinases (RTKs, e.g., PDGFR and IGF-IR), and protein kinase C, among other targets. Treatment of Colo 205 cells with Src kinase inhibitor I (Calbiochem) induced cell clustering and adhesion in Colo 205 and LoVoCdx2−/− cells (Fig. 4A and data not shown). However, Cdx2 expression in LoVo and Colo 205 cells was not associated with changes in total Src or activated phosphorylated Src levels. This suggests that Cdx2 does not reduce Src kinase activity to induce E-cadherin function.
Fig. 4.
Several protein kinase inhibitors can phenocopy Cdx2's effects on E-cadherin and cell-cell adhesion in Colo 205 cells. A: phase-contrast images of cells treated for 24 h with 10 μM Src kinase inhibitor I or vehicle control. Cells appear to cluster when treated with the inhibitor only. Whole cell lysates collected at 24 h demonstrated reductions in Src phosphorylation (Y416) with the inhibitor but not in total Src levels. However, Cdx2 expression did not alter Src levels or its phosphorylation at Y416. Western blot analysis of Colo-MIGR1 control, Colo-MIGR-Cdx2, LoVo, and LoVoCdx2−/− cells for Src and phosphorylated (p) Src shows no changes in their levels with Cdx2 expression. α-Tubulin served as loading control in both. B: Western blots of whole cell lysates from Colo 205 cells receiving the control MIGR1 or MIGR-Cdx2 retroviruses and from LoVo and LoVoCdx2−/− cells. C: phase-contrast images of Colo-MIGR1 cells treated with vehicle, the Rac inhibitor (NSC23766), or the Rho kinase inhibitor (Y 27632) for 24 h. Cells appear to cluster only when treated with the inhibitor. Clustering Colo-MIGR-Cdx2 cells in vehicle are included as a control.
We investigated other cell signaling pathways as well. There were no changes in activated or total MAPK p42/44 levels with Cdx2 expression (data not shown). Rho and Rac small GTPases are known to initiate and expand cadherin-mediated cell-cell contacts (37). Also, Rac1 activation has been associated with disruption of E-cadherin function by trafficking it to intracellular vesicles (1, 20, 29). Cdx2 expression did not change total Rac and Rho protein levels, but Cdx2 expression did increase Rac phosphorylation (at S71) in both cell lines (Fig. 4B). Phosphorylation at S71 has been linked to reduced Rac activity (26). Treatment of Colo-MIGR1 cells with inhibitors to Rho (Y27632) or Rac1 (NSC23766) did phenocopy Cdx2 and induce cell-cell clustering (Fig. 4C). Together, these findings suggest that Cdx2 expression regulates Rac activity to induce E-cadherin trafficking to the cell membrane.
Cdx2 expression inhibits PDGFR and IGF-IR activity to promote E-cadherin function.
A number of kinase pathways converge on Rho and Rac, including RTKs (21, 48, 49). RTKs are frequently aberrantly active in colon cancer cells. Their activation can result in the disruption of E-cadherin function. To identify activated RTKs, we incubated cell lysates with the human phosphorylated RTK profiler array (R & D Systems, Minneapolis, MN; Fig. 5). Several active candidate RTKs were identified in our cells, but only the hepatocyte growth factor (HGF) receptor (c-Met) was reliably altered with Cdx2 expression (Table 1, Fig. 5B). However, treatment of our cells with a c-Met inhibitor (SU11274) did not alter cell adhesion (Fig. 5C and data not shown).
Fig. 5.
A: human phosphorylated receptor tyrosine kinase (RTK) profiler array identified a number of RTKs active in Colo 205 and LoVo cells. A: fresh protein lysates from Colo-MIGR1, Colo-MIGR-Cdx2, LoVo, and LoVoCdx2−/− cells were incubated with phosphorylated RTK profiler array. After the array was washed, it was probed with the detection antibody and chemiluminescence reagents and exposed to X-ray film. Results from 1 of 2 experiments are presented. B: Western blots for total and activated c-Met levels in lysates from Colo-MIGR1, Colo-MIGR-Cdx2, LoVo, and LoVoCdx2−/− cells. Tubulin is included as a loading control. C: phase-contrast images of Colo-MIGR-Cdx2 and Colo-MIGR1 cells treated with 5 μM SU11274 (a c-Met inhibitor) or vehicle control for 24 h.
Table 1.
