Abstract
Nonalcoholic fatty liver (NAFL) is a common liver disease, associated with insulin resistance. Betaine has been tested as a treatment for NAFL in animal models and in small clinical trials, with mixed results. The present study aims to determine whether betaine treatment would prevent or treat NAFL in mice and to understand how betaine reverses hepatic insulin resistance. Male mice were fed a moderate high-fat diet (mHF) containing 20% of calories from fat for 7 (mHF) or 8 (mHF8) mo without betaine, with betaine (mHFB), or with betaine for the last 6 wk (mHF8B). Control mice were fed standard chow containing 9% of calories from fat for 7 mo (SF) or 8 mo (SF8). HepG2 cells were made insulin resistant and then studied with or without betaine. mHF mice had higher body weight, fasting glucose, insulin, and triglycerides and greater hepatic fat than SF mice. Betaine reduced fasting glucose, insulin, triglycerides, and hepatic fat. In the mHF8B group, betaine treatment significantly improved insulin resistance and hepatic steatosis. Hepatic betaine content significantly decreased in mHF and increased significantly in mHFB. Betaine treatment reversed the inhibition of hepatic insulin signaling in mHF and in insulin-resistant HepG2 cells, including normalization of insulin receptor substrate 1 (IRS1) phosphorylation and of downstream signaling pathways for gluconeogenesis and glycogen synthesis. Betaine treatment prevents and treats fatty liver in a moderate high-dietary-fat model of NAFL in mice. Betaine also reverses hepatic insulin resistance in part by increasing the activation of IRS1, with resultant improvement in downstream signaling pathways.
Keywords: high fat, hepatic steatosis, insulin receptor substrate, triglyceride
nonalcoholic fatty liver(NAFL) is defined as the presence of increased fat in the liver that is not caused by alcohol consumption. Most commonly, NAFL is associated with obesity (4, 43, 53) and insulin resistance/diabetes (45). NAFL is present in ∼17–33% of Americans and is associated with liver cirrhosis (17) and an increased risk of atherosclerosis (8) and hepatocellular carcinoma (3, 24). The pathogenesis of hepatic fat accumulation in NAFL is incompletely understood. Proposed mechanisms include increased fat consumption, persistent lipolysis in adipocytes (increased delivery of fat to liver), increased de novo lipogenesis in the liver, and decreased export of very low-density lipoprotein (VLDL) from hepatocytes (14–16, 46).
There is no effective or generally accepted treatment for NAFL. Betaine is a naturally occurring dietary compound that is also synthesized in vivo from choline. In vivo, betaine acts as a methyl donor for the conversion of homocysteine to methionine and it also functions as an osmolyte. Oral betaine treatment has been evaluated in the treatment of alcoholic liver disease (5–6, 28, 32). In animal models of NAFLD, betaine treatment improved insulin and glucose levels (48), whereas betaine administration to humans with nonalcoholic steatohepatitis decreased indexes of steatosis (1). The mechanisms by which betaine improved hepatic steatosis are incompletely understood. Betaine's effect on remethylation of homocysteine (28), oxidative stress and transsulfuration reactions (33–34), activation of AMP-activated protein kinase (AMPK) (48), and restoring phosphatidylcholine (PC) generation (31) have been studied in animal models of fatty liver. Recently, Wang et al. (52) reported that betaine treatment of a high-fat mouse model improved adipocyte insulin signaling. However, the effects of betaine on hepatic insulin signaling, insulin-stimulated gluconeogenesis, glycogen synthesis, and lipogenesis in the animal models of NAFL have not been reported.
In the present study, we produced NAFL with insulin resistance in male mice by feeding a nutritionally complete diet containing 20% calories from fat for 7 mo as described in our previous study (40). We sought to determine whether betaine, when given orally with a high-fat diet, would prevent NAFL and whether when given after NAFL had developed it would reverse NAFL. We also sought to understand where in the insulin signaling pathway betaine would act to reverse hepatic insulin resistance in NAFL.
METHODS
All the chemicals were from Sigma Chemical (St. Louis, MO) unless otherwise noted.
Animal Experiment
The study was approved by the Animal Use Committee/IACUC at the VA Long Beach Healthcare System prior to the start and annually thereafter, and all applicable institutional and governmental regulations concerning the ethical use of animals were followed. One-month-old male mice, 75% Balb/c and 25% B6D2F2, from our breeding colony were used (39). Six groups of male mice, 10 in each group were studied: groups 1-3 were fed for 7 mo whereas groups 4-6 were fed for 8 mo. The first group [standard fat (SF)] was fed standard rodent chow (Teklad 8604) which contains 9% of calories from fat, 33% from protein, and 53% from carbohydrate. The second group [moderately high fat (mHF)] was fed rodent chow for pregnant and lactating mice (Teklad 8626), which is a nutritionally complete diet containing ∼20% of calories from fat, 23% from protein, and 50% from carbohydrate. The third group was fed Teklad 8626 and had 1.5% betaine hydrochloride (wt/vol) added to the drinking water (mHFB). The fourth group was fed Teklad 8604 for 8 mo (SF8). The fifth group was fed Teklad 8626 for 8 mo (mHF8). The sixth group was fed Teklad 8626 for 8 mo, with 1.5% betaine added to the drinking water for the final 6 wk (mHF8B). All diets contained fat from both animal and vegetable sources and contained ∼0.01% cholesterol, and fructose was not added. Gross energy of Teklad 8604 is 3.94 and Teklad 8626 is 4.33 kcal/g. Compared with other high-fat mouse models for NAFL this diet contains moderate fat content. All mice were allowed free access to food and water. Mice were killed between 8 and 10 AM with or without overnight fasting. Blood obtained from the heart at euthanasia was used for biochemical tests. Liver and visceral fat were removed and weighed, samples were placed in formalin for histological examination, and the remainder were snap frozen and stored at −80°C.
Histology
Coded hematoxylin and eosin-stained liver sections were scored for inflammation, necrosis and fat by an experienced hepatopathologist (S. W. French) in a blinded manner according to the previously described scoring system (40).
Insulin and Glucose Tolerance Test
Two weeks before euthanasia, mice were fasted overnight and blood was drawn from the saphenous vein for assessments of fasting plasma insulin (Ultra Sensitive Rat Insulin ELISA kit, Crystal Chem, Downers Grove, IL) and glucose (AccuCheck Glucometer, Roche Diagnostics). One week before euthanasia, a glucose tolerance test was performed on fasted mice by intraperitoneal injection of 1.5 mg glucose/g body wt followed by measurement of blood glucose (tail) at every 15 min for first hour and every 30 min for the second hour (AccuCheck Glucometer, Roche Diagnostics).
Measurement of Hepatic Triglyceride
Liver triglycerides were estimated as described previously (30). Briefly, liver tissue (0.03–0.05 g) was homogenized in 0.8 ml of phosphate buffer (50 mM sodium phosphate, 20 mM EDTA, and 20 mM NaCl, pH 7.4). The homogenate was extracted with 2.4 ml of n-butanol and di-isopropyl ether (40:60) for 1 h at room temperature and centrifuged for 2 min at 2,000 rpm at room temperature. The organic layer was removed and triglycerides were measured in an automated analyzer (Hitachi Instruments, San Jose, CA).
