Abstract
Many liver sinusoidal endothelial cell (LSEC)-dependent processes, including drug-induced liver injury, ischemia-reperfusion injury, acute and chronic rejection, fibrosis, and the HELLP (hemolytic anemia, elevated liver enzymes, low platelet count) syndrome, may have a lobular distribution. Studies of the mechanism of this distribution would benefit from a reliable method to isolate LSEC populations from different regions. We established and verified a simple method to isolate periportal, midlobular, and centrilobular LSEC. Three subpopulations of LSEC were isolated by immunomagnetic separation on the basis of CD45 expression. Flow cytometry showed that 78.2 ± 2.3% of LSEC were CD45 positive and that LSEC could be divided into CD45 bright (28.6 ± 2.7% of total population), dim (49.6 ± 1.0%), and negative populations (21.8 ± 2.3%). Immunohistochemistry confirmed that in vivo expression of CD45 in LSEC had a lobular distribution with enhanced CD45 staining in periportal LSEC. Cell diameter, fenestral diameter, number of fenestrae per sieve plate and per cell, porosity, and lectin uptake were significantly different in the subpopulations, consistent with the literature. Endocytosis of low concentrations of the LSEC-specific substrate, formaldehyde-treated serum albumin, was restricted to CD45 bright and dim LSEC. Acetaminophen was more toxic to the CD45 dim and negative populations than to the CD45 bright population. In conclusion, CD45 is highly expressed in periportal LSEC, low in midlobular LSEC, and negative in centrilobular LSEC, and this provides an easy separation method to isolate LSEC from the three different hepatic regions. The LSEC subpopulations obtained by this method are adequate for functional studies and drug toxicity testing.
Keywords: liver circulation, drug-induced liver injury, acetaminophen, CD45, endocytosis
the liver sinusoidal endothelial cell (LSEC) has been implicated in many forms of (often zonated) liver injury, including drug-induced liver injury, ischemia-reperfusion injury, acute and chronic rejection, fibrosis, and the HELLP (hemolytic anemia, elevated liver enzymes, low platelet count) syndrome (1, 4, 5, 7, 8, 10, 11, 13, 16, 18, 20, 23, 26, 27, 30). A zonated injury that originates in the sinusoid could be due to differences in LSEC across the lobule, but other possibilities include regional differences in metabolism by neighboring hepatocytes and oxygen or substrate gradients across the lobule. A simple method to isolate viable LSEC subpopulations would greatly facilitate in-depth studies of LSEC-dependent, zonated injury but would also allow studies of the physiology of LSEC across the lobule. It is well established that there are differences between periportal and centrilobular LSEC in morphology (24, 25, 29), antigen expression (22), and lectin affinity (3). However, to the best of our knowledge, the only functional difference that has been demonstrated to date is a lobular difference in endocytosis (2, 21).
CD45, the common leukocyte antigen, is expressed both in vivo and in vitro in normal rat LSEC (15, 19). Furthermore, there is subpopulation heterogeneity in CD45 expression in LSEC (19). The present study examines LSEC for expression pattern of CD45; determines whether LSEC can be separated into periportal, midlobular, and centrilobular subpopulations based on CD45 expression level; examines differences in endocytosis in the subpopulations; and investigates whether the subpopulations obtained are adequate to study the zonated toxicity of acetaminophen.
MATERIALS AND METHODS
Reagents
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. Antibodies used were mouse anti-rat CD45 antibody (BD Pharmingen, San Diego, CA, catalog no. 554875), fluorescein isothiocyanate (FITC)-conjugated mouse anti-rat CD45 (BD Pharmingen, catalog no. 554877), phycoerythrin (PE)-conjugated mouse anti-rat CD45 (BD Pharmingen, catalog no. 554878), mouse anti-rat CD31 antibody (Abcam, Cambridge, MA), rabbit anti-rat von Willebrand factor (vWF) antibody (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-mouse IgG FITC conjugate (BD Pharmingen), donkey anti-mouse IgG, PE conjugate and donkey anti-rabbit IgG, rhodamine conjugate (Santa Cruz Biotechnology). FITC-labeled formaldehyde-treated serum albumin (FITC-FSA) was a kind gift from Dr. Bård Smedsrød, University of Tromsø, Norway.
Animals
Male Sprague-Dawley rats (body weight 220–250 g) were obtained from Bantin and Kingman Laboratories (Fremont, CA). All protocols were reviewed and approved by the Animal Care and Use Committee at the University of Southern California (USC) to ensure ethical and humane treatment of the animals. This study followed the guidelines outlined in the NIH “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
Cell Isolation
LSEC were first isolated by collagenase perfusion, iodixanol density gradient centrifugation, and centrifugal elutriation as previously described (12). Yields of LSEC are on average 100 million sinusoidal endothelial cell per 10 g liver with viability of >95% and purity of >98%, as determined by positive staining for fluorescent acetylated low-density lipoprotein and a negative peroxidase stain to reveal contaminating Kupffer cells. Total LSEC were then further separated on the basis of CD45 expression by immunomagnetic separation by using an autoMACS Pro separator (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. After blocking with FcR blocking reagent, LSEC were incubated with 0.2 ml (1:10) of FITC-conjugated mouse anti-rat CD45 or PE-conjugated mouse anti-rat CD45 for 45 min at 4°C with gentle shaking, washed, and then incubated with 0.2 ml (1:10) anti-FITC MicroBeads (Miltenyi Biotec, Auburn, CA) or anti-PE MicroBeads (Miltenyi Biotec) for another 45 min at 4°C with gentle shaking. Cells were washed, resuspended in 0.5 ml buffer, and subjected to magnetic separation. The autoMacs “Possel” program was used to separate CD45 bright cells from CD45 dim and negative populations. CD45 dim and negative populations were further separated using the autoMacs “Possel_s” program. Cells were cultured on rat-tail collagen-coated plates (400,000 cells/cm2) in Dulbecco's minimal essential medium (DMEM)-low glucose with 10% fetal bovine serum (FBS). Cells usually attached to the culture dish within 3 h of plating.
Real-Time PCR
Rat bone marrow mononuclear cells (BMMC) were obtained from rat femur with depletion of red blood cells. Total RNA of LSEC and BMMC were prepared by using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Total RNA of rat aortic endothelial cells (RAEC) was purchased from Cell Applications (San Diego, CA). cDNA was prepared using RT2 First Strand Kit from SABiosciences (Frederick, MD). Real-time PCR was performed on the ABI Prism 7900HT sequence Detection System (Applied Biosystems, Carlsbad, CA) using Real-Time RT2qPCR Primer Assays (SABiosciences) with β-actin as a reference control. All primers were purchased from SABiosciences.
Fluorescence Staining
Normal rat liver was snap frozen in liquid nitrogen and embedded in optimal cutting temperature compound. Cryostat sections (10 μm) were fixed with cold acetone for 10 min and blocked with 5% rabbit serum for 30 min. Sections were incubated with CD45 antibody (1:50) for 2 h followed by a rabbit anti-mouse IgG FITC conjugate (1:100) for 45 min. Controls were stained with purified mouse IgG1 κ-isotype control antibody (BD Pharmingen). Slides were mounted with Prolong Gold Antifade reagent (Invitrogen, Carlsbad, CA). Images were taken with a Zeiss LSM 510 confocal microscope (Carl Zeiss Micro Imaging, Thornwood, NY).
Immunocytochemistry
Cells were cultured overnight and coverslips were washed, fixed in 4% paraformaldehyde (PF) and cold methanol, and incubated with mouse anti-rat CD31 antibody (1:50), or rabbit anti-rat vWF antibody (1:50) for 2 h at room temperature. Coverslips were washed in PBS and incubated with either donkey anti-mouse IgG, PE conjugated (1:100), or donkey anti-rabbit IgG, rhodamine conjugated (1:50), for 45 min at room temperature. Controls were incubated with isotype-control IgG at the same concentration as the primary antibody. Coverslips were mounted on slides with Prolong Gold Antifade reagent (Invitrogