Abstract
Lactobacillus reuteri (L. reuteri) is a probiotic that inhibits the severity of enteric infections and modulates the immune system. Human-derived L. reuteri strains DSM17938, ATCC PTA4659, ATCC PTA 5289, and ATCC PTA 6475 have demonstrated strain-specific immunomodulation in cultured monocytoid cells, but information about how these strains affect inflammation in intestinal epithelium is limited. We determined the effects of the four different L. reuteri strains on lipopolysaccharide (LPS)-induced inflammation in small intestinal epithelial cells and in the ileum of newborn rats. IPEC-J2 cells (derived from the jejunal epithelium of a neonatal piglet) and IEC-6 cells (derived from the rat crypt) were treated with L. reuteri. Newborn rat pups were gavaged cow milk formula supplemented with L. reuteri strains in the presence or absence of LPS. Protein and mRNA levels of cytokines and histological changes were measured. We demonstrate that even though one L. reuteri strain (DSM 17938) did not inhibit LPS-induced IL-8 production in cultured intestinal cells, all strains significantly reduced intestinal mucosal levels of KC/GRO (∼IL-8) and IFN-γ when newborn rat pups were fed formula containing LPS ± L. reuteri. Intestinal histological damage produced by LPS plus cow milk formula was also significantly reduced by all four strains. Cow milk formula feeding (without LPS) produced mild gut inflammation, evidenced by elevated mucosal IFN-γ and IL-13 levels, a process that could be suppressed by strain 17938. Other cytokines and chemokines were variably affected by the different strains, and there was no toxic effect of L. reuteri on intestinal cells or mucosa. In conclusion, L. reuteri strains differentially modulate LPS-induced inflammation. Probiotic interactions with both epithelial and nonepithelial cells in vivo must be instrumental in modulating intrinsic anti-inflammatory effects in the intestine. We suggest that the terms anti- and proinflammatory be used only to describe the effects of a probiotic in the living host.
Keywords: formula, intestine, cytokine, endotoxin, probiotic
the commensal intestinal microbiota represents a major modulator of intestinal homeostasis. The immune system at both the systemic and mucosal levels can be modulated by bacteria in the gut. Disruption of the normal interaction of microbiota with the intestinal epithelium and mucosal immune system may result in severe intestinal inflammation. Probiotics are live microorganisms that, when ingested, produce a therapeutic or preventative health benefit (34). There is emerging clinical evidence for the beneficial effect of probiotics in preventing and/or treating gastrointestinal diseases, including necrotizing enterocolitis, infectious and antibiotic-associated diarrhea, and allergic diseases (25). The use of probiotics is of particular relevance to pediatric investigators, because infants are especially vulnerable to gastrointestinal disease during the delicate process of intestinal maturation.
Probiotic bacteria have multiple and diverse influences on the host (19). Different organisms can influence the intestinal luminal environment, epithelial and mucosal barrier function, and the mucosal immune system. They exert their effects on numerous cell types involved in the innate and adaptive immune responses, such as immune cells (dendritic cells, monocytes/macrophages, T cells) and epithelial cells.
Lactobacillus reuteri is a promising therapy for many different conditions, including diarrheal disease (26), infantile colic (27), eczema (1), “episodes of workplace illness” (32), and Helicobacter pylori infection (8). L. reuteri is considered an endogenous organism of the human gastrointestinal tract and is present on the mucosa of the gastric corpus and antrum, duodenum, and ileum (24, 33). It has been known that L. reuteri produces a potent antibacterial compound, reuterin, that is capable of inhibiting a wide spectrum of microorganisms (31). An anti-inflammatory action of L. reuteri has been shown by previous studies documenting inhibition of experimental colitis in transgenic IL-10-deficient mice (17), as well as reduction of levels of the proinflammatory cytokine TNF-α in mice with colitis (22). Furthermore, studies have shown that live L. reuteri has a potent inhibitory effect on TNF-α-induced IL-8 expression in human intestinal epithelial cells (16). The above studies, although defining important potentially therapeutic effects, did not define the effects of specific L. reuteri strains or examine effects in vivo.
The modulation of TNF-α production by L. reuteri secreted factors is strain dependent (10, 13). Anti-inflammatory L. reuteri strains ATCC PTA 6475 and ATCC PTA 5289 suppressed TNF-α production by bacterial lipopolysaccharide (LPS)-activated monocytoid cells. In contrast, immunostimulatory L. reuteri strains ATCC 55730 and CF48–3A did not suppress TNF-α production by LPS-activated monocytoid cells (10, 13). However, the anti- or proinflammatory effects of L. reuteri strains on intestinal epithelial cells and in the intestines in vivo have not been reported.
In this article, we show evidence that L. reuteri strains differentially affect LPS-induced IL-8 response in intestinal epithelial cells and intestinal inflammatory cytokine and chemokine production in newborn rats. We also demonstrate that all four strains reduced the intestinal damage produced by LPS in newborn rats.
MATERIALS AND METHODS
Cell culture and IL-8 (CINC-1) inhibition experiments.
The IPEC-J2 cell line was obtained from Dr. Douglas G. Burrin, USDA/ARS Children's Nutrition Research Center at Baylor College of Medicine. IPEC-J2 is a nontransformed intestinal cell line originally developed by H. M. Berschneider from jejunal epithelium isolated from a neonatal, unsuckled piglet and maintained as a continuous culture (28). Rat small intestinal cell line IEC-6 was purchased from American Tissue Culture Collection (Manassas, VA). Cells were grown in DMEM with high d-glucose (4.5 g/l) (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Atlanta Bio, Lawrenceville, GA) and maintained in an atmosphere of 5% CO2 at 37°C. Cells (1 × 105 per well) were cultured in sterile 24-well flat-bottom plates for 24 h before treatment. Cells were treated with LPS from Escherichia coli subtype 0111:B4 at the concentration of 5 μg/ml for different time courses or at different concentrations for 16 h. For L. reuteri inhibition experiments, cells were cultured in fresh antibiotic- and serum- free medium and treated for 16 h with LPS and the probiotic L. reuteri strains, at a multiplicity of infection (MOI) of 100 (for IPEC-J2) or 10 (for IEC-6) cells. Supernatants were then harvested and sterilized through a 0.22-μm filter, in preparation for a quantikine porcine IL-8 or rat CXCL1/CINC-1 ELISA (R&D Systems, Minneapolis, MN). The results were demonstrated by a pictogram of cytokine production per 10,000 cells. Cell viability was detected by using TACS XTT Cell Proliferation Assay (Trevigen, Gaithersburg, MD), which based on the cleavage of tetrazolium salts, XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carbox-anilide] to formazan in the mitochondria of metabolically active cells, according to manufacturer's instructions. Cell apoptosis was detected by using APO-BrdU TUNEL Assay kit (Invitrogen, Molecular Probes, Eugene, OR) according to the manufacturer's protocol. Briefly, detached and trypsinized cells were washed and fixed with 1% (wt/vol) paraformaldehyde after being treated with LPS or L. reuteri strains. Fixed sample cells and positive and negative control cells were treated with DNA-labeling solution containing TdT enzyme and BrdUTP, followed by staining with Alexa Fluor 488 dye-labeled anti-bromodeoxyuridine (BrdU) antibody. The samples were analyzed by flow cytometry. The assay includes apoptotic negative and positive control cells for staining quality control.
Bacterial strains and preparations.