Serum Triglyceride, Cholesterol, and ALT Measurement
Serum was separated from the blood drawn from heart by centrifugation at 10,000 rpm for 10 min. Serum was diluted with normal saline and analyzed for triglyceride, cholesterol, and alanine transaminase (ALT) by an automated analyzer (Hitachi).
Cell Culture Experiments
HepG2 cells were maintained in normal glucose (5.5 mM) Dulbecco's modified Eagle's medium (DMEM) medium with 10% fetal bovine serum (FBS) and antibiotics. FBS-free DMEM medium was adjusted to 20 mM betaine, pH 7.3, and then filtered before use. For high-glucose media, FBS-free DMEM medium was adjusted to 54.5 mM glucose with d-glucose and filtered before use. To get desired concentrations of betaine and glucose, the stock DMEM medium containing 20 mM betaine and 54.5 mM glucose or 5.5 mM glucose were mixed appropriately. Cells were exposed to the test media for 24 h, followed by insulin induction (10 nM, 10 min). For phosphoinositol 3-kinase (PI3K) inhibition studies, prior to insulin induction, cells were exposed to 5 nM wortmannin for 1 h to inhibit PI3K.
Protein Extraction, SDS-PAGE, and Western Blotting
Protein extraction from mouse liver.
Liver protein was extracted by homogenizing 50 mg of frozen tissue in 0.5 ml of cell lysis buffer (Cell Signaling, Beverly, MA) with protease and phosphatase inhibitors (Roche Diagnostics, Indianapolis, IN). The homogenate was centrifuged at 13,500 rpm for 15 min at 4°C. The supernatant was collected, total protein was measured by Bio-Rad Dc protein assay (Bio-Rad Laboratories, Hercules, CA) and the protein concentration was adjusted to 5 mg/ml with the lysis buffer. Insulin receptor substrate 1 (IRS1) protein was immunoprecipitated as described previously (30).
Protein extraction from HepG2 cells.
The cells were washed three times with cold PBS and collected in PBS, centrifuged at 10,000 rpm for 10 min at 4°C. The pellet was dissolved in 1× lysis buffer (Cell Signaling) containing protease and phosphatase inhibitors (Roche). Cells in lysis buffer were sonicated briefly and centrifuged at 10,000 rpm for 10 min at 4°C. The total protein content was measured by Bio-Rad Dc protein assay and the concentration was adjusted to 2 mg/ml.
SDS-PAGE and Western blotting.
SDS-PAGE was performed by denaturing proteins at 95°C for 3 min in Laemmli sample buffer containing 62.5 mM Tris, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, and 5% β-mercaptoethanol. The proteins were fractionated in 10% SDS-PAGE at 100 V for 2–5 h and were transferred to nitrocellulose membrane in transfer buffer (25 mM Tris, pH 8.3, 192 mM glycine, 20% methanol) at 35 V overnight at 4°C. Following transfer, the membranes were blocked with 5% bovine serum albumin (BSA) in TBST (0.05 M Tris pH 7.6, 0.9% NaCl, 0.1% Tween-20) for 1 h. Primary antibodies for insulin receptor substrate-1 (IRS1), phosphotyrosine-IRS1, protein kinase B (PKB/Akt), phospho-PKB/Akt, glycogen synthase kinase 3α (GSK3α), phospho-GSK3α, forkhead box O1 (FoxO1α), phospho-FoxO1α, AMPK, phospho-AMPK, acetyl CoA carboxylase (ACC), phospho-ACC, and β-actin (Cell Signaling) were used at 1:1,000–5,000 dilutions in TBST containing 5% BSA overnight at 4°C. Membranes were exposed to secondary antibodies conjugated with horseradish peroxidase (Calbiochem, San Diego, CA) at a dilution of 1:5,000 in 5% BSA in TBST for 1 h at room temperature. Signals were detected with ECL Detection Kit (GE Healthcare, Buckinghamshire, UK) and exposed to Blue Basic Autorad film (Bioexpress, Kaysville, UT). The relative density of the bands of the Western blots was measured by using UN-SCAN-IT software (version 6.1).
Measurement of Hepatic Betaine, Dimethylglycine, and Choline
The extraction of choline, betaine, and dimethylglycine from mouse liver was carried out as described by Holm et al. (22) with modifications based on our instrumentation. Briefly, pulverized frozen (−80°C) mouse liver (20 mg) was combined with acetonitrile (185 μl) containing 0.1% formic acid to precipitate the proteins. This was followed by the addition of a mixture of deuterium-labeled internal standards (10 μl) containing 0.2 mM each of d9-choline, d9-betaine, and d6-dimethylglycine. After vortexing, a pellet mixer (VWR International, West Chester, PA) was used to disrupt the tissues and the samples were centrifuged (10,600 g for 15 min at 4°C). The supernatant (150 μl) was then transferred to a vial from which 10 μl was injected into a LC-MS/MS system consisting of a TSQ Quantum mass spectrometer (Thermo, San Jose, CA) equipped with a refrigerated Accela autosampler (Thermo) and an Accela pump with degasser (Thermo). Metabolites were separated by HPLC using a Prevail silica column (150 × 2.1 mm, 5 μm; Grace, Deerfield, IL) with matching guard column (4.6 × 25 mm, 5 μm). The mobile phase was run under isocratic conditions (500 μl/min) and contained acetonitrile (81%) and ammonium formate (15 mM) with 0.1% formic acid. The mass spectrometer was operated in positive ion electrospray mode. The metabolites of interest were detected in a multiple reaction monitoring mode of the tandem mass spectrometer with the following transitions: betaine, m/z 118/59; d9-betaine, m/z 127/59; choline, m/z 104/60; d9-choline, m/z 113/69; dimethylglycine, m/z 104/58; d6-dimethylglycine, m/z 110/64. Quantification of choline compounds was performed by comparing samples with the signals obtained from the choline compound standards using Xcalibur software (Thermo). Quality assurance was monitored through the use of duplicate sampling and in-house control materials.
Measurement of Hepatic Glycogen
Liver glycogen was estimated as described previously (30). Briefly, liver (0.05 g) was homogenized in 5% trichloroacetic acid (TCA) (1 ml) and centrifuged at 3,000 rpm for 10 min at room temperature. Resulting supernatant (100 μl) was mixed with an equal volume of 10 N KOH and placed in boiling water for 1 h. The mixture was cooled to room temperature and mixed with glacial acetic acid (50 μl) and H2O (750 μl). This mixture was placed on ice and 100 μl of this was added drop by drop to 200 μl of ice cold anthrone reagent and left on ice for 5 min. The tube was placed in a boiling water bath for 10 min and then cooled to room temperature. A blank was prepared by replacing the liver sample with 100 μl of 5% TCA. The absorbance was read at 650 nm. Glucose standard (0.125, 0.25, 0.5, 1, and 2 mg/ml) was used to calculate the amount of glucose in liver samples.
