Abstract
Spectrin is an important structural component of the membrane skeleton that underlies and supports the erythrocyte plasma membrane. It is composed of nonidentical alpha (Mr 240,000) and beta (Mr 220,000) subunits, each of which contains multiple homologous 106-amino acid segments. We report here the isolation and characterization of a human erythroid-specific beta-spectrin cDNA clone that encodes parts of the beta-9 through beta-12 repeat segments. This cDNA was used as a hybridization probe to assign the beta-spectrin gene to human chromosome 14 and to begin molecular analysis of the gene and its mRNA transcripts. RNA transfer blot analysis showed that the reticulocyte beta-spectrin mRNA is 7.8 kilobases in length. Southern blot analysis of genomic DNA revealed the presence of restriction fragment length polymorphisms (RFLPs) within the beta-spectrin gene locus. The isolation of human spectrin cDNA probes and the identification of closely linked RFLPs will facilitate analysis of mutant spectrin genes causing congenital hemolytic anemias associated with quantitative and qualitative spectrin abnormalities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Birkenmeier C. S., Bodine D. M., Repasky E. A., Helfman D. M., Hughes S. H., Barker J. E. Remarkable homology among the internal repeats of erythroid and nonerythroid spectrin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5671–5675. doi: 10.1073/pnas.82.17.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cioe L., Curtis P. Detection and characterization of a mouse alpha-spectrin cDNA clone by its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1367–1371. doi: 10.1073/pnas.82.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzer T. L., Palek J. Partial spectrin deficiency in hereditary pyropoikilocytosis. Blood. 1986 Apr;67(4):919–924. [PubMed] [Google Scholar]
- Conboy J., Kan Y. W., Shohet S. B., Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9512–9516. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis P. J., Palumbo A., Ming J., Fraser P., Cioe L., Meo P., Shane S., Rovera G. Sequence comparison of human and murine erythrocyte alpha-spectrin cDNA. Gene. 1985;36(3):357–362. doi: 10.1016/0378-1119(85)90191-x. [DOI] [PubMed] [Google Scholar]
- Dhermy D., Lecomte M. C., Garbarz M., Bournier O., Galand C., Gautero H., Feo C., Alloisio N., Delaunay J., Boivin P. Spectrin beta-chain variant associated with hereditary elliptocytosis. J Clin Invest. 1982 Oct;70(4):707–715. doi: 10.1172/JCI110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell. 1983 Sep;34(2):503–512. doi: 10.1016/0092-8674(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. Erythroid spectrin, brain fodrin, and intestinal brush border proteins (TW-260/240) are related molecules containing a common calmodulin-binding subunit bound to a variant cell type-specific subunit. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4002–4005. doi: 10.1073/pnas.79.13.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Huebner K., Palumbo A. P., Isobe M., Kozak C. A., Monaco S., Rovera G., Croce C. M., Curtis P. J. The alpha-spectrin gene is on chromosome 1 in mouse and man. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3790–3793. doi: 10.1073/pnas.82.11.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles W. J., Morrow J. S., Speicher D. W., Zarkowsky H. S., Mohandas N., Mentzer W. C., Shohet S. B., Marchesi V. T. Molecular and functional changes in spectrin from patients with hereditary pyropoikilocytosis. J Clin Invest. 1983 Jun;71(6):1867–1877. doi: 10.1172/JCI110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Coetzer T. L., Palek J., Jacob H. S., Luban N. Sp alpha I/65: a new variant of the alpha subunit of spectrin in hereditary elliptocytosis. Blood. 1985 Sep;66(3):706–709. [PubMed] [Google Scholar]