Biogaia (Stockholm, Sweden) provided human-derived L. reuteri strains including ATCC DSM 17938, ATCC PTA 4659, ATCC PTA 5289, and ATCC PTA 6475. L. reuteri ATCC DSM 17938 is a strain of plasmid-cured ATCC 55730 from Peruvian mother's milk. ATCC PTA 4659 and ATCC PTA 6475 were isolated from the breast milk of healthy Finnish women. ATCC PTA 5289 is an oral isolate from a healthy Japanese woman. L. reuteri bacteria were cultured in deMan-Rogosa-Sharpe (MRS; Difco, Detroit, MI) medium at 37°C for 24 h, then plated in MRS agar at specific serial dilutions and grown at 37°C for 48 h for counting the colonies. Quantitative analysis of bacteria in culture media was determined by using a standard curve of bacterial colony-forming units (CFU)/ml on MRS agar plate relative to visible absorbance (600 nm; Eppendorf Photometer, Eppendorf, Hamburg, Germany) of bacterial culture media. Desired numbers of bacteria in culture media were harvested by centrifugation at 1,500 g for 15 min and resuspended in PBS for cell study or in formula for animal feeding.
Animal model and experimental design.
All in vivo experiments were performed using newborn Sprague-Dawley rat pups (Harlan Laboratories, Indianapolis, IN) weighing 5–6 g. Studies were approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston (HSC-AWC-07-124). Newborn rat pups (1 day old) were separated from their mothers, housed in an incubator, and gavage fed for 3 days with either 1) a special rodent formula, 2) formula containing L. reuteri, 3) formula containing LPS from E. coli 0111:B4 (Sigma, St. Louis, MO, at the dose of 0.25 mg·kg body wt−1·day−1) (12), or 4) LPS in combination with one of the four designated L. reuteri strains (106 CFU·g body wt−1·day−1). The formula consisted of 15 g Similac 60/40 (Ross Pediatrics, Columbus, OH) in 75 ml of Esbilac canine milk replacement (Pet-Ag, Hampshire, IL) (14). Each group had 8–15 newborn rat pups. Rat pups were euthanized on day 4.
Tissue harvest.
The gastrointestinal tract was carefully removed, and last 4 cm of terminal ileum was excised. A portion of each sample was formalin fixed, paraffin embedded, microtome-sectioned at 5 μm, and stained with hematoxylin and eosin (H&E) for histological evaluation. Part of ileum for each animal was washed with cold PBS, pH 7.4, and fresh frozen immediately in liquid nitrogen for RNA and protein isolation.
Intestinal morphology.
We focused our microscopy studies on the ileum because this is a region that is most highly susceptible to ischemic injury (for example in necrotizing enterocolitis). Each stained H&E section was observed and the whole images were recorded at ×100 magnification. Villus length and density (villi per unit length) were measured by use of Imaging Tool software. Approximately 5–10 villi were measured for each stained H&E section, and each rat had three stained sections (15–30 villi per rat). Villus length was measured from the tip of the villus to the beginning of the crypt. Villus density was measured by counting the number of villi populating half of the total bowel circumference for each stained H&E section. The measurement was performed by two different examiners unaware of group assignment. Villus length and density were calculated and represented as means ± SE.
Tissue preparation for cytokine protein assay.
Frozen ileal tissues were homogenized in 0.4 ml of lysis buffer containing protease inhibitors (14). The homogenates were centrifuged at 14,000 g for 10 min at 4°C after incubation on ice for 30 min. The supernatants were removed for detection of protein concentration and cytokine measurement. Cytokines were assessed by using MSD Rat Demonstration Multiplex (Meso Scale Discovery, Gaithersburg, MD). Each well of the 96-well plate-based multiplex assay contained antibodies to a cytokine (IFN-γ, TNF-α, IL-1β, IL-13, IL-5, or IL-4), or chemokine keratinocyte-derived chemokine/growth-related oncogene [KC/GRO, also known as rat cytokine-induced neutrophil chemoattractant-1 (CINC-1), or CXCL1, which has similar function as human IL-8]. The assay was run according to the manufacturer's instructions. Total protein in tissue lysates was measured by using Bio-Rad Dc Protein Assay (Bio-Rad Laboratories, Hercules, CA). The results from cytokine measurements in tissue lysates were expressed as picograms of cytokine per milligram total protein.
mRNA expression by qRT-PCR.
RNA was isolated from frozen tissue samples by use of TRIzol (Invitrogen, Carlsbad, CA), followed by RNA purification and On-Column DNase digestion (Qiagen, Valencia, CA) according to the manufacturer's protocols. The quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) was performed with the rat inflammatory cytokines RT2 profiler PCR and SYBR green/ROY qPCR master mix (SABiosciences, Frederick, MD). All qRT-PCR reactions were run at the Quantitative Genomics Core Laboratory (UTHSC-Houston Medical School, Houston, TX) by utilizing a 7700 Detector (Applied Biosystems, Foster City, CA). The threshold cycle (Ct) value for each well was obtained by using the instrument's software. Data analysis by the ΔΔCt method was automatically performed by PCR Array Data Analysis Web Portal provided by SABiosciences. To determine the fold change in gene expression, the normalized expression of each gene of interest (GOI) in the experimental sample was divided by the normalized expression of the same GOI in the control sample. The GOIs assessed in this study included IFN-γ (NM_138880), IL-13 (NM_053828), TNF-α (NM_012675), and IL-1β (NM_031512). Chemokines measured in this study included Cxcl2 (NM_053647), Ccl2 (NM_031530), Ccl12 (XM_213425), Cxcl12 (NM_022177), Ccl19 (XM_342824), Cx3Cr1 (NM_133534), and IL8ra (NM_019310). Five housekeeping genes (HKG) were used: ribosomal protein, large P1 (Rplp1, NM_001007604); hypoxanthine guanine phosphoribosyl transferase (Hprt, NM_012583); ribosomal protein L13A (Rpl13a, NM_173340); lactate dehydrogenase A (Ldha, NM_017025); and β-actin (Actb, NM_031144). We used the average Ct value of all housekeeping genes that were not influenced by our experimental conditions for normalization with the ΔΔCt method. The calculation was as follows: 2−ΔΔCt = 2−[Ct (GOI) − Ct (HKG)] expt/2−[Ct (GOI) − Ct (HKG)] control. In our study, the fold changes of transcripts of each group were compared with the changes in LPS-fed group. To monitor the quality of tested samples, the controls for genomic DNA, reverse transcription, and positive PCR were examined simultaneously. All the samples passed the tests for quality control.
Statistics.
Experimental results are expressed as means ± SE. Statistical analyses were performed with one-way ANOVA using GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA). Dunnett's and Tukey's multiple-comparison tests were used for comparison of multiple groups with a control group. A P value of <0.05 was considered statistically significant.
RESULTS
L. reuteri strains differentially affected LPS-induced IL-8 production in cultured intestinal epithelial cell lines.
Most intestinal cell lines derived from colon carcinomas have tolerance to LPS. We initially examined the effects of LPS on IL-8 secretion in IPEC-J2 small intestinal cells. In our study, IPEC-J2 cells continuously expressed IL-8 at a detectable level (Supplemental Fig. S1A; the online version of this article contains supplemental data). When the cells were incubated with LPS at incremental doses for 16 h, IL-8 levels in the cell culture supernatant increased in a dose-dependent manner (Supplemental Fig. S1B). When the cells were treated with LPS at 5 μg/ml for different periods of time, the level of IL-8 increased in a linear manner for up to 72 h (Supplemental Fig. S1C). These results indicated that IPEC-J2 intestinal cells are highly responsive to LPS, producing IL-8 in both dose- and time-dependent fashion.
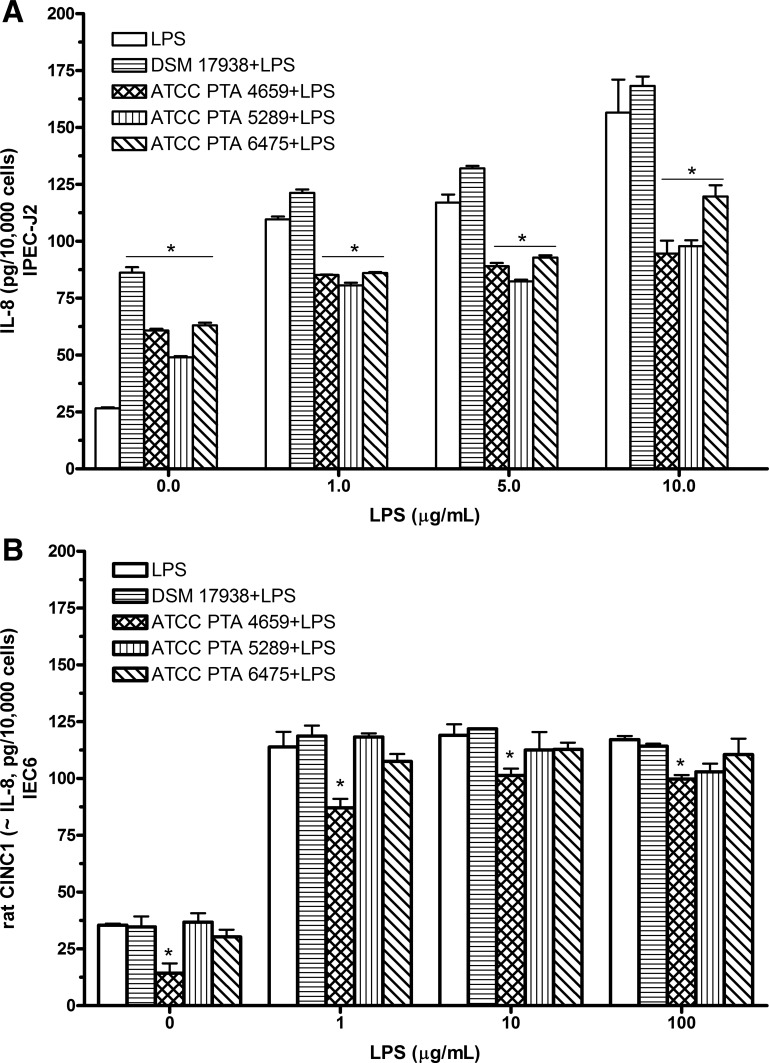
To assess the effects of L. reuteri strains on LPS-induced IL-8 production in cultured intestinal cells, IPEC-J2 cells were incubated in fresh antibiotic-free, serum-free medium with L. reuteri at a MOI of 102 in combination with different concentrations of LPS for 16 h. All the L. reuteri strains alone without combination with LPS stimulated IL-8 production in IPEC-J2 cells compared with untreated control cells. However, in combination with different doses of LPS, three L. reuteri strains (ATCC PTA 4659, 5289, and 6475) significantly inhibited LPS-induced IL-8 production. However, the level of IL-8 secretion remained high when IPEC-J2 cells were treated with DSM 17938 with LPS (Fig. 1A).
Fig. 1.
IL-8 [cytokine-induced neutrophil chemoattractant-1 (CINC-1)] production from cultured intestinal epithelial cells after treatment with Lactobacillus reuteri strains in combination with LPS. Cells (1 × 105 cells/well) were treated with Lactobacillus reuteri strains in combination with indicated different concentrations of LPS for 16 h, respectively. IL-8 (or CINC-1) levels in culture supernatants were examined by ELISA assay for porcine IL-8 or rat CINC-1. A: IPEC-J2 cells. All L. reuteri strains (ATCC PTA 4659, 5289, and 6475) except DSM 17938 suppressed LPS-induced IL-8 secretion by IPEC-J2 cells. B: IEC-6 cells. Only strain 4659 inhibited LPS-induced CINC-1 production by IEC-6 cells. Data are represented as means ± SE, N = 4–6. *P < 0.05 L. reuteri strain + LPS compared with LPS.
We also examined the effect of L. reuteri strains on LPS-induced CINC-1 (equivalent to IL-8) in rat intestinal epithelial cell line IEC-6. The baseline level of CINC-1 production by IEC-6 cells was inhibited by L. reuteri strain 4659. LPS significantly increased CINC-1 levels at the concentrations of 1–100 μg/ml after 16 h treatment. Interestingly, we observed a completely different effect of L. reuteri strains on LPS-induced CINC-1 in rat IEC-6 compared with that in IPEC-J2 cells. Only L. reuteri strain 4659 significantly reduced LPS-induced CINC-1 in rat IEC-6 cells (Fig. 1B).
In summary, in vitro studies in intestinal epithelial cells demonstrated that L. reuteri strains have intrinsic proinflammatory activity in cultured cells, but the strains differentially inhibit LPS-induced IL-8 production. The commercially available L. reuteri strain DSM 17938, which did not suppress LPS-induced IL-8 secretion, emerged as different from L. reuteri strain 4659. The latter strain was unique in that it significantly inhibited LPS-induced IL-8 production both in IPEC-J2 and IEC-6 cells.
L. reuteri strains prevented LPS-induced cell death in IPEC-J2 cells.
Because L. reuteri strains could have reduced IL-8 production by a nonspecific toxic effect on IPEC-J2 cells, we measured cell number after treatment of intestinal cells with L. reuteri strains in combination with LPS by XTT Assay. We found that LPS (10 μg/ml)-treated cells had ∼20% more nonviable cells than untreated control cells, P < 0.05. LPS-induced death of a similar cell line, IPEC-1 cells, was previously reported by another group (6). All the L. reuteri strains in combination with LPS treatment significantly increased cell number, compared with cells treated with LPS without the probiotic (P < 0.05) (Supplemental Fig. S2). Interestingly, DSM 17938 conferred a protective effect on cell number in the presence of LPS, similar to that of other L. reuteri strains, even though the level of IL-8 secretion remained high when IPEC-J2 cells were treated with 17938 plus LPS. In IEC-6 cells, we noted that LPS could not produce cell death, even though IEC-6 cells responded to LPS treatment (100 μg/ml) with increased CINC-1 production.
To explore whether the cell death induced by LPS is due to apoptosis, we measured apoptotic cells by TdT-mediated dUTP nick-end labeling (TUNEL) staining. There was no significant difference in the percentage of cells undergoing apoptosis (as measured by BrdU labeling) in cells treated with LPS (4.8 ± 2.79% apoptosis) compared with untreated control cells (6.6 ± 4.35% apoptosis). In addition, there was no significant increase number of the apoptotic cells during treatment with any of the 4 L. reuteri strains. In summary, the reduction of cytokine production by L. reuteri strains was not produced by a general toxic or proapoptotic effect on cultured intestinal cells.