Measurement of Total Plasma Homocysteine
Total plasma homocysteine (tHcy; free plus protein bound) was assayed by HPLC with fluorescence detection according to Ubbink et al. (50).
Measurement of Hepatic S-Adenosylemethionine and S-Adenosylhomocystine
Fresh liver (∼0.05 g) was homogenized with 10 volumes of perchloric acid (0.4 M). Homogenates were centrifuged and the clear perchloric acid extract stored at −20°C until analysis. The S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) measurements were determined by HPLC coupled to UV detection as previously reported (7).
Statistical Analysis
Unpaired Student's t-test was used to analyze the data in consultation with a statistician at the Southern California Institute for Research and Education. SigmaStat for Windows version 2.03 statistical software was used.
RESULTS
Liver Betaine, Choline, and Dimethylglycine Concentrations
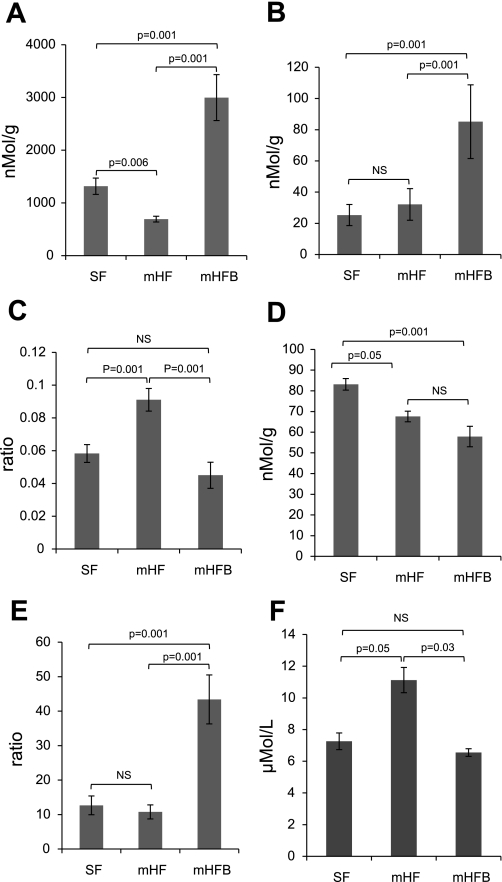
Betaine concentration in livers decreased ∼50% in mice fed mHF diet (P < 0.01 vs. SF). The concentration of betaine in the liver of mHFB was approximately five times higher than that in mHF and three times as high as in SF (P < 0.001 vs. mHF and vs. SF) (Fig. 1A). Dimethylglycine (DMG) is produced when betaine donates a methyl group. The concentration of DMG was similar in SF and mHF mice but increased approximately four times in mHFB mice (Fig. 1B). The DMG-to-betaine ratio was similar in SF and mHFB but was significantly increased in mHF (Fig. 1C). Choline is the precursor of betaine in the biosynthetic pathway of betaine synthesis. The choline concentration in the liver was decreased in mHF and mHFB compared with SF (Fig. 1D). The ratio of betaine to choline was similar in SF and mHF although greater in mHFB (Fig. 1E).
Fig. 1.
Concentrations of betaine (A), dimethylglycine (DMG; B), DMG-to-betaine ratio (C), choline (D), betaine-to-choline ratio in liver (E), and fasting total plasma homocysteine (tHcy) (F) of animals fed standard-fat diet (SF), moderately high-fat diet (mHF), and mHF with betaine (mHFB). The liver concentrations of betaine, DMG, and choline and plasma concentration of tHcy were measured by HPLC methods described in methods. Values are means ± SE of n = 10 per group (n = 5 per group for tHcy). NS, not significant.
Plasma tHcy, Liver SAM, and SAH Levels
In the methionine cycle, the enzyme betaine homocysteine methyl transferase (BHMT) uses a methyl group from betaine to convert homocysteine to methionine. The fasting plasma tHcy was 7.3 μmol/l in SF. Fasting plasma tHcy level was significantly higher in mHF animals (P < 0.05) and was normalized in mHFB group (Fig. 1F). Hepatic SAM level was increased in mHFB (P = 0.03). Hepatic SAH level was not significantly different between mHF and mHFB (P = 0.3) (Table 1). Because of technical problems, hepatic SAM and SAH levels were not available in SF mice.
Table 1.
Physical and biochemical characteristics of SF, mHF, and mHFB
| SF | mHF | mHFB | |
|---|---|---|---|
| Body wt, g | 27.7 ± 0.4ab | 33.5 ± 1.4a | 32.1 ± 0.5b |
| Liver wt, g | 1.4 ± 0.03ab | 2.0 ± 0.1a | 2.1 ± 0.08b |
| Liver wt/body wt, % | 4.92 ± 0.14ab | 6.29 ± 0.18a | 6.56 ± 0.26b |
| Visceral fat wt, g | 0.30 ± 0.03ab | 1.40 ± 0.1ac | 0.70 ± 0.08bc |
| ALT, IU/ml | 43.3 ± 5.4a | 54.5 ± 3.5ac | 44.5 ± 2.0c |
| Total pathology score | 0.8 ± 0.4a | 3.5 ± 0.6ac | 0.7 ± 0.4c |
| Fasting serum insulin, mg/dld | 0.24 ± 0.02ab | 0.62 ± 0.1ac | 0.33 ± 0.02bc |
| Fasting serum glucose, mg/dld | 120.6 ± 4.1a | 158.8 ± 7.1bac | 129.2 ± 5.0c |
| Fasting serum triglyceride, mg/dle | 128 ± 12ab | 184 ± 18.9ac | 151 ± 9.5bc |
| Fasting serum cholesterol, mg/dle | 122 ± 19ab | 171.2 ± 14ac | 110 ± 16bc |
| Liver triglyceride, mg/g liver | 80.33 ± 18.2ab | 161.50 ± 16.2ac | 101.42 ± 11.3bc |
| Liver glycogen, % | 1.25 ± 0.5b | 1.29 ± 0.4c | 2.82 ± 0.5bc |
| Fasting liver SAM, nmol/g livere | 34.71 ± 4.6c | 49.44 ± 4.0c | |
| Fasting liver SAH, nmol/g livere | 65.0 ± 4.8 | 72.4 ± 6.6 |
Values are means ± SE; n = 10 per group.
Standard-fat diet (SF) vs. moderately high-fat diet (mHF);
SF vs. mHF with betaine added to drinking water (mHFB);
mHF vs. mHFB =P < 0.05;
fasting insulin and fasting glucose were measured 2 wk before euthanasia;
fasting triglyceride, cholesterol, S-adenosylmethionine (SAM), and S-adenosylhomocysteine (SAH) were measured at the end of the experiment (n = 5 per group). ALT, alanine aminotransferase.