- Lawler J., Liu S. C., Palek J., Prchal J. A molecular defect of spectrin in a subset of patients with hereditary elliptocytosis. Alterations in the alpha-subunit domain involved in spectrin self-association. J Clin Invest. 1984 Jun;73(6):1688–1695. doi: 10.1172/JCI111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Liu S. C., Palek J., Prchal J. Molecular defect of spectrin in hereditary pyropoikilocytosis. Alterations in the trypsin-resistant domain involved in spectrin self-association. J Clin Invest. 1982 Nov;70(5):1019–1030. doi: 10.1172/JCI110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Palek J., Liu S. C., Prchal J., Butler W. M. Molecular heterogeneity of hereditary pyropoikilocytosis: identification of a second variant of the spectrin alpha-subunit. Blood. 1983 Dec;62(6):1182–1189. [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Erythrocyte and brain forms of spectrin in cerebellum: distinct membrane-cytoskeletal domains in neurons. Science. 1983 Jun 17;220(4603):1295–1296. doi: 10.1126/science.6190228. [DOI] [PubMed] [Google Scholar]
- Lebo R. V., Gorin F., Fletterick R. J., Kao F. T., Cheung M. C., Bruce B. D., Kan Y. W. High-resolution chromosome sorting and DNA spot-blot analysis assign McArdle's syndrome to chromosome 11. Science. 1984 Jul 6;225(4657):57–59. doi: 10.1126/science.6587566. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Linnenbach A. J., Speicher D. W., Marchesi V. T., Forget B. G. Cloning of a portion of the chromosomal gene for human erythrocyte alpha-spectrin by using a synthetic gene fragment. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2397–2401. doi: 10.1073/pnas.83.8.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. The red cell membrane skeleton: recent progress. Blood. 1983 Jan;61(1):1–11. [PubMed] [Google Scholar]
- Moon R. T., Lazarides E. beta-Spectrin limits alpha-spectrin assembly on membranes following synthesis in a chicken erythroid cell lysate. Nature. 1983 Sep 1;305(5929):62–65. doi: 10.1038/305062a0. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Ngai J., Wold B. J., Lazarides E. Tissue-specific expression of distinct spectrin and ankyrin transcripts in erythroid and nonerythroid cells. J Cell Biol. 1985 Jan;100(1):152–160. doi: 10.1083/jcb.100.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian V., Evans J. P., Gratzer W. B. A case of elliptocytosis associated with a truncated spectrin chain. Br J Haematol. 1985 Sep;61(1):31–39. doi: 10.1111/j.1365-2141.1985.tb04057.x. [DOI] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):45–87. [PubMed] [Google Scholar]
- Palek J., Lux S. E. Red cell membrane skeletal defects in hereditary and acquired hemolytic anemias. Semin Hematol. 1983 Jul;20(3):189–224. [PubMed] [Google Scholar]
- Repasky E. A., Granger B. L., Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982 Jul;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- Riederer B. M., Zagon I. S., Goodman S. R. Brain spectrin(240/235) and brain spectrin(240/235E): two distinct spectrin subtypes with different locations within mammalian neural cells. J Cell Biol. 1986 Jun;102(6):2088–2097. doi: 10.1083/jcb.102.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
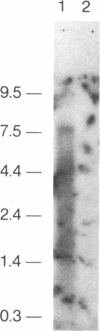
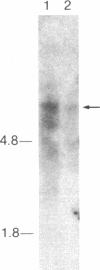
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. W., Josephs R., Steck T. L. Ultrastructure of unit fragments of the skeleton of the human erythrocyte membrane. J Cell Biol. 1984 Sep;99(3):810–821. doi: 10.1083/jcb.99.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
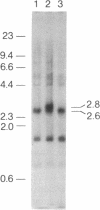
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Temple G. F., Chang J. C., Kan Y. W. Authentic beta-globin mRNA sequences in homozygous betaO-thalassemia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3047–3051. doi: 10.1073/pnas.74.7.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasenius V. M., Saraste M., Knowles J., Virtanen I., Lehto V. P. Sequencing of the chicken non-erythroid spectrin cDNA reveals an internal repetitive structure homologous to the human erythrocyte spectrin. EMBO J. 1985 Jun;4(6):1425–1430. doi: 10.1002/j.1460-2075.1985.tb03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D., Chemla Y., Herzberg M. Isolation of poly(A)+ RNA by paper affinity chromatography. Anal Biochem. 1984 Sep;141(2):329–336. doi: 10.1016/0003-2697(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Yoshino H., Marchesi V. T. Isolation of spectrin subunits and reassociation in vitro. Analysis by fluorescence polarization. J Biol Chem. 1984 Apr 10;259(7):4496–4500. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]