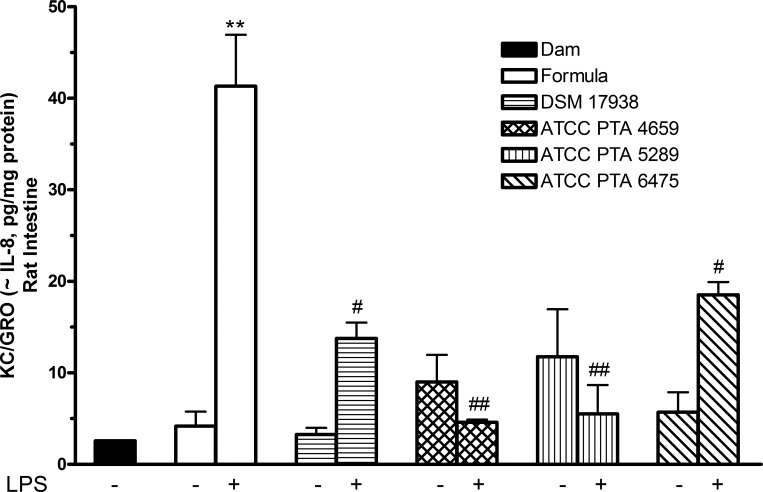
L. reuteri strains reduce LPS-induced KC/GRO production in rat intestines.
To compare the effects of L. reuteri strains on LPS effects in cell culture with the response to LPS in vivo, 1-day-old rat pups were separated from their mothers and fed with cow milk-based formula containing different L. reuteri strains in the presence or absence of LPS for 3 days. Ileal tissues were collected, and the levels of KC/GRO (Cxcl1 or CINC-1), which has similar function to human IL-8 (5) in tissue lysates, were examined. KC/GRO, released after NF-κB pathway activation, has been suggested as a major early signal in the enterotoxic cascade and has been implicated in the pathogenesis of distal organ damage originating in the intestine (12). As shown in Fig. 2, intestinal KC/GRO levels in rat pups fed with formula containing LPS (without probiotics) was significantly increased compared with KC/GRO level in rat pups fed with either formula or on the dam (P < 0.01). Interestingly, in contrast to the results shown in the cell culture studies, none of the L. reuteri strains given alone increased intestinal KC/GRO production compared with either breast milk or formula feeding. Additionally, all four L. reuteri strains significantly inhibited LPS-induced KC/GRO production in rat intestine (P < 0.01).
Fig. 2.
Effects of L. reuteri strains on LPS-induced chemokine keratinocyte-derived chemokine/growth-related oncogene (KC/GRO) production by newborn rat intestine. One-day-old rat pups were dam-fed or fed with formula or formula + LPS (0.25 mg·kg body wt−1·day−1) or formula + L. reuteri (106 colony-forming units·g body wt−1·day−1) ± LPS for 3 days. KC/GRO production in ileal tissue lysates were assayed by using a MSD rat multiplex assay. Data are represented as means ± SE, N = 8–10 animals per group. Formula + LPS group compared with either dam-fed or formula-fed control: **P < 0.01. Formula + L. reuteri + LPS group compared with formula + LPS group: #P < 0.05, ##P < 0.01. LPS feeding significantly increased the level of KC/GRO in rat intestine, compared with either breast milk or formula feeding. None of the L. reuteri strains given alone increased intestinal KC/GRO production. All measured L. reuteri strains significantly inhibit LPS-induced KC/GRO production in rat intestines.
L. reuteri strains differentially affect Th1-type and Th2-type cytokines in rat intestine.
LPS as a ligand of TLR4 activates Toll-like receptor signaling pathways, leading to activation of NF-κB transcription. This activation results in the subsequent upregulation of inflammatory cytokines and chemokines that can be associated with autoimmune and allergic disorders. In subsequent experiments, we measured the impact of L. reuteri strains or LPS-stimulated Th1-type cytokines IFN-γ, TNF-α, and IL-1β and Th2-type cytokines IL-4, IL-5, and IL-13 in the rat intestine.
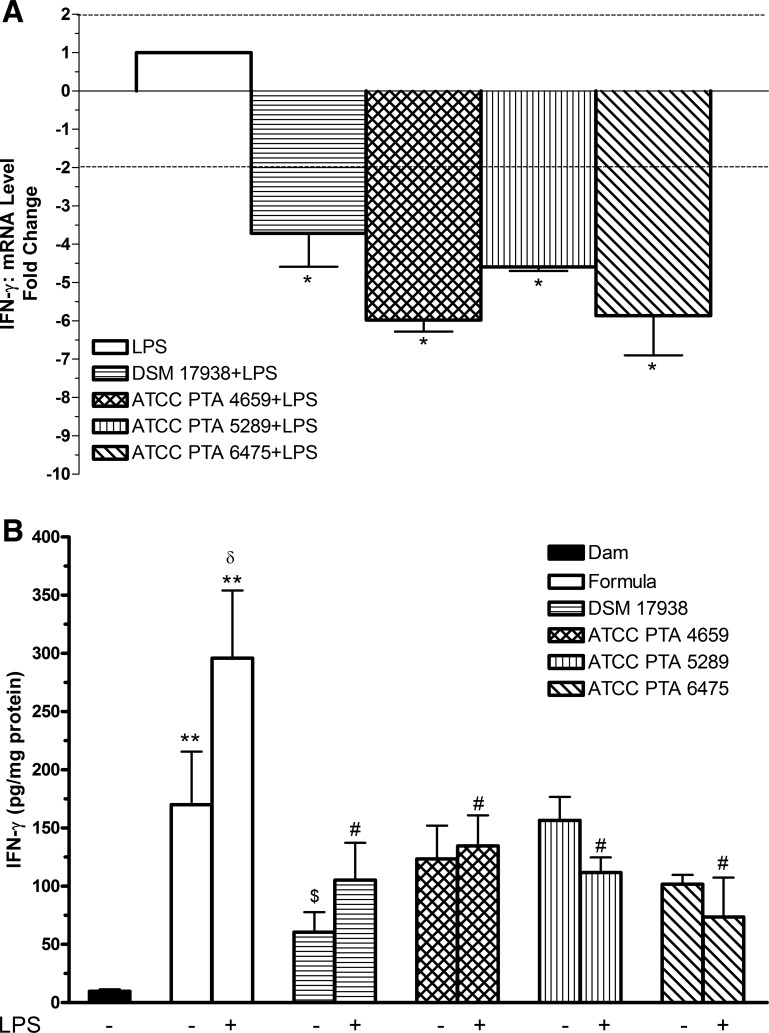
To clarify the effects of LPS and L. reuteri strains on IFN-γ mRNA in the intestines, qRT-PCR was performed. We found that all four L. reuteri strains significantly downregulated IFN-γ mRNA expression induced by LPS (P < 0.05) (Fig. 3A). We further examined IFN-γ protein level (Fig. 3B). The results indicated that 1) IFN-γ production significantly increased in the intestines of rat pups fed with formula compared with dam feeding (P < 0.01); 2) the levels of IFN-γ dramatically increased in rat pups fed with formula containing LPS alone compared with formula alone (P < 0.05); 3) this elevation due to formula feeding alone could be reduced by L. reuteri strain DSM 17938 (P < 0.05), but not by the other three L. reuteri strains; and 4) when rat pups were fed with formula containing the different L. reuteri strains plus LPS, levels of IFN-γ were significantly decreased by all L. reuteri strains compared with formula containing LPS alone (P < 0.05).