Body Weight, Liver Weight, and Abdominal Fat Weight
Mice fed mHF weighed significantly more than SF (P < 0.01) but not different than mHFB (P > 0.3) (Table 1). Liver-to-body weight ratio was higher in mHF and mHFB (P < 0.05) compared with SF. Visceral fat weight was significantly higher in mHF compared with SF (P = 0.001). mHFB significantly decreased visceral fat weight compared with mHF (P < 0.05); there was no significant difference in visceral fat weight between mHFB and SF.
Liver Injury
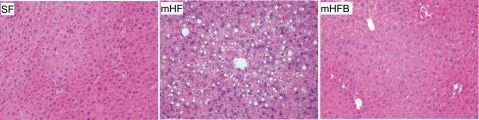
Serum ALT level was significantly higher in mHF compared with SF (P < 0.05); betaine treatment normalized ALT levels. On histological examination, macro- and microvesicular fat were increased in mHF compared with SF. Supplementation of betaine significantly reduced hepatic fat accumulation. Total pathology score (includes macro fat, micro fat, inflammation, and necrosis) showed greater liver injury in mHF than SF and mHFB (Table 1, Fig. 2).
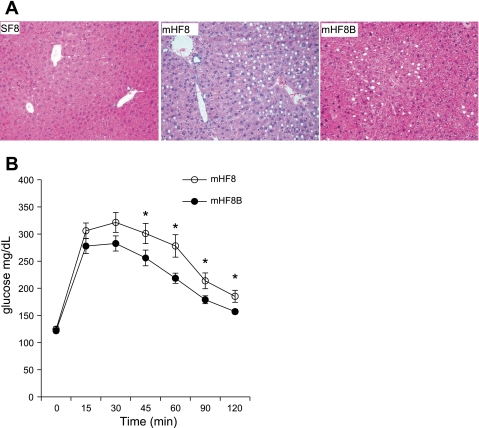
Fig. 2.
Hematoxylin and eosin (H&E)-stained liver sections of mice fed SF, mHF, and mHFB, showing macrovesicular and microvesicular fat accumulation. Histology of liver from SF shows normal liver; mHF liver shows 2+ macrovesicular fat and 2+ microvesicular fat predominately in the perivenular areas; and mHFB liver shows no macrovesicular or microvesicular fat accumulation. Original magnification ×20.
Plasma Glucose, Insulin, and Glucose Tolerance Test
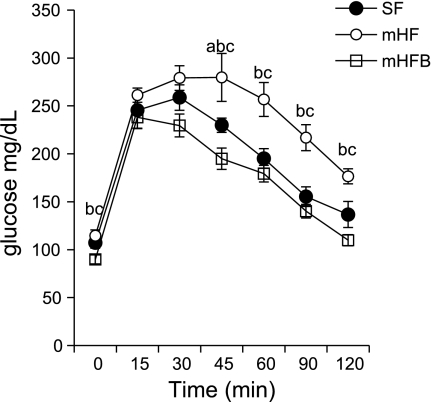
Fasting blood glucose (P = 0.04) and insulin (P = 0.001) levels were significantly higher in mHF compared with SF. Betaine treatment reduced fasting glucose to normal and reduced fasting insulin toward normal (P = 0.02 vs. mHF). During glucose tolerance testing, blood glucose level was significantly higher in mHF compared with mice fed SF or mice fed mHFB at all time points from 45 min through 2 h (Table 1, Fig. 3).
Fig. 3.
Glucose tolerance test. Animals were fasted overnight, and 0 time glucose was measured by tail snip. Mice were injected with 1.5 mg glucose/g body wt ip. Blood glucose was measured every 15 min for the 1st hour and every 30 min for the 2nd hour. Values are means ± SE of n = 10 per group. aSF vs. mHF; bSF vs. mHFB; cmHF vs. mHFB (P = <0.05).
Triglyceride and Cholesterol Levels
The liver triglyceride level increased approximately twofold in HF (P < 0.01 vs. SF); addition of betaine reduced liver triglyceride content toward normal (P < 0.05 vs. HF; P = not significant vs. SF) (Table 1). The fasting serum triglyceride level increased significantly in HF (P < 0.001 vs. SF) and decreased after betaine treatment (P < 0.02 HFB vs. HF) but remained higher than SF (P < 0.05). Serum cholesterol level increased in HF (P < 0.004 vs. SF) and returned to normal with betaine treatment (Table 1).
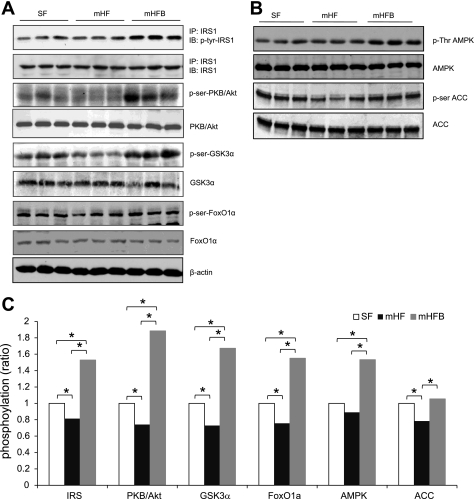
IRS1 and PKB/Akt Activation
By Western blot, the hepatic content of total IRS1 was similar in SF, mHF, and mHFB. The level of tyrosine-phosphorylated IRS1 in mHFB was significantly increased compared with mHF (Fig. 4, A and C). Hepatic total PKB/Akt content was similar in all three groups. Compared with SF, mice fed mHF had decreased levels of serine phosphorylated-PBK/Akt (activated) whereas mHFB had greater levels of phosphorylated PKB/Akt (Fig. 4, A and C).
Fig. 4.
Betaine reverses hepatic insulin resistance induced by mHF. Activation of insulin signal proteins in the livers of SF, mHF, and mHFB were determined by Western blotting. Protein isolation, immunoprecipitation (IP), and Western blot were done as described in methods. IB, immunoblot. A: activation of insulin receptor substrate 1 (IRS1) (p-tyrIRS1 vs. IRS1), PKB/Akt (p-ser308 PKB/Akt vs. PKB/Akt), GSK3α (p-ser GSK3 α vs. GSK3α) and FoxO1α (p-serFoxO1α vs. FoxO1α) were measured by Western blotting with specific antibodies. β-Actin was used for loading control. B: activation of AMPK (p-ser AMPK vs. AMPK) and inactivation of acetyl CoA carboxylase (ACC) (p-ser ACC vs. ACC) were measured by Western blotting. C: density of the bands were measured and ratio of phosphorylated to total was calculated and normalized with SF (*P < 0.05).
Gluconeogenesis
The nuclear factor FoxO1α, which is under control by insulin via PKB/Akt, regulates the expression of phosphoenolpyruvate carboxykinase (PEPCK), the rate-limiting enzyme in the formation of glucose from pyruvate (42). In conformity with phosphorylation of PKB/Akt, the level of phosphorylated FoxO1α was higher in mHFB compared with mHF (Fig. 4, A and C), whereas there was no difference in total FoxO1α between the three groups.