Results from human phosphorylated RTK array studies
| Cell Type |
||
|---|---|---|
| Colo-MIGR-Cdx2/Colo-MIGR1 | LoVoCdx2−/−/LoVo | |
| HGFR (c-Met) | Increased vs. Colo-MIGR1* | Increased vs. LoVoCdx2−/− |
| IGF-IR | Present* | Not detected |
| PDGFRα | Present* | Not detected |
| EGFR | Present* | Present* |
| ErbB2 | Present | Unknown |
| ErbB3 | Present* | Present* |
| ErbB4 | Present | Not detected |
| FGFR3 | Present | Present |
| Insulin receptor | Present | Present |
| Dtk | Present | Not detected |
| VEGFR3 | Present | Present |
| MuSK | Present | Present |
| EphA1R | Present | Present |
| EphA2R | Present* | Present* |
| EphB2R | Present | Present |
| c-Ret | Not detected | Present |
Two separate lysates from each cell line (Colo-MIGR1, Colo-MIGR-Cdx2, LoVo, and LoVoCdx2−/− cells) were incubated with the arrays. Receptor tyrosine kinases (RTKs) were scored as to whether, in all 4 Colo arrays (or all 4 LoVo arrays), the tyrosine-phosphorylated protein product could be detected (present) or could not be detected (not detected). Detection of phosphorylated ErbB2 in LoVo cells was weak in 2 lysates and not the others and was labeled unknown.
Validated by Western blotting. HGFR, hepatocyte growth factor receptor; IGF-IR, IGF-I receptor; PDGFR, PDGF receptor; EGFR, EGF receptor; FGFR, FGF receptor; VEGFR, VEGF receptor.
Next, we studied other RTKs detected in our array. No changes were noted in the expression and phosphorylation state of EGFR1, ErbB3, and EphA2R with Cdx2 expression (Fig. 6A). Nor did the addition of inhibitors to the EGFR (PD153035) or FGFR/VEGFR (PD173074) induce cell-cell adhesion in Colo 205 cells (Fig. 6B). However, Colo 205 cells were strongly induced to cluster with the addition of an inhibitor to the PDGFR (Fig. 7, A and B). This cell-cell adhesion mimicked that seen with Cdx2. Importantly, Cdx2 expression in Colo 205 cells was associated with a significant reduction in PDGFRα activation, as indicated by the reduced levels of phosphorylated PDGFRα (Fig. 7C). Cdx2 expression did not reduce PDGFRα total levels but induced its removal from the membrane compartment (Fig. 7D), effectively inhibiting it. A downstream effector of the PDGFR signaling pathway, the CrkL adaptor protein (3, 34), was similarly lost from the membrane compartment with Cdx2 expression. PDGFRβ was not detected in the Colo 205 cells (data not shown).
Fig. 6.
Some RTK inhibitors cannot phenocopy Cdx2's effects on cell-cell adhesion in Colo 205 cells. A: Western blots showing total and activated EGF receptor (EGFR), ErbB3, and EphA2R levels in lysates from Colo-MIGR1, Colo-MIGR-Cdx2, LoVo, and LoVoCdx2−/− cells. Tubulin is included as a loading control. B: phase-contrast images of Colo-MIGR1 cells treated for 24 h with 1 μM EGFR1 inhibitor, FGFR-VEGFR inhibitor, or vehicle control.
Fig. 7.
Cdx2 induces E-cadherin function and cell-cell adhesion in Colo 205 cells by inhibiting PDGF receptor (PDGFR) activity. A: phase-contrast images of Colo-MIGR1 cells treated for 24 h with 10 μM PDGFR inhibitor V or vehicle control. B: whole cell lysates were prepared from treated Colo-MIGR1 cells at 24 h and then subjected to Western blot analysis for phosphorylated and total PDGFRα levels. C: whole cell lysates from Colo-MIGR1 and Colo-MIGR-Cdx2 cells were subjected to Western blot analysis for phosphorylated and total PDGFRα levels. D: fractionated cell protein lysates were prepared from Colo-MIGR1 and Colo-MIGR-Cdx2 cells. Levels of PDGFRα were determined in the membrane fraction, as were levels of the downstream effector CrkL, an SH2 and SH3 adaptor-containing protein. Tubulin in the cytoplasmic fraction served as a protein quality control.
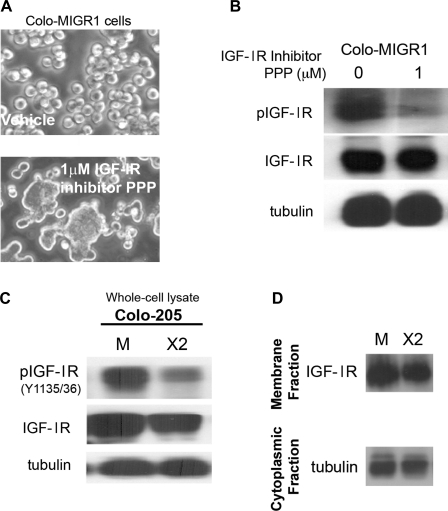
IGF-IR was similarly identified as active by the array in Colo 205 cells. Treatment of Colo-MIGR1 cells with PPP, a potent inhibitor of IGF-IR kinase activity, induced cell-cell adhesion (Fig. 8, A and B). Cdx2 expression was associated with a significant reduction in IGF-IR phosphorylation, indicating reduced kinase activity. However, Cdx2 expression did not reduce IGF-IR levels or its localization in the membrane compartment (Fig. 8, C and D). Thus, Cdx2 appears to induce E-cadherin activity and Colo 205 cell-cell adhesion by reducing IGF-IR and PDGFRα activity in Colo 205 cells.