Fig. 3.
Effects of L. reuteri strains on LPS-induced IFN-γ levels in rat intestine. A: IFN-γ mRNA expression levels from the ilea of rat pups with L. reuteri strains and LPS feeding. RNA was isolated from the ileum of newborn rats. Quantitative RT-PCR (qRT-PCR) analysis was performed to determine the expression level (fold change) of IFN-γ comparing L. reuteri + LPS-fed rats with LPS-fed rats. Data are represented as means ± SE, N = 6. The dotted lines show cutoff values of fold change: > +2 indicating upregulation, < −2 downregulation. *P < 0.05 vs. LPS control. All 4 L. reuteri strains significantly downregulated mRNA expression of IFN-γ induced by LPS. B: IFN-γ protein level in rat intestine. IFN-γ protein in ileal tissue lysates were assayed by using a MSD rat multiplex assay. Data are represented as means ± SE, N = 8–10. Formula ± LPS-fed groups compared with dam-fed control: **P < 0.01. Formula + LPS compared with formula group: δP < 0.05. Formula + L. reuteri groups compared with formula-fed group: $P < 0.05. L. reuteri + LPS groups compared with LPS group: #P < 0.05. All 4 L. reuteri strains inhibited LPS-induced IFN-γ production. Only L. reuteri DSM 17938 significantly reduced formula-induced inflammation.
LPS-induced TNF-α or IL-1β production was significantly decreased differentially by L. reuteri strains, with inhibited by ATCC PTA 4659 and 5289 (TNF-α) or DSM 17938 and ATCC PTA 6475 (IL-1β), compared with formula containing LPS alone (P < 0.05).
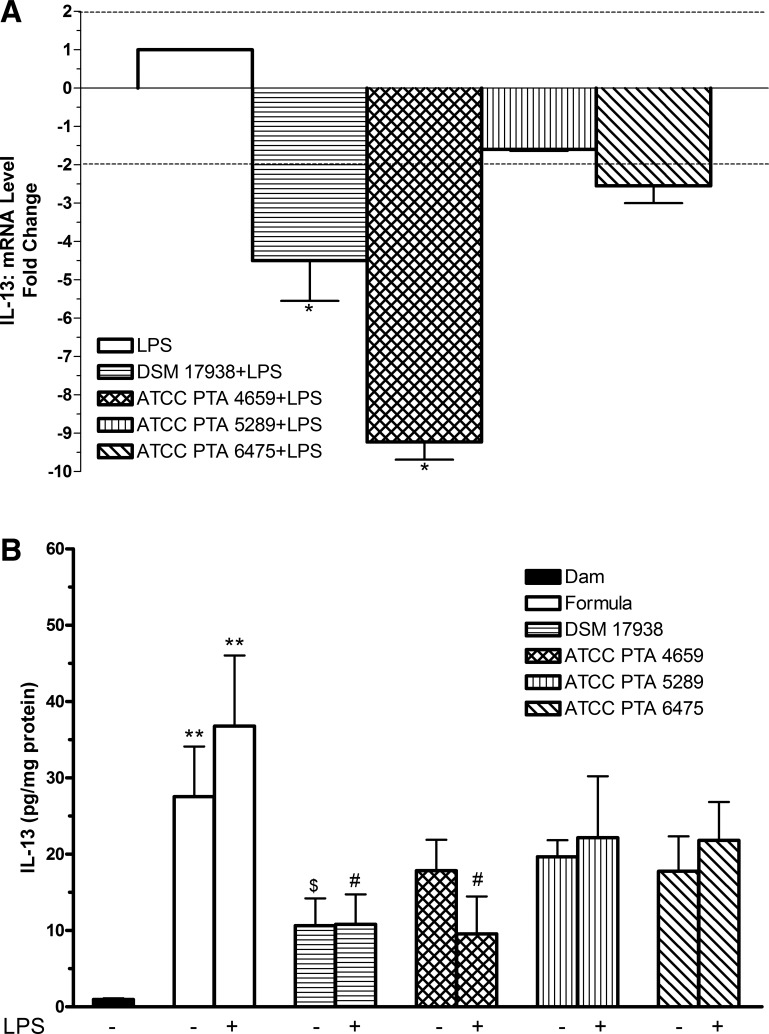
The mRNA level of IL-13 in the intestines was significantly downregulated in rats fed with formula containing LPS and L. reuteri strain DSM 17938 or ATCC PTA 4659, compared with formula containing LPS alone (P < 0.05) (Fig. 4A). IL-13 protein level in the intestine was dramatically elevated in rats fed with formula alone, to about the same extent as the formula containing LPS (Fig. 4B). When comparing the group of rats fed with formula containing both LPS and L. reuteri with the groups of rats fed with formula with LPS and no probiotics, we found that only L. reuteri strains DSM 17938 or ATCC PTA 4659 significantly reduced the levels of IL-13, corresponding to the reduction in IL-13 mRNA level. Only strain DSM 17938 provided a significant reduction of formula-enhanced IL-13 level.
Fig. 4.
Effects of L. reuteri strains on LPS-induced IL-13 levels in rat intestine. A: IL-13 mRNA expression levels from the ilea of rat pups with L. reuteri strains and LPS feeding. qRT-PCR analysis was performed to determine the expression level (fold changes) of IL-13 comparing L. reuteri + LPS-fed rats with LPS-fed rats. Data are represented as means ± SE, N = 6. Dotted lines show cutoff values of fold change: > +2 indicating upregulation, < −2 downregulation. *P < 0.05 vs. LPS control. L. reuteri strains DSM 17938 and ATCC PTA 4659 significantly downregulated mRNA expression of IL-13 induced by LPS. B: IL-13 protein level in rat intestine. IL-13 protein in ileal tissue lysates were assayed by using a MSD rat multiplex assay. Data are represented as means ± SE, N = 8–10. Formula ± LPS-fed groups compared with dam-fed control: **P < 0.01. Formula + L. reuteri groups compared with formula-fed group: $P < 0.05. L. reuteri + LPS groups compared with LPS group: #P < 0.05. L. reuteri strains DSM 17938 and ATCC PTA 4659 inhibit LPS-induced IL-13 production. Only L. reuteri strain DSM 17938 significantly reduced formula-stimulated IL-13.
Similar effects of formula and LPS were observed for levels of other traditional “Th2” cytokines, IL-4 and IL-5, in the intestine. Inhibitory effects of L. reuteri strain DSM 17938 on formula- or LPS-induced IL-4 and IL-5 production were again seen. However, strain ATCC PTA 6475, but not 4659, in combination with LPS also significantly reduced IL-4 and IL-5 levels compared with LPS alone (P < 0.05). In summary, L. reuteri strains differentially affected LPS-induced cytokine production in the intestine of rat pups fed with formula.
L. reuteri strains DSM 17938 and ATCC PTA 4659 differentially regulate LPS-induced chemokine profiles.
Because the immunomodulatory activities of L. reuteri strains differed in vitro, we further compared whether these two strains differentially regulate LPS-induced inflammatory chemokines in vivo. RNA was isolated from the ileal tissues of rat pups subjected to different feeding probiotics. Quantitative RT-PCR analysis was performed to determine the expression profile of 40 different chemokines and chemokine receptors.
Rat pups were fed formula containing LPS and DSM 17938 or ATCC PTA 4659. The effects of DSM 17938 or ATCC PTA 4659 on LPS were compared with LPS alone (Table 1). The cutoff values of fold change compared with LPS alone group were defined as greater than +2, indicating upregulation, and less than −2, indicating downregulation. Our results showed that mRNA expression of 7 chemokines among 40 induced by LPS were differentially regulated by L. reuteri strains DSM 17938 and ATCC PTA 4659. DSM 17938 significantly upregulated the expression of Cxcl2, Ccl2, and Ccl12, whereas it downregulated LPS-induced expression of Cxcl12, Ccl19, and IL8ra. Conversely, strain 4659 significantly downregulated LPS-induced expression of five chemokines including Ccl2, Ccl12, Ccl19, Cx3Cr1, and IL8ra compared with either LPS or DSM 17938 (P < 0.05) (Table 1).
Table 1.
Up- or downregulation of mRNA expression of chemokines in rat intestines
| Fold Change | Cxcl2 | Ccl2 | Ccl12 | Cxcl12 | Ccl19 | Cx3Cr1 | IL8ra |
|---|---|---|---|---|---|---|---|
| DSM 17938† | 5.9 ± 1.1* | 3.1 ± 1.4* | 2.7 ± 0.9* | −5.2 ± 1.1* | −3.8 ± 1.3* | −1.6 ± 1.4 | −8.7 ± 1.2* |
| ATCC PTA 4659 | 0.7 ± 0.3 | −2.5 ± 0.1* | −13.8 ± 0.6* | −1.5 ± 0.1 | −10.9 ± 0.5* | −9.6 ± 0.4* | −13.8 ± 0.7* |
Fold change, Lactobacillus reuteri + LPS compared with LPS alone. The cutoff values of fold change are ±2, > +2 indicating upregulation, < −2 indicating downregulation. Numbers represent means ± SE; n = 6 animals for each group.
P < 0.05, comparing DSM 17938 or ATCC PTA 4659 + LPS with LPS alone;
P < 0.05, comparing DSM 17938 + LPS with ATCC PTA 4659 + LPS for all listed chemokines.
L. reuteri strains improved LPS-induced intestinal morphological damage.
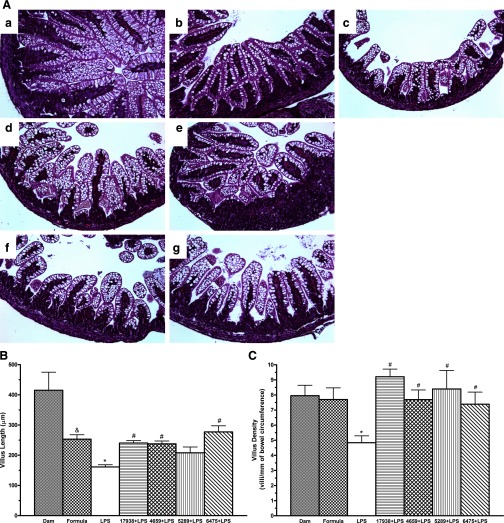
To compare intestinal morphology among the treatment groups, ileal villus length and density were measured (Fig. 5). Ileal villus length in formula-fed rats was significantly reduced compared with dam-fed rat pups. We assume this finding reflected better nutrition in the dam-fed rat pups that were continuously consuming breast milk (P < 0.05). Villus length in LPS-fed rats was found to be significantly shortened compared with either dam-fed (P < 0.01) or formula-fed rats that did not receive LPS (P < 0.05). The feeding of all L. reuteri strains except 5289 when fed to the LPS-fed rat pups were associated with significantly improved villus length (P < 0.05). In fact, feeding of the probiotic was associated with recovery of the villus length to that of a normal formula-fed rat (Fig. 5B).
Fig. 5.
Effects of L. reuteri strains on intestinal morphology. Ileal morphology was observed by light microscopy. Ileal villus length and villus density were measured. A: ileal morphology. a: dam-fed (n = 8). b: formula-fed (n = 9). c: formula + LPS (n = 11). d: formula + L. reuteri DSM 17938 + LPS (n = 10). e: formula + L. reuteri ATCC PTA 4659 + LPS (n = 10). f: formula + L. reuteri ATCC PTA 5289 + LPS (n = 10). g: formula + L. reuteri ATCC PTA 6475 + LPS (n = 10). Magnification = ×200. B: comparison of villous length (in μm) among different treatment groups. C: comparison of villous density (number of villi per unit intestinal circumference) among different groups. Note: &P < 0.05 formula-fed without LPS rats compared with dam-fed rats; *P < 0.05 rat pups fed with formula containing LPS compared with formula-fed without LPS; #P < 0.05 comparing LPS without L. reuteri strains compared with LPS with L. reuteri strains. The data are expressed as means ± SE. Feeding of 4 L. reuteri strains was associated with improved villus length and density after LPS damage.
Even though formula feeding, compared with dam feeding, was associated with a shortening of villus length, villus density (expressed as the number of villi per millimeter of bowel circumference) was not reduced. However, villus density in LPS-fed rat ileum was significantly decreased compared with villus density of dam-fed or formula-fed rats (P < 0.05). Of importance, villus densities were significantly increased in rats fed with all four L. reuteri strains (P < 0.05) (Fig. 5C).
In summary, all L. reuteri strains (DSM 17938, ATCC PTA 4659, 5289, and 6475) were associated with restoration toward a more normal villus structure following LPS injury.
DISCUSSION
We examined the effects of different commensal-derived probiotic L. reuteri strains (DSM 17938, ATCC PTA 4659, ATCC PTA 5289 and ATCC PTA 6475) on intestinal epithelial cells and in the intestine of newborn rats. We asked whether these strains could modulate cytokine production and LPS-induced intestinal inflammation. Several important findings emerged from this study: 1) Even though L. reuteri strain (DSM 17938) did not inhibit LPS-induced IL-8 production in cultured intestinal cells, all strains significantly reduced the intestinal levels of KC/GRO (∼IL-8) and IFN-γ when newborn rat pups fed with LPS and/or L. reuteri. 2) Intestinal histological damage produced by LPS addition to the cow milk formula was also significantly reduced by all four strains. 3) Cow milk formula feeding, even without LPS, in newborn rat pups produced mild gut inflammation, increasing the levels of inflammatory cytokine IFN-γ and IL-13, a process that could be suppressed by L. reuteri strain DSM 17938.
Importance of determining enterocyte response to LPS.
The intestinal epithelial cell lining is the first line of defense against pathogens. It can synthesize and secrete many mediators that play a role in homeostasis and innate immune responses (11, 30). LPS or endotoxin is an integral component of the outer membrane of all gram-negative bacteria. LPS binds to Toll-like receptor-4 (TLR4) and activates several protein kinase signaling pathways, subsequently generating inflammatory cytokines and other mediators (9). Previous studies looking at epithelial responses to LPS were controversial. Several studies have reported that intestinal epithelial cells (IEC) are unresponsive to LPS because they express low levels of TLR4 (2, 29), whereas others have noted that IEC are responsive to LPS (3, 21). In our study, small intestinal cells (both piglet IPEC-J2 and rat IEC-6) responded to LPS exposure by secreting IL-8 into the culture medium. We noted that IEC-6 cells produced the IL-8 equivalent CINC-1 at concentrations of 1 μg/ml or 100 μg/ml of LPS after 16-h treatment, without cell death and in a dose-independent manner. However, IPEC-J2 cells responded to LPS by producing IL-8 in a dose-dependent manner. In addition, we observed that ∼20% of IPEC-J2 cells were nonviable after LPS treatment, probably because IPEC-J2 cells (derived from the jejunum of unsuckled newborn piglets) may be more sensitive to LPS than IEC-6 cells (derived from the rat intestinal crypts). A similar LPS injury in another piglet intestinal epithelial cell line was reported by others (6). These piglet intestinal cells therefore provided a useful cell model to study the effect of L. reuteri strains on LPS-induced IL-8 production.