Glycogen Synthesis
PKB/Akt also regulates glycogen synthesis by phosphorylation (inactivation) of GSK3α, the rate-limiting enzyme in glycogen synthesis (12). Total GSK3α was similar in all groups. The level of serine-21 phosphorylated-GSK3α (inactive GSK3α) was increased in mHFB compared with mHF and SF (Fig. 4, A and C). Inactivation of GSK3α would be expected to increase glycogen synthesis. Consistent with this possibility, liver glycogen content was significantly increased in mHFB compared with mHF and SF (Table 1).
AMPK Activation and De Novo Fat Synthesis
AMPK phosphorylation (activation) suppresses fatty acid synthesis by inhibiting ACC, the rate-limiting enzyme in the fatty acid synthesis pathway. There was no significant difference in hepatic total AMPK in SF, mHF, and mHFB. The ratio of phosphorylated-AMPK to total AMPK was increased in mHFB compared with SF and mHF. In addition, the level of phosphorylated ACC was significantly higher in mHFB compared with SF and mHF, consistent with possible suppression of fatty acid synthesis by betaine in mice fed high-fat diet (Fig. 4, B and C).
Body Weight, Liver Weight, Visceral Fat Weight, and Liver Injury in Animals with 6 Wk Betaine Supplementation After the Development of NAFL
Since betaine administration in conjunction with a mHF diet prevented insulin resistance and NAFL, we hypothesized that betaine could reverse insulin resistance and liver steatosis in mice with established NAFL and insulin resistance. To test this hypothesis, animals were fed mHF for 8 mo to develop fatty liver and insulin resistance. Half of the mice received 1.5% betaine in drinking water for the last 6 wk (mHF8B) and the other half received water (mHF8) without betaine. A control group was maintained on SF for 8 mo (SF8). Mice in the mHF8 and mHF8B groups were significantly heavier and had increased liver-to-body weight ratio compared with mice in the SF group. Also, mHF8 had significantly higher ALT, fasting triglycerides, fasting cholesterol, visceral fat, and liver total pathology scores than SF8. Betaine supplementation for 6 wk did not reduce body weight or liver weight. However, betaine treatment significantly reduced ALT, liver triglyceride, fat accumulation in liver, and total pathology score as assessed by liver histology. Also, visceral fat weight decreased significantly with betaine treatment (Table 2, Fig. 5A).
Table 2.
Physical and biochemical characteristics of SF8, mHF8, and mHF8B
| SF8 | mHF8 | mHF8B | |
|---|---|---|---|
| Body wt, g | 28.47 ± 3.0ab | 33.41 ± 2.70a | 33.68 ± 3.4b |
| Liver wt, g | 0.93 ± 0.2ab | 1.44 ± 0.40a | 1.36 ± 0.22b |
| Liver wt/body wt, % | 3.2 ± 0.2ab | 4.3 ± 0.01a | 4.0 ± 0.003b |
| visceral fat wt, g | 0.38 ± 0.3a | 1.114 ± 0.30ac | 0.34 ± 0.23c |
| ALT, IU/ml | 37.23 ± 5.26a | 44.80 ± 4.44ac | 34.29 ± 6.97c |
| Pathology score | 0.83 ± 0.57ab | 3.75 ± 1.83ac | 2.50 ± 1.40bc |
| Glucose, mg/dld | 109.33 ± 10.61a | 123.81 ± 17.44ac | 94.17 ± 11.27bc |
| Fasting serum triglyceride, mg/dle | 113.73 ± 43.63a | 196.75 ± 77.73ab | 114.33 ± 47.16b |
| Fasting serum cholesterol, mg/dle | 74.00 ± 15.79a | 127.5 ± 14.57ab | 73.33 ± 30.66b |
| Liver triglycerides, mg/g liver | 76 ± 21.42a | 163.25 ± 25.3ab | 120.67 ± 17.9ac |
Values are means ± SE; n = 10 per group.
8-mo SF (SF8) vs. 8-mo mHF (mHF8);
SF8 vs. 8-mo mHF with betaine added to drinking water for the final 6 wk (mHF8B);
mHF8 vs. mHF8B (P < 0.05);
Fasting glucose was measured 2 wk before euthanasia;
fasting triglyceride and cholesterol were measured at the end of the experiment (n = 5 per group).
Fig. 5.
A: H&E-stained liver sections of mice fed 8-mo SF (SF8), 8-mo mHF (mHF8), and 8-mo mHF with betaine in drinking water for the final 6 wk (mHF8B) showing macrovesicular and microvesicular fat accumulation. Histology of liver from SF shows normal liver; mHF8 liver shows 2+ macrovesicular fat and 2+ microvesicular fat predominately in the perivenular areas; and mHF8B liver shows significantly lower macrovesicular or microvesicular fat accumulation. Original magnification ×20. B: glucose tolerance test. Animals were fasted overnight and 0 time glucose was measured by tail snip. Mice were injected with 1.5 mg glucose/g body wt ip. Blood glucose was measured every 15 min for the 1st hour and every 30 min for the 2nd hour. Values are means ± SE of n = 10 per group. *P < 0.05.
Plasma Glucose and Insulin Resistance in NAFL Animals with 6 Wk Betaine Supplementation
Fasting blood glucose level was significantly reduced with 6 wk betaine treatment (Table 2). Glucose tolerance test on mHF8 and mHF8B animals showed significantly lower blood glucose levels at 45, 60, and 120 min after glucose injection (Fig. 5B) in mice that received betaine.
Betaine Alleviates Insulin Resistance In Vitro
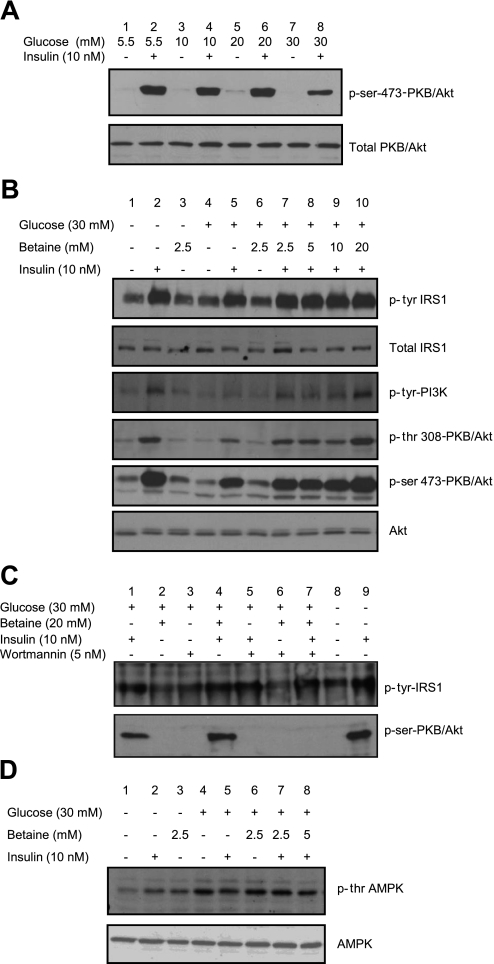
We further evaluated the effect of betaine on insulin-related biochemical pathways in Hep G2 cells made insulin resistant by exposure to 30 mM glucose for 24 h. When insulin-resistant HepG2 cells were induced with 10 nM insulin for 10 min, the activation of PKB/Akt was decreased by 50% compared with HepG2 cells in 5.5 mM glucose (Fig. 6A, lane 8). Addition of betaine to insulin-resistant HepG2 cells restored activation of IRS1, PI3K, and PKB/Akt by 50–100% depending on the concentration of the betaine added (Fig. 6B, lanes 7-10). Inhibition of PI3K with wortmannin prevented the reversal of activation of PKB/Akt by betaine (Fig. 6C, lane 7), demonstrating that betaine's effect in reversal of insulin resistance is upstream of PI3K.