Fig. 8.
Cdx2 expression inhibits IGF-I receptor (IGF-IR) activation in Colo 205 cells, resulting in enhanced E-cadherin function and cell-cell adhesion. A: phase-contrast images of Colo-MIGR1 cells treated for 24 h with 1 μM picropodophyllin (PPP), an IGF-IR inhibitor, or vehicle control. B: whole cell lysates were prepared from treated Colo-MIGR1 cells at 24 h and then subjected to Western blot analysis for phosphorylated and total IGF-IR levels. C: whole cell lysates from Colo-MIGR1 and Colo-MIGR-Cdx2 cells were subjected to Western blot analysis for phosphorylated and total IGF-IR levels. D: fractionated cell protein lysates from Colo-MIGR1 and Colo-MIGR-Cdx2 cells were examined for levels of IGF-IR in the membrane fraction. α-Tubulin in the cytoplasmic fraction served as a protein quality control.
Reduction in c-Abl kinase activation by Cdx2 promotes cell-cell adhesion in Colo 205 and LoVo cells.
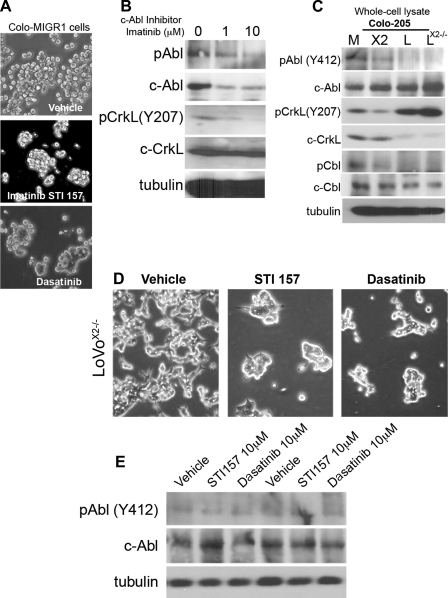
While we succeeded in identifying several RTKs in Colo 205 cells regulated by Cdx2 that influence cell-cell adhesion, neither was detected in the LoVo cells by Western blot studies of LoVo and LoVoCdx2−/− whole cell lysates (data not shown). A downstream effector common to PDGFR and the IGF-IR, as well as other RTKs, is the c-Abl tyrosine kinase (42). Previously, a novel Src/Abl kinase inhibitor induced a dense spheroid clustering of Colo 205 cells (14). We therefore investigated whether c-Abl activity regulated cell adhesion in Colo 205 and LoVo cells. Treatment of Colo-MIGR1 cells with inhibitors of the c-Abl kinase (STI157 or dasatinib) for 24 h induced the cell-cell adhesion phenotype (Fig. 9, A and B). STI157 reduced phosphorylated Abl and total c-Abl levels. This treatment also reduced the level of CrkL phosphorylation. CrkL is an adaptor protein that is activated by Abl kinase phosphorylation and is an important effector for c-Abl kinase activity (42, 48).
Fig. 9.
Induction of cell-cell adhesion by Cdx2 is mediated by reductions in c-Abl kinase activity. A: phase-contrast images of Colo-MIGR1 cells treated with the c-Abl kinase inhibitors STI157 (10 mM) and dasatinib (10 mM) for 24 h show cell-cell clustering. B: Western blots of lysates obtained after 24 h with STI157. C: whole cell lysates from Colo-MIGR1, Colo-MIGR-Cdx2, LoVo, and LoVoCdx2−/− cells were subjected to Western blot analysis for phosphorylated and total Abl kinase, phosphorylated and total CrkL, and phosphorylated and total c-Cbl levels. D: phase-contrast images of LoVoCdx2−/− cells treated with the c-Abl kinase inhibitors STI157 (10 mM) and dasatinib (10 mM) for 24 h shows cell-cell clustering. E: Western blots of lysates from LoVoCdx2−/− cells obtained after 24 h with STI157 and probed for phosphorylated and total c-Abl.
Cdx2 expression reduced phosphorylated Abl levels and CrkL phosphorylation in Colo 205 cells, both consistent with diminished Abl kinase activity (Fig. 9C). Moreover, the levels of phosphorylated c-Cbl, another adaptor protein activated by Abl kinase, were similarly reduced. Together these findings support the conclusion that Cdx2 expression in Colo 205 cells reduces c-Abl kinase activity.
Our findings in LoVo cells are similar. While phosphorylated Abl and phosphorylated c-Cbl appear to be below the level at which we can detect them, we clearly observe a reduction in CrkL phosphorylation in the LoVo cells compared with the LoVoCdx2−/− cells (Fig. 9C). Moreover, treatment of the LoVoCdx2−/− cells with the Abl kinase inhibitors did induce greater cell-cell adhesion (Fig. 9, D and E). To better establish the role for c-Abl or CrkL in Colo 205 and LoVo cell-cell adhesion, we used shRNA targeting vectors. Using shRNA sequences in pLKO.1 vectors (Sigma-Aldrich; c-Abl clones 2 and 3, CrkL clone 2), we significantly reduced c-Abl and CrkL levels in our cells (Fig. 10). Some clones had no effect and served as additional controls (CrkL clone 1). In Colo-MIGR and LoVoCdx2−/− cells, knocking down c-Abl or CrkL levels was associated with induction of cell-cell adhesion (Fig. 10). In the Colo 205 cells, the Abl kinase knockdown was associated not only with diminished phosphorylated Abl and phosphorylated CrkL, but also reduced phosphorylated (Y489) β-catenin (Fig. 10B). The CrkL knockdown did not, in contrast, reduce phosphorylated Abl or phosphorylated (Y489) β-catenin. These observations are consistent with β-catenin being a phosphorylation target of the Abl kinase at Y489 (27). We conclude that the c-Abl kinase, a common downstream effector of RTK activity, acts to disrupt E-cadherin function in Colo 205 and LoVo cells. Cdx2 expression in both cell lines is associated with diminished Abl kinase activity, likely due to Cdx2-mediated reductions in upstream RTK activity.
Fig. 10.
Reductions in c-Abl kinase activity by Cdx2 induces cell-cell adhesion in LoVoCdx2−/− cells. A: phase-contrast images of Colo-MIGR1 cells after infection with lentiviruses and selection. Viruses direct expression of short interfering RNA (shRNA) targeting c-Abl, CrkL, or the scrambled control. B: Western blots of lysates from Colo-MIGR1 cells after infection with shRNA lentiviruses and puromycin selection. Blots were probed for phosphorylated and total c-Abl, phosphorylated and total CrkL, and phosphorylated and total β-catenin, as well as α-tubulin loading control. C: phase-contrast images of LoVoCdx2−/− cells after infection with lentiviruses and selection. Viruses direct expression of shRNA targeting c-Abl, c-CrkL, or the scrambled control.
Cdx2 enhances caveolae function to diminish RTK activity and restore E-cadherin to the membrane compartment.
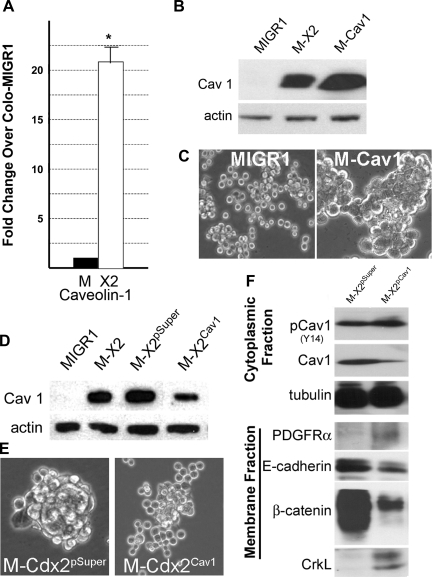
Caveolae are specialized plasma membrane microdomains that have been implicated in a variety of physiological processes, including membrane trafficking, cell motility, and signal transduction (including RTKs) (30, 36, 38, 47). Caveolins are the principal protein components of caveolae. We investigated caveolin-1 levels in our Colo 205 and LoVo cell lines. LoVoCdx2−/− cells expressed abundant caveolin-1, but Colo 205 cells had little detectable caveolin-1 expression (data not shown). Retroviral-mediated expression of Cdx2 significantly increased caveolin-1 mRNA and protein levels in the Colo 205 cells (Fig. 11, A and B). Directed expression of a caveolin-1 cDNA (kindly provided by Phoebe Fielding, University of California San Francisco) using a retroviral vector in Colo 205 cells did induce cell-cell adhesion, as observed with Cdx2 expression (Fig. 11, B and C). We next targeted caveolin-1 expression in Colo-MIGR-Cdx2 cells using a shRNA vector. The vector reduced caveolin-1 levels by about two-thirds compared with the control (Fig. 11D), and Colo-MIGR-Cdx2 cells infected with this retrovirus lost their cell-cell adhesion phenotype (Fig. 11E). Moreover, in fractionated protein lysates, caveolin-1 total protein levels are diminished, but phosphorylated caveolin levels remain unchanged, consistent with an overall relative increase in phosphorylated caveolin-1 levels (Fig. 11F). More importantly, in the membrane fraction, we observed increased PDGFR and CrkL levels and reduced E-cadherin and β-catenin protein levels (Fig. 11F). This pattern is similar to that seen in wild-type Colo 205 cells in the absence of Cdx2 expression (Figs. 2 and 7). We conclude that Cdx2 promotes E-cadherin function and cell-cell adhesion in Colo 205 cells by enhancing caveolin-1 protein levels, leading to diminished RTK activity, resulting in increased localization of E-cadherin to the cell membrane compartment.
Fig. 11.
Cdx2 regulates RTK activity and adherens junction function by inducing caveolin-1 mRNA and protein in Colo 205 cells. A: quantitative SYBR Green RT-PCR analysis of caveolin-1 gene expression in Colo 205 cells. Total RNA was isolated from Colo-MIGR-Cdx2 and control Colo-MIGR1 cells. PCR control was the phosphoprotein 36B4. n = 6 samples. *Significantly different from Colo-MIGR1 cells (P < 0.001). B: caveolin-1 protein levels in control Colo-MIGR1 cells, Colo-MIGR-Cdx2 cells, and cells treated with the MSCV-Cav1 virus (M-Cav1) to express a caveolin-1 cDNA. C: phase-contrast images of Colo-MSCV-Cav1 cells and control Colo-MIGR1 cells demonstrating cell-cell adhesion induced by caveolin-1 protein expression. D: effects of a shRNA vector targeting caveolin-1. Western blots show caveolin-1 levels in protein lysates obtained from control Colo-MIGR1 cells, Colo-MIGR-Cdx2 cells, Colo-MIGR-Cdx2 cells treated with the empty shRNA vector (M-X2pSuper), and Colo-MIGR-Cdx2 cells treated with the knockdown vector (M-X2Cav1). E: phase-contrast images showing effects of caveolin-1 reduction on cell-cell adhesion in Colo-MIGR-Cdx2 cells. F: fractionated cell protein lysates from Colo-MIGR-Cdx2Cav1 and control Colo-MIGR-Cdx2pSuper cells were examined for levels of phosphorylated and total caveolin-1 protein in the cytoplasmic fraction and for levels of PDGFR, E-cadherin, β-catenin, and CrkL in the membrane fraction. α-Tubulin in the cytoplasmic fraction served as a protein quality control.
DISCUSSION
The homeodomain transcription factor Cdx2 is required for intestinal differentiation and columnar morphogenesis (11–13, 25). Cdx2 has been suggested to be a tumor suppressor (4, 6) or to have tumor suppressor properties (16, 17, 19) in the colon. Cdx2 has been implicated as an antagonist of EMT in colon cancer (15), and this effect depends on its promotion of strong cell-cell adhesion interactions. The observations reported here significantly advance our understanding of Cdx2 regulation of E-cadherin function. We establish for the first time a well-defined mechanism by which Cdx2 regulates E-cadherin in two distinct colon cancer cell lines. Moreover, we found that Cdx2 expression can influence RTK activity without directly regulating its expression. This is a powerful and novel function for Cdx2, one with implications for Cdx2 regulation of colon cancer and normal intestinal cell proliferation, migration, and survival.
Cdx2 regulates E-cadherin indirectly by modulating RTK activity at the cell surface.
One challenge to understanding how Cdx2 regulates E-cadherin function has been the absence of an obvious transcriptional mechanism. Several cell-cell adhesion proteins have been identified as transcriptional targets for Cdx2 (12, 23, 39). Despite the suggestion from one early study that E-cadherin expression was regulated by Cdx2 (28), we found no evidence for this in studies of several colon cancer cell lines (11, 12, 25). Fundamentally, the restoration of E-cadherin and the catenin proteins to the membrane compartment by Cdx2 expression is a novel mechanism. Our ability to establish this relationship in Colo 205 and LoVo cells implies that this mechanism is broadly operative in colon cancer and intestinal cells. In addition, we identified a pathway of kinases and adaptor proteins that links several cell surface RTKs to regulators of E-cadherin function (Fig. 12). In Colo 205 cells only, Cdx2 influences the activity of this pathway by enhancing caveolin-1 expression levels, which leads to the silencing of PDGFR and IGF-IR tyrosine kinase activity. This is an unexpected finding. While Cdx2 has been identified as a transcriptional activator of the heparin-binding EGF gene (45), no previous reports have suggested that Cdx2 regulates RTK activity in any manner.
Fig. 12.
Model for Cdx2-mediated regulation of E-cadherin function and cell-cell adhesion in colon cancer and intestinal epithelial cells. Left: in the absence of Cdx2 expression, RTKs, such as PDGFR (only colon cancer cells), IGF-IR, and others, are active, phosphorylated, and in turn activate downstream targets, including c-Abl (phosphorylated Abl). Abl kinase in turn phosphorylates targets and alters their function, including c-Cbl (pCbl), CrkL (pCrkL), and β-catenin (pβ-cat). E-cadherin (E-Cad) function is disrupted in 2 ways: Y489-phosphorylated β-catenin does not interact with E-cadherin, and, perhaps more significantly, pAbl and pCrkL can modulate Rho and Rac activity to block trafficking of E-cadherin to the cell surface membrane (20, 29, 48, 49). β-Catenin released from E-cadherin and tyrosine-phosphorylated β-catenin can translocate to the nucleus, where it modulates target gene expression. TCF, T cell factor. Right: in the presence of Cdx2 expression, caveolin-1 and, possibly, other factors that reduce RTK activation, such as IGF-IR, are expressed and promote the withdrawal of other RTKs, such as PDGFR, from the surface membrane. Without the RTK stimulus, c-Abl and its effector and adaptors are also dephosphorylated, possibly by protein tyrosine phosphatase 1B (PTP-1B) (11). Normal Rho and Rac function are restored, and E-cadherin is transported to the surface membrane, where it can engage in cell-cell adhesion. Moreover, Cdx2 induces the expression of other cell-cell adhesion proteins, including LI-cadherin (LI-Cad), claudin-2, and desmocollin-2 (DSC2).
The diminished PDGFR and IGF-IR activity leads to reduction in activity of c-Abl kinase, as well as activity of downstream effectors that are known to modulate E-cadherin function (48). Nearly all these factors have been reported to regulate E-cadherin function (35, 42, 48); however, their control by Cdx2 is novel, as is their placement by us within the context of colon cancer cell-cell adhesion. Additionally, this finding resolves a long-standing unresolved question regarding Cdx2's tumor-suppressor qualities. Aoki et al. (4) reported that Cdx2 heterozygous knockout mice were prone to more polyps in the APCMin/+ mouse model because of increased mTOR activity (6). However, how Cdx2 haploinsufficiency led to increased mTOR kinase activity was never resolved. We suggest, on the basis of our observations, that reduction of Cdx2 levels may lead to increased RTK activity and, via phosphatidylinositol 3-kinase, elevation of mTOR kinase activity as well.
One limitation of this study was our inability to identify RTKs in the LoVo cells that performed a role similar to that of PDGFR and IGF-IR or to confirm caveolin-1 regulation by Cdx2 in these cells. One possibility is that PDGFR and IGF-IR are present in LoVo cells at low levels that we cannot detect by Western blotting, but even at these levels, they serve to regulate E-cadherin function and are, in turn, regulated by Cdx2. Alternatively, it is possible that the modest expression of the Cdx2 homolog Cdx1 contributed to our difficulties with the LoVo cells. Cdx1 can substitute for Cdx2 and promote E-cadherin function in the Colo 205 cells (11, 25). It may therefore be obscuring some of the effects from the LoVo Cdx2 knockout. However, even in LoVo cells, Cdx2 expression appears to modulate RTK activity. This is suggested by our observations that 1) the disruption of E-cadherin in LoVoCdx2−/− and Colo 205 cells was due to removal of E-cadherin and catenins from the cell membrane compartment and 2) LoVoCdx2−/− cells became adhesive when treated with Abl kinase inhibitors or a targeted reduction in c-Abl or CrkL by shRNA vectors. These findings support the conclusion that the LoVoCdx2−/− cells require RTK inputs through the c-Abl kinase pathway to disrupt E-cadherin function.
Cdx2 regulation of IGF-IR and other RTKs in the intestinal epithelium.
IGF-IR is expressed in normal intestinal epithelium, including crypts (2). It is conceivable then that Cdx2 expression regulates intestinal IGF-IR levels and activity. In addition, most studies suggest that Cdx2 levels are diminished (but not lost) in intestinal epithelial cells as they progress to cancer (19, 24, 32). Some of the increase in IGF-IR levels with progression to cancer might therefore be explained by the reduction in Cdx2 levels. These lower Cdx2 levels may still be sufficient to repress the IGF-binding protein-3 promoter (7), further stimulating IGF-IR activity.
Of great interest was the role of caveolin-1. Caveolae are important modulators of a variety of cell surface receptors (30, 36, 38, 47): they are required for signaling in some instances and silencing in others. Cellular and tissue context likely plays a role in these divergent effects. It is clear, however, that they are important regulators of RTK activity. We therefore suspect broader effects by Cdx2 on RTK activities that may impact other aspects of intestinal and colon cancer cell biology, from metabolism and cell proliferation to cell migration and adhesion. From a therapeutic perspective, targeting c-Abl kinase and RTKs in poorly differentiated colon cancers with little Cdx2 expression may significantly impede tumor metastasis and EMT by enhancing E-cadherin function.
Several questions remain unanswered from our work. The role of the Cdx2 homolog Cdx1 in these processes remains unstudied. In our previous studies, restoration of Cdx1 expression in Colo 205 cells yielded a phenotype similar to that for Cdx2 (11, 25). We speculate that, in Colo 205 cells, restoration of Cdx1 (in the absence of Cdx2) would similarly induce caveolin-1 levels, which would, in turn, modulate PDGFR and IGF-IR activity and PDGFR and E-cadherin membrane localization. We have preliminarily demonstrated induction of caveolin-1 protein by Cdx1 expression in Colo 205 cells (data not shown) and are examining these Colo-MIGR-Cdx1 cells for changes in PDGFR and IGF-IR as well. Lastly, the regulation of caveolin-1 gene expression by Cdx1 and Cdx2 may have important implications for intestinal epithelial cell biology. Sequence analysis of the human and murine caveolin-1 promoters reveals that they contain canonical Cdx2 binding sites (data not shown).