The LPS-fed newborn rat as an animal model.
An animal model in which artificial LPS feeding of neonatal rat pups results in intestinal inflammation has been developed by Neu and colleagues (12, 35). We fed cow milk formula to neonatal rat pups as early as 1 day old and observed significant intestinal inflammation, even without LPS, which was augmented by LPS. We underscore that LPS feeding is not a model for neonatal necrotizing enterocolitis (NEC). Others have used a model of NEC in which the addition of LPS to cow milk formula feeding is combined with hypoxia and hypothermia to provoke severe intestinal damage (4, 23). In separate experiments, we are studying whether L. reuteri strains modify NEC in a similar model (7, 18). However, in the NEC model described by Ford and coworkers (7, 18), mortality rates in some litters approached 80%. Our present “injury model” of artificial LPS feeding reproducibly produced villus atrophy and a robust expression of cytokine mRNAs and proteins that was not associated with any significant mortality over a 3-day period.
Our group has consistently observed that feeding cow milk formula to rat pups produces an inflammatory response. We have previously shown that artificial feeding in the absence of hypoxic challenge produced almost as much inflammatory cytokine production within the mucosa as artificial feeding in the presence of hypoxia. In fact, exposure of neonatal rat pups to hypoxia in dam-fed pups did not induce significant cytokine production in the intestine (14).
Differential modulation of inflammation by L. reuteri strains.
L. reuteri strains have been shown to differentially modulate TNF-α production by LPS activated human monocytes/macrophages (10, 13). In the absence of LPS, the secreted factors or formed biofilms of L. reuteri strain ATCC 55730 stimulated TNF-α production. In contrast, L. reuteri strain ATCC PTA 6475 yielded undetectable levels of TNF-α by monocytoid THP1 cells. However, in LPS-activated THP1-cells, L. reuteri strain 6475 robustly suppressed TNF production (13). L. reuteri strains were therefore divided into two subsets with respect to their action on leukocytes: immunosuppressive (ATCC PTA 6475) and immunostimulatory (ATCC 55730) (10, 13).
We, too, found that cultured intestinal cells responded differently to L. reuteri strains in the absence of LPS. All L. reuteri strains stimulated IL-8 production in IPEC-J2 but not in IEC-6 cells. We speculate that adult rat intestinal crypt cells (IEC-6) are more tolerant to LPS than newborn enterocytes (IPEC-J2). More importantly, LPS-stimulated intestinal cells responded differentially to L. reuteri strains with respect to LPS-induced IL-8 production. All L. reuteri strains except strain 17938 (“proinflammatory”) inhibited LPS-induced IL-8 production in IPEC-J2 cells; however, only strain 4659 (“anti-inflammatory”) inhibited LPS-induced CINC-1 production in IEC-6 cells. These findings indicate a difference between the action of L. reuteri strains on intestinal epithelial cells and the monocytoid cells studied by Versalovic and coworkers (10, 13). The differential aspects of these two strains in IEC are summarized in Table 2. All strains reduced the effects of LPS feeding in the probiotic-fed animals.
Table 2.
Differential effects of 2 L. reuteri strains on LPS-increased cytokine production
| Cells |
Rat Pups |
|||||
|---|---|---|---|---|---|---|
| IPEC-J2 |
IEC-6 |
Th1 |
Th2 |
|||
| L. reuteri Strains | IL-8 | CINC1 | KC/GRO | IFN-γ | IL-13 | IL-5 |
| DSM 17938 | ||||||
| LPS− | ↑↑ | ↔ | ↔ | ↓ | ↓ | ↓ |
| LPS+ | ↔ | ↔ | ↓ | ↓ | ↓ | ↓ |
| ATCC PTA 4659 | ||||||
| LPS− | ↑ | ↓ | ↔ | ↔ | ↔ | ↔ |
| LPS+ | ↓ | ↓ | ↓↓ | ↓ | ↓ | ↔ |
Direction of arrow indicates the direction of effect on cytokine level (increase or decrease). Horizontal arrows indicate no effect of the probiotic. Double arrows indicate stronger effect.
In our LPS-fed neonatal rat pup model of gut inflammation, different strains of L. reuteri had more subtle differences in their effects on the intestine in vivo. An important difference was seen in their impact on the IL-8 equivalent KC/GRO. All strains of L. reuteri were without effect on KC/GRO levels when fed in the formula. Furthermore, each strain inhibited LPS feeding-induced KC/GRO expression. Thus the “early” signal of KC/GRO as a chemokine sentinel of gut injury would be expected to be ameliorated in vivo by any of the strains we tested. Examining cow milk formula-stimulated IFN-γ production in the neonatal rat intestine, we found that only L. reuteri strain DSM 17938 suppressed its level in the mucosa. In the ileum, IFN-γ levels of animals fed with other L. reuteri strains remained as high as IFN-γ levels in the ilea of those with formula feeding. Addition of LPS to the formula further increased intestinal IFN-γ levels, but this increase was significantly inhibited by all L. reuteri strains at both the mRNA and protein levels.
Chemokines are expressed in a tissue-specific manner to guide leukocyte migration. Although it was not a primary focus of the present studies, we also determined whether L. reuteri strains affected mucosal levels of a panel of chemokines (CXC), in addition to their effects on chemokine KC/GRO/IL-8. We focused on two strains that have distinct features in vitro: strain 17938 (proinflammatory) and strain 4659 (anti-inflammatory). We found that a variety of chemokines were differentially affected the two L. reuteri strains, with an overall decrease in intestinal levels following probiotic feeding.
More complex relationships between the different probiotic strains and IL-13 production were seen. Both IL-13 and IL-5 are traditionally classified as Th2 cytokines, involved in allergic and antiparasitic reactions. However, in the neonatal rat pup intestine, cytokine output represents innate immune responsiveness to endotoxic or formula challenge. We have previously shown that virtually all Th1 and Th2 cytokine mRNAs were elevated our model of NEC (14). Formula feeding with or without LPS was equally injurious with respect to IL-13 production. Two of the four L. reuteri strains, DSM 17938 and ATCC PTA 4659, significantly reduced LPS-induced IL-13 at the mRNA and protein levels. Effects of the different strains on IL-5 levels were virtually identical to those on IL-13, except significant inhibition by DSM 17938 and ATCC PTA 6475 on IL-5 levels were observed. Therefore, all four strains of L. reuteri had anti-inflammatory effects on LPS-stimulated cytokine secretion, but the most consistent inhibition was produced by strain DSM 17938, which inhibited milk feeding-associated and LPS + milk feeding-associated inflammation.
LPS-induced histological injury in our newborn rats was mainly observed as distortion of overall villus architecture (with reduced villus length and villus density), but not by an inflammatory infiltrate, as described by other studies (12, 35). For this reason, we did not evaluate the accumulation and infiltration of different immune cell types in the mucosa in response to the LPS-induced inflammatory process. However, the effect of L. reuteri strains on different immune cell types in the mucosa during inflammation should be further evaluated in future studies.
In summary, regardless of the anti-inflammatory or proinflammatory nature of the L. reuteri strains in cultured intestinal cells, all strains (DSM 17938, ATCC PTA 4659, 5289, and 6475) ameliorated intestinal injury produced by LPS in vivo. The reduction in injury correlated with an improved histological appearance of the mucosal villi following LPS injury and by lower levels of mucosal cytokines.
We also reviewed limited data from four animals that were fed on the dam but also gavage-fed probiotic strain 17938. These animals had mild increases in cytokines such as IL-1β and TNF-α (data not shown) too, compared with the dam-fed animals, indicating that mild inflammation from a probiotic may be beneficial whereas severe inflammation may be reduced by the same probiotic.
Implications of our findings to in vivo probiotic signaling.
Our studies have implications about terminology referring to probiotic effects in the intestine. They indicate that it is appropriate to evaluate different strains of the same probiotic carefully, because their effects may differ. However, the terms anti- or proinflammatory should only be based on their impact on the host that ingests them. Although strain 17938 enhanced intestinal cellular IL-8 levels, feeding the same strain to rat pups produced a strong anti-inflammatory signal in vivo with respect to the same cytokine. This observation supports other observations implicating emerging major role of lamina propria cells in probiotic signaling. Key cells in this immunomodulatory response are mucosal dendritic cells and T cells in the lamina propria. Work by O'Mahony and colleagues (15) has recently shown that dendritic cell sampling of Bifidobacterium breve resulted in the proliferation of intestinal regulatory T-cells (Treg), which are known to provide key anti-inflammatory signals. Importantly, these probiotic-conditioned Tregs could be transferred adoptively to probiotic-naive animals and were able to transmit immunosuppressive properties (20). The mechanisms by which L. reuteri strains interact with intestinal epithelial cells and immune cells and modify their mutual interaction need to be further explored.
GRANTS
This work was supported in part by Department of Pediatrics of the University of Texas Health Science Center at Houston Medical School and Public Health Service Grant DK56338, which funds the Texas Medical Center Digestive Diseases Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Gregory L. Shipley from the Quantitative Genomics Core Laboratory, University of Texas (UT) Medical School at Houston, TX for assisting in qRT-PCR technique and analysis of transcript profiling results; Cellular & Molecular Morphology Core, the Texas Medical Center Digestive Disease Center at Houston, TX for performing histological preparations; Dr. Douglas G. Burrin from USDA/ARS Children's Nutrition Research Center at Baylor College of Medicine, Houston, TX for providing IPEC-J2 cells, and Biogaia (Stockholm, Sweden) for providing the different strains of probiotic L. reuteri. Dr. Dat Q. Tran from the Pediatric Research Center of UT Medical School at Houston, TX critically reviewed the manuscript. Dr. Sanker Surendran helped to develop the neonatal feeding technique.
REFERENCES
- 1. Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm MC, Bjorksten B, Oldaeus G. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 119: 1174–1180, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 167: 1609–1616, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 164: 966–972, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg 41: 144–149, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Harada K, Toyonaga A, Mitsuyama K, Sasaki E, Tanikawa K. Role of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, in rat experimental colitis. Digestion 55: 179–184, 1994. [DOI] [PubMed] [Google Scholar]
- 6. Haynes TE, Li P, Li X, Shimotori K, Sato H, Flynn NE, Wang J, Knabe DA, Wu G. L-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37: 131–142, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 63: 117–123, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Imase K, Tanaka A, Tokunaga K, Sugano H, Ishida H, Takahashi S. Lactobacillus reuteri tablets suppress Helicobacter pylori infection—a double-blind randomised placebo-controlled cross-over clinical study. Kansenshogaku Zasshi 81: 387–393, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Johnson GB, Brunn GJ, Samstein B, Platt JL. New insight into the pathogenesis of sepsis and the sepsis syndrome. Surgery 137: 393–395, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 9: 35, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest 100: 6–10, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li N, Liboni K, Fang MZ, Samuelson D, Lewis P, Patel R, Neu J. Glutamine decreases lipopolysaccharide-induced intestinal inflammation in infant rats. Am J Physiol Gastrointest Liver Physiol 286: G914–G921, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Lin YP, Thibodeaux CH, Pena JA, Ferry GD, Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis 14: 1068–1083, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G442–G450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyons A, O'Mahony D, O'Brien F, Macsharry J, Sheil B, Ceddia M, Russell WM, Forsythe P, Bienenstock J, Kiely B, Shanahan F, O'Mahony L. Bacterial strain-specific induction of Foxp3(+) T regulatory cells is protective in murine allergy models. Clin Exp Allergy 40: 811–819, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Ma D, Forsythe P, Bienenstock J. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun 72: 5308–5314, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116: 1107–1114, 1999. [DOI] [PubMed] [Google Scholar]
- 18. Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15: 300–310, 2009. [DOI] [PubMed] [Google Scholar]
- 20. O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A, Sherlock G, Macsharry J, Kiely B, Shanahan F, O'Mahony L. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog 4: e1000112, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126: 1054–1070, 2004. [DOI] [PubMed] [Google Scholar]
- 22. Pena JA, Li SY, Wilson PH, Thibodeau SA, Szary AJ, Versalovic J. Genotypic and phenotypic studies of murine intestinal lactobacilli: species differences in mice with and without colitis. Appl Environ Microbiol 70: 558–568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radulescu A, Zorko NA, Yu X, Besner GE. Preclinical neonatal rat studies of heparin-binding EGF-like growth factor in protection of the intestines from necrotizing enterocolitis. Pediatr Res 65: 437–442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reuter G. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol 2: 43–53, 2001. [PubMed] [Google Scholar]
- 25. Ruemmele FM, Bier D, Marteau P, Rechkemmer G, Bourdet-Sicard R, Walker WA, Goulet O. Clinical evidence for immunomodulatory effects of probiotic bacteria. J Pediatr Gastroenterol Nutr 48: 126–141, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Saavedra J. Probiotics and infectious diarrhea. Am J Gastroenterol 95: S16–S18, 2000. [DOI] [PubMed] [Google Scholar]
- 27. Savino F, Pelle E, Palumeri E, Oggero R, Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 119: e124–e130, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Schierack P, Nordhoff M, Pollmann M, Weyrauch KD, Amasheh S, Lodemann U, Jores J, Tachu B, Kleta S, Blikslager A, Tedin K, Wieler LH. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol 125: 293–305, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated upregulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun 71: 3503–3511, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr Opin Microbiol 2: 99–105, 1999. [DOI] [PubMed] [Google Scholar]
- 31. Talarico TL, Dobrogosz WJ. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob Agents Chemother 33: 674–679, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tubelius P, Stan V, Zachrisson A. Increasing work-place healthiness with the probiotic Lactobacillus reuteri: a randomised, double-blind placebo-controlled study. Environ Health 4; 25: 1–5, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 70: 1176–1181, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vanderhoof JA, Young R. Probiotics in the United States. Clin Infect Dis 46, Suppl 2: S67–S72, 2008. [DOI] [PubMed] [Google Scholar]
- 35. Zhang L, Li N, des Robert C, Fang M, Liboni K, McMahon R, Caicedo RA, Neu J. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J Pediatr Gastroenterol Nutr 42: 545–552, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.