Fig. 6.
Activation of insulin signal proteins in HepG2 cells with betaine. A: HepG2 cells were exposed to different concentrations of glucose. Insulin resistance was measured by induction with 10 nM insulin for 10 min and determination of the activated PKB/Akt (p-Ser473PKB/Akt vs. -PKB/Akt) by Western blot. Cells exposed to 30 mM glucose showed ∼50% reduction in the activation of PKB/Akt (lane 8). B: betaine reverses insulin resistance in HepG2 cells. Control cells (exposed to normal glucose, lanes 1-3) or insulin-resistant cells (exposed to 30 mM glucose, lanes 4-10) with betaine (lanes 3, 6-10) or without betaine (lanes 1, 2, 4-5) were maintained for 24 h and induced with 10 nM insulin for 10 min (lanes 2, 5, 7-10). The activation of insulin signal proteins IRS1 (p-tyrIRS1 vs. IRS1), PI3K (p-tyrPI3K), and PKB/Akt (p-tyr308 and p-ser473 PKB/Akt vs. PKB/Akt) were measured by Western blot. C: activation of insulin signal by betaine is upstream of PKB/Akt. HepG2 cells were exposed to 30 mM glucose (lanes 1-7) or normal glucose (lanes 8-9) with betaine (lanes 2, 4, and 6-7) or without betaine (lanes 1, 3, 5, and 8-9) for 24 h. Cells were inhibited with wortmannin for 1 h (lanes 3 and 5-7) or not inhibited (lanes 1, 2, 4, and 8-9). Cells were either induced with insulin to activate insulin signal (lanes 1, 4-5, 7, and 9) or without induction (lanes 2-3, 6, and 8). Activation of IRS1 (p-tyrIRS1) was observed with insulin induction (lanes 1, 4-5, 7, and 9). Insulin-induced activation of PKB/Akt was absent when PI3K was inhibited (lane 5) and even in the presence of betaine (lane 7). D: AMPK is activated with addition of betaine. Cells were exposed to normal glucose (lanes 1-3) or 30 mM glucose (insulin resistant, lanes 4-10) with betaine (lanes 3, 6-10) or without betaine (lane 1, 2, 4, and 5) and maintained for 24 h and induced with 10 nM insulin for 10 min. The activation of AMPK (p-ser AMPK vs. AMPK) was measured by Western blotting. AMPK was activated when exposed to 30 mM glucose (lane 4) and suppressed with insulin induction (lane 5). The suppression was reversed by betaine concentrations of 2.5–5 mM (lanes 7-8).
Betaine Stimulated Phosphorylation of AMPK In Vitro
When HepG2 cells were grown in high-glucose media, phosphorylated AMPK was higher than it was in cells grown in normal-glucose media (Fig. 6D, lane 4). The addition of 10 nM insulin resulted in 50% decrease in activation of AMPK in insulin-resistant cells (Fig. 6D, lane 5). When betaine 2.5 and 5 mM was added to insulin-resistant HepG2 cells, the inhibition of AMPK phosphorylation by 10 nM insulin was suppressed (Fig. 6D, lanes 6-8), suggesting that betaine might reduce hepatic fat synthesis via AMPK induced inhibition of ACC and consequent reduction in de novo lipogenesis.
DISCUSSION
In the present study we investigated the ability of betaine to prevent and treat insulin resistance and hepatic fat accumulation in a moderate high-fat dietary model of NAFL in mice. We also investigated the effect of betaine on insulin signaling in an in vitro model of insulin-resistant HepG2 cells. The important findings were that betaine supplementation prevented insulin resistance and hepatic steatosis when given in conjunction with a moderate high-fat diet and reversed insulin resistance and steatosis when administered following the development of NAFL. Betaine also reversed insulin resistance in insulin-resistant HepG2 cells. Both in vivo and in vitro betaine increased activation of IRS1, normalizing the downstream pathways involved in gluconeogenesis and glycogen synthesis. We also found that hepatic betaine concentration decreased in NAFL and increased significantly with oral betaine supplementation.
The moderately high-fat dietary model used in our experiments mimics the pathophysiology of NAFL in humans, including consumption of a nutritionally complete diet and the development of obesity, insulin resistance, increased serum ALT, and histological hepatic steatosis. Administration of betaine with the mHF diet prevented many of the pathophysiological abnormalities in NAFL. Betaine treatment slightly reduced body weight but significantly reduced serum ALT and hepatic fat measured histologically and biochemically. Similar to our observation, Song and coworkers (48) reported decreased hepatic fat accumulation with betaine treatment in a high-sucrose model of NAFL. Betaine treatment also reduced the visceral fat mass. Overall, betaine administration in conjunction with a mHF diet prevented liver injury and hepatic fat accumulation whereas betaine treatment reduced steatosis when given to mice with NAFL that continued to consume a high-fat diet.
Insulin resistance being a major pathophysiological abnormality in NAFL, we examined the effect of betaine treatment on systemic insulin resistance. As reported previously (40), mice fed a mHF diet had elevated fasting serum insulin levels and abnormal glucose tolerance compared with mice fed the SF diet, consistent with systemic insulin resistance. Betaine prevented and reversed the increase in fasting serum insulin and glucose and the abnormal glucose tolerance in mice fed mHF diet. The improvement in systemic insulin resistance with betaine was accompanied by improvement in serum triglyceride and cholesterol levels.