In summary, we establish a novel mechanism by which the intestine-specific transcription factor Cdx2 regulates E-cadherin function. Our data suggest that Cdx2 expression can reduce RTK activity by modulating caveolin-1 levels. This leads to increased trafficking of E-cadherin and p120- and β-catenin to the cell membrane. This mechanism has broad implications for Cdx2's role in promoting columnar morphogenesis and preventing EMT in colorectal carcinogenesis. It also predicts that Cdx2 can influence intestinal cell metabolism, motility, and survival on the basis of this ability to modulate RTK function.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-068366 (J. P. Lynch) and also by National Cancer Institute Program Project P01 Grant DE-12467 and the Morphology, Cell Culture, and Molecular Biology Core Facilities of the Center for Molecular Studies in Digestive and Liver Disease at the University of Pennsylvania (P30-DK-50306).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of S. Funakoshi: Division of Gastroenterology, Keio University School of Medicine, Tokyo, Japan.
REFERENCES
- 1.Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell 12: 847–862, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison AS, McIntyre MA, McArdle C, Habib FK. The insulin-like growth factor type 1 receptor and colorectal neoplasia: insights into invasion. Hum Pathol 38: 1590–1602, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Δ716Cdx2+/− compound mutant mice. Nat Genet 35: 323–330, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Aono S, Nakagawa S, Reynolds AB, Takeichi M. p120ctn acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J Cell Biol 145: 551–562, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonhomme C, Duluc I, Martin E, Chawengsaksophak K, Chenard MP, Kedinger M, Beck F, Freund JN, Domon-Dell C. The Cdx2 homeobox gene has a tumour suppressor function in the distal colon in addition to a homeotic role during gut development. Gut 52: 1465–1471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun SY, Chen F, Washburn JG, Macdonald JW, Innes KL, Zhao R, Cruz-Correa MR, Dang LH, Dang DT. CDX2 promotes anchorage-independent growth by transcriptional repression of IGFBP-3. Oncogene 26: 4725–4729, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Dang DT, Mahatan CS, Dang LH, Agboola IA, Yang VW. Expression of the gut-enriched Kruppel-like factor (Kruppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2. Oncogene 20: 4884–4890, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang LH, Chen F, Ying C, Chun SY, Knock SA, Appelman HD, Dang DT. CDX2 has tumorigenic potential in the human colon cancer cell lines LoVo and SW48. Oncogene 25: 2264–2272, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Elston MS, Gill AJ, Conaglen JV, Clarkson A, Cook RJ, Little NS, Robinson BG, Clifton-Bligh RJ, McDonald KL. Nuclear accumulation of E-cadherin correlates with loss of cytoplasmic membrane staining and invasion in pituitary adenomas. J Clin Endocrinol Metab 94: 1436–1442, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Ezaki T, Guo RJ, Li H, Reynolds AB, Lynch JP. The homeodomain transcription factors Cdx1 and Cdx2 induce E-cadherin adhesion activity by reducing β- and p120-catenin tyrosine phosphorylation. Am J Physiol Gastrointest Liver Physiol 293: G54–G65, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Funakoshi S, Ezaki T, Kong J, Guo RJ, Lynch JP. Repression of the desmocollin 2 gene in colorectal cancer cells is relieved by the homeodomain transcription factors Cdx1 and Cdx2. Mol Cancer Res 6: 1478–1490, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell 16: 588–599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golas JM, Lucas J, Etienne C, Golas J, Discafani C, Sridharan L, Boghaert E, Arndt K, Ye F, Boschelli DH, Li F, Titsch C, Huselton C, Chaudhary I, Boschelli F. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res 65: 5358–5364, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gross I, Duluc I, Benameur T, Calon A, Martin E, Brabletz T, Kedinger M, Domon-Dell C, Freund JN. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene 27: 107–115, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Gross I, Duluc I, Benameur T, Calon A, Martin E, Brabletz T, Kedinger M, Domon-Dell C, Freund JN. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene 27: 107–115, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Guo R, Funakoshi S, Lee HH, Kong J, Lynch JP. The intestine-specific transcription factor Cdx2 inhibits β-catenin/TCF transcriptional activity by disrupting the β-catenin/TCF protein complex. Carcinogenesis 31: 159–166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo RJ, Huang E, Ezaki T, Patel N, Sinclair K, Wu J, Klein PS, Suh E, Lynch JP. Cdx1 inhibits human colon cancer cell proliferation by reducing β-catenin/TCF transcriptional activity. J Biol Chem 279: 36865–36875, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther 3: 593–601, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Hage B, Meinel K, Baum I, Giehl K, Menke A. Rac1 activation inhibits E-cadherin-mediated adherens junctions via binding to IQGAP1 in pancreatic carcinoma cells. Cell Commun Signal 7: 23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans 33: 891–895, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Hecht A, Torbey CF, Korsmo HA, Olsen WA. Regulation of sucrase and lactase in developing rats: role of nuclear factors that bind to two gene regulatory elements. Gastroenterology 112: 803–812, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Hinoi T, Lucas PC, Kuick R, Hanash S, Cho KR, Fearon ER. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology 123: 1565–1577, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Hinoi T, Tani M, Lucas PC, Caca K, Dunn RL, Macri E, Loda M, Appelman HD, Cho KR, Fearon ER. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol 159: 2239–2248, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller MS, Ezaki T, Guo RJ, Lynch JP. Cdx1 or Cdx2 expression activates E-cadherin-mediated cell-cell adhesion and compaction in human Colo 205 cells. Am J Physiol Gastrointest Liver Physiol 287: G104–G114, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kwon T, Kwon DY, Chun J, Kim JH, Kang SS. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J Biol Chem 275: 423–428, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol 17: 459–465, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lorentz O, Duluc I, Arcangelis AD, Simon-Assmann P, Kedinger M, Freund JN. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J Cell Biol 139: 1553–1565, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozano E, Frasa MA, Smolarczyk K, Knaus UG, Braga VM. PAK is required for the disruption of E-cadherin adhesion by the small GTPase Rac. J Cell Sci 121: 933–938, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell 4: 499–515, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Lynch J, Keller M, Guo R, Yang D, Traber PG. Cdx1 inhibits the proliferation of human colon cancer cells by reducing cyclin D1 gene expression. Oncogene 22: 6395–6407, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Mallo GV, Rechreche H, Frigerio JM, Rocha D, Zweibaum A, Lacasa M, Jordan BR, Dusetti NJ, Dagorn JC, Iovanna JL. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int J Cancer 74: 35–44, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke JD. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun 314: 1001–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto T, Yokote K, Take A, Takemoto M, Asaumi S, Hashimoto Y, Matsuda M, Saito Y, Mori S. Differential interaction of CrkII adaptor protein with platelet-derived growth factor α- and β-receptors is determined by its internal tyrosine phosphorylation. Biochem Biophys Res Commun 270: 28–33, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Mauro L, Surmacz E. IGF-I receptor, cell-cell adhesion, tumour development and progression. J Mol Histol 35: 247–253, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Miotti S, Tomassetti A, Facetti I, Sanna E, Berno V, Canevari S. Simultaneous expression of caveolin-1 and E-cadherin in ovarian carcinoma cells stabilizes adherens junctions through inhibition of Src-related kinases. Am J Pathol 167: 1411–1427, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 36: 149–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orlichenko L, Weller SG, Cao H, Krueger EW, Awoniyi M, Beznoussenko G, Buccione R, McNiven MA. Caveolae mediate growth factor-induced disassembly of adherens junctions to support tumor cell dissociation. Mol Cell 20: 4140–4152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC. Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1α. J Biol Chem 277: 21361–21370, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Salahshor S, Naidoo R, Serra S, Shih W, Tsao MS, Chetty R, Woodgett JR. Frequent accumulation of nuclear E-cadherin and alterations in the Wnt signaling pathway in esophageal squamous cell carcinomas. Mod Pathol 21: 271–281, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Silberg DG, Furth EE, Taylor JK, Schuck T, Chiou T, Traber PG. CDX1 protein expression in normal, metaplastic, and neoplastic human alimentary tract epithelium. Gastroenterology 113: 478–486, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Sirvent A, Benistant C, Roche S. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol Cell 100: 617–631, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Soubeyran P, Andre F, Lissitzky JC, Mallo GV, Moucadel V, Roccabianca M, Rechreche H, Marvaldi J, Dikic I, Dagorn JC, Iovanna JL. Cdx1 promotes differentiation in a rat intestinal epithelial cell line. Gastroenterology 117: 1326–1338, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Suh E, Chen L, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol 14: 7340–7351, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uesaka T, Lu H, Katoh O, Watanabe H. Heparin-binding EGF-like growth factor gene transcription regulated by Cdx2 in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 283: G840–G847, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Wahl JK, Kim YJ, 3rd, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem 278: 17269–17276, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto M, Toya Y, Jensen RA, Ishikawa Y. Caveolin is an inhibitor of platelet-derived growth factor receptor signaling. Exp Cell Res 247: 380–388, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Zandy NL, Pendergast AM. Abl tyrosine kinases modulate cadherin-dependent adhesion upstream and downstream of Rho family GTPases. Cell Cycle 7: 444–448, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci USA 104: 17686–17691, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]