We also examined the effect of betaine on activation of insulin signaling pathways in the liver and found that betaine increased tyrosine phosphorylation of IRS1, an early step in insulin signaling. Presumably, the increase in activation of IRS1 was the mechanism by which betaine normalized downstream pathways, including activation of PKB/Akt. PKB/Akt is critical in glucose homeostasis because it controls both gluconeogenesis and glycogen synthesis. PBK/Akt regulates gluconeogenesis by inactivation of the transcription factor FoxO1α, the major nuclear factor regulating expression of PEPCK, the rate-limiting enzyme in gluconeogenesis (42). PEPCK overexpression in the liver increases hepatic glucose production (44, 49, 51), whereas knockout of PEPCK reduces hepatic glucose production (47). Our study found that betaine administration led to inactivation of FoxO1α via PKB/Akt, suggesting a reduction in gluconeogenesis, which also correlated with improved glucose levels in mHFB mice. PKB/Akt also regulates glycogen synthesis by phosphorylation (inactivation) of GSK3α, the rate-limiting enzyme in glycogen synthesis (12). In parallel with the increase in PKB/Akt activation, inactivation of GSK3α was greater in mHFB suggesting increased glycogen synthesis. This possibility was supported by finding an increase in glycogen content in the liver of betaine treated mice. Our finding of reduced GSK3α activity and increased hepatic glycogen content are consistent with other studies reporting increased glycogen content when GSK3α is inhibited (10, 29, 38). Overall, decreased production of glucose by inhibition of gluconeogenesis and increased use of glucose in glycogen synthesis could contribute to improved serum glucose level with betaine administration despite of consumption of a moderate high-fat diet.
ACC and fatty acid synthase, the two rate-limiting enzymes in fatty acid synthesis, are regulated by AMPK, an enzyme whose activation is regulated by insulin. The hepatic level of phosphorylated (activated) AMPK was decreased and is consistent with the increase in de novo lipogenesis in NAFL (14–16). Betaine restored phosphorylation of AMPK toward normal. The increase in activated AMPK with betaine supplementation was associated with an increase in phosphorylated (inactivated) ACC, suggesting decreased hepatic fatty acid synthesis with betaine treatment. Our findings are similar to a prior report of betaine-induced increase in hepatic AMPK activation and suppressed ACC activation in the liver of mice with NAFL induced by dietary sucrose (48). Overall, our findings, and those of Song et al. (48), suggest that betaine may reduce the increased hepatic de novo lipogenesis that is present in NAFL.
To determine whether betaine acts directly to reduce insulin resistance, we studied the effect of betaine in an established in vitro insulin-resistant (hyperglycemic) HepG2 cell model. As expected, IRS1 phosphorylation and other downstream insulin-related pathways, including activation of PKB/Akt, were inhibited in the insulin-resistant HepG2 cells. Betaine increased activation of IRS1 in the insulin-resistant HepG2 cells, demonstrating that it acts at an early point in the insulin signaling cascade. Betaine treatment also increased phosphorylation of PKB/Akt. To confirm that activation of PKB/Akt was via the insulin-activated PI3K pathway, we inhibited PI3K with wortmannin (21) and found that phosphorylation of PKB/Akt was completely blocked. This suggests that betaine is an enhancer of IRS1 phosphorylation but not an independent activator of PKB/Akt. PKB/Akt activation requires phosphorylation on threonine 308 by PDK1 (2) and serine 473 by mTOR-RICTOR (23), both of which are regulated by insulin via PI3K activation. Using specific antibodies, we found that phosphorylation of both sites was decreased in insulin-resistant HepG2 cells and that betaine increased phosphorylation at both sites. Betaine also reversed the inhibition of AMPK phosphorylation. These in vitro findings suggest that betaine acts directly on hepatocytes to reverse insulin resistance starting with increased tyrosine phosphorylation of IRS1, acts through PI3K, and includes activation of PKB/Akt.
Betaine is an intracellular osmolyte involved in cell volume regulation and osmosignaling that also serves as a methyl donor for the conversion of homocysteine to methionine (11, 20, 36, 54). Betaine, being a long-term osmolyte, could influence macromolecular crowding in maintaining intracytoplasmic normal osmotic pressure (13, 19). Although we did not define the mechanism by which betaine improves IRS1 phosphorylation, each of these roles could potentially be important. Recently, protein N-arginine methyltransferase 1 (PRMT-1) mediated methylation of heterogenous nuclear ribonucleoprotein (hnRNPQ) has been implicated in insulin receptor trafficking and insulin signaling (25–27). PRMT-1 transfers a methyl group from SAM to hnRNPQ, leading to internalization and sustained activation of the insulin receptor. It is possible that betaine, via regeneration of methionine, the precursor of SAM, could increase the availability of methyl groups for PRMT-1. On the other hand, hyperosmolality, as would be present in hyperglycemia, has been shown to inhibit IRS1 phosphorylation and PI3K activation in 3T3L adipocytes (9, 18) as well as inhibit other insulin-mediated effects such as activation of mitogen-activated protein kinase phosphatase expression in rat hepatoma cells (37). Our finding that the concentration of betaine in the liver of mHFB is nearly five times higher than in the liver of mHF and three times as high as the level in SF suggests that it may be acting as an osmolyte as well.
A decrease in hepatic betaine in NAFL has not been reported previously. Although we did not explore the mechanism for decreased hepatic betaine content, betaine's participation in several biochemical pathways might explain this finding. Administration of a high-fat diet would increase the demand for PC, the predominant phospholipid in lipoprotein particles, including VLDL. Choline, the precursor of betaine, is required for the synthesis of PC via Kennedy pathway (41). An increased demand for PC would decrease the amount of choline available for betaine biosynthesis. Alternatively, PC can be synthesized by the phosphatidylethanolamine N-methyltransferase (PEMT) pathway where betaine can serve as a source of methyl groups for the SAM-dependent sequential methylation of phosphatidylethanolamine (PE) (31, 55). Noga and Vance (41) reported that the PEMT pathway, and not the Kennedy pathway, provided the additional PC in male mice fed a high-fat, high-cholesterol diet. Thus the diminished hepatic betaine concentration may arise either from decreased availability of the precursor molecule choline or from increased use of the methyl donor betaine. The diminished hepatic concentration of betaine in NAFL is most likely due to its increased use as a methyl donor, a view that is also supported by the increased ratio of dimethylglycine to betaine in mHF mice in the present study. DMG concentration was higher in mHFB, which showed increased use of betaine as methyl donor; however, the DMG-to-betaine ratio was lower than in mHF because of higher concentration of betaine in mHFB. Furthermore, the inability of betaine supplementation to increase hepatic choline level to normal in mHFB suggests that the low hepatic choline content in mHF may not be due to increased synthesis of betaine, but more likely due to use of choline in PC synthesis via Kennedy pathway. The use of betaine as methyl donor was also supported by normalization of plasma tHcy levels in mHFB mice. Song et al. (48) have also reported normalization of homocysteine levels in NAFL mice created by feeding a high-sucrose diet. Presumably, the mechanism for reduced tHcy level was through increased conversion to methionine through remethylation by the BHMT pathway. In our study betaine increased hepatic SAM level by ∼44% compared with ∼57% increase in betaine-fed mice on the high-sucrose diet model of NAFL (48) and ∼291% in a higher fat (70%) rat model of NAFL (35). The variation in hepatic SAM with betaine feeding between our data and that of others may relate to the differences in the diet, severity of liver steatosis, and other experimental variables. Similar to Song et al., we did not find a difference in hepatic SAH level with betaine feeding.
In conclusion, ad libitum feeding of a nutritionally complete, moderately high-fat diet to male mice produced insulin resistance, obesity, and histological NAFL. Mice with NAFL have decreased hepatic betaine content. Oral betaine supplementation prevented hepatic betaine deficiency and reversed insulin resistance and fatty liver without significantly changing body weight. Both in vivo and in vitro betaine reversed insulin resistance by increasing IRS1 phosphorylation as well as improving the downstream pathways related to gluconeogenesis and glycogen synthesis.
GRANTS
Financial support was provided by the Southern California Institute of Research and Education, Long Beach, CA and by the University of Southern California Research Center for Alcoholic Liver and Pancreatic Diseases Morphology Core grant NIH/NIAAA p50-011999-08. Additional financial assistance was provided by the Vicki and Joshi Krishna Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol 96: 2711–2717, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7: 261–269, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 346: 1221–1231, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 107: 1103–1109, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ. Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin Exp Res 17: 552–555, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Barak AJ, Beckenhauer HC, Tuma DJ. Betaine, ethanol, and the liver: a review. Alcohol 13: 395–398, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Bottiglieri T. Isocratic high performance liquid chromatographic analysis of S-adenosylmethionine and S-adenosylhomocysteine in animal tissues: the effect of exposure to nitrous oxide. Biomed Chromatogr 4: 239–241, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol 25: 1045–1050, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chen D, Fucini RV, Olson AL, Hemmings BA, Pessin JE. Osmotic shock inhibits insulin signaling by maintaining Akt/protein kinase B in an inactive dephosphorylated state. Mol Cell Biol 19: 4684–4694, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cline GW, Johnson K, Regittnig W, Perret P, Tozzo E, Xiao L, Damico C, Shulman GI. Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes 51: 2903–2910, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Craig SA. Betaine in human nutrition. Am J Clin Nutr 80: 539–549, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Del Monte U. Swelling of hepatocytes injured by oxidative stress suggests pathological changes related to macromolecular crowding. Med Hypotheses 64: 818–825, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab 282: E46–E51, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab 29: 478–485, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43: S99–S112, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Gual P, Gonzalez T, Gremeaux T, Barres R, Le Marchand-Brustel Y, Tanti JF. Hyperosmotic stress inhibits insulin receptor substrate-1 function by distinct mechanisms in 3T3–L1 adipocytes. J Biol Chem 278: 26550–26557, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Hall D, Minton AP. Macromolecular crowding: qualitative and semiquantitative successes, quantitative challenges. Biochim Biophys Acta 1649: 127–139, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Haussinger D. Osmosensing and osmosignaling in the liver. Wien Med Wochenschr 158: 549–552, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol 194: 243–256, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 49: 286–294, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hresko RC, Mueckler mTOR M. RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3–L1 adipocytes. J Biol Chem 280: 40406–40416, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Iannaccone R, Piacentini F, Murakami T, Paradis V, Belghiti J, Hori M, Kim T, Durand F, Wakasa K, Monden M, Nakamura H, Passariello R, Vilgrain V. Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: helical CT and MR imaging findings with clinical-pathologic comparison. Radiology 243: 422–430, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki H. Impaired PRMT1 activity in the liver and pancreas of type 2 diabetic Goto-Kakizaki rats. Life Sci 85: 161–166, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki H. Involvement of PRMT1 in hnRNPQ activation and internalization of insulin receptor. Biochem Biophys Res Commun 372: 314–319, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki H, Yada T. Protein arginine methylation regulates insulin signaling in L6 skeletal muscle cells. Biochem Biophys Res Commun 364: 1015–1021, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124: 1488–1499, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kaidanovich-Beilin O, Eldar-Finkelman H. Long-term treatment with novel glycogen synthase kinase-3 inhibitor improves glucose homeostasis in ob/ob mice: molecular characterization in liver and muscle. J Pharmacol Exp Ther 316: 17–24, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kathirvel E, Morgan K, French SW, Morgan TR. Overexpression of liver-specific cytochrome P4502E1 impairs hepatic insulin signaling in a transgenic mouse model of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 21: 973–983, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol 46: 314–321, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Kharbanda KK, Todero SL, Ward BW, Cannella JJ, 3rd, Tuma DJ. Betaine administration corrects ethanol-induced defective VLDL secretion. Mol Cell Biochem 327: 75–78, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Kim SK, Kim YC. Effects of betaine supplementation on hepatic metabolism of sulfur-containing amino acids in mice. J Hepatol 42: 907–913, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kim SK, Seo JM, Chae YR, Jung YS, Park JH, Kim YC. Alleviation of dimethylnitrosamine-induced liver injury and fibrosis by betaine supplementation in rats. Chem Biol Interact 177: 204–211, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Kwon do Y, Jung YS, Kim SJ, Park HK, Park JH, Kim YC. Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr 139: 63–68, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem 43: 732–744, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Lornejad-Schafer MR, Schafer C, Graf D, Haussinger D, Schliess F. Osmotic regulation of insulin-induced mitogen-activated protein kinase phosphatase (MKP-1) expression in H4IIE rat hepatoma cells. Biochem J 371: 609–619, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacAulay K, Hajduch E, Blair AS, Coghlan MP, Smith SA, Hundal HS. Use of lithium and SB-415286 to explore the role of glycogen synthase kinase-3 in the regulation of glucose transport and glycogen synthase. Eur J Biochem 270: 3829–3838, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology 36: 122–134, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Morgan K, Uyuni A, Nandgiri G, Mao L, Castaneda L, Kathirvel E, French SW, Morgan TR. Altered expression of transcription factors and genes regulating lipogenesis in liver and adipose tissue of mice with high fat diet-induced obesity and nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 20: 843–854, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Noga AA, Vance DE. Insights into the requirement of phosphatidylcholine synthesis for liver function in mice. J Lipid Res 44: 1998–2005, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 423: 550–555, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology 118: 1117–1123, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Rosella G, Zajac JD, Baker L, Kaczmarczyk SJ, Andrikopoulos S, Adams TE, Proietto J. Impaired glucose tolerance and increased weight gain in transgenic rats overexpressing a non-insulin-responsive phosphoenolpyruvate carboxykinase gene. Mol Endocrinol 9: 1396–1404, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 77: 43–50, 2003 [DOI] [PubMed] [Google Scholar]
- 47.She P, Burgess SC, Shiota M, Flakoll P, Donahue EP, Malloy CR, Sherry AD, Magnuson MA. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes 52: 1649–1654, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, McClain CJ. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am J Physiol Gastrointest Liver Physiol 293: G894–G902, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thorburn AW, Baldwin ME, Rosella G, Zajac JD, Fabris S, Song S, Proietto J. Features of syndrome X develop in transgenic rats expressing a non-insulin responsive phosphoenolpyruvate carboxykinase gene. Diabetologia 42: 419–426, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Ubbink JB, Hayward Vermaak WJ, Bissbort S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr 565: 441–446, 1991 [DOI] [PubMed] [Google Scholar]
- 51.Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA 91: 9151–9154, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z. Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 298: G634–G642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 12: 1106–1110, 1990 [DOI] [PubMed] [Google Scholar]
- 54.Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol 148: 1–80, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J 370: 987–993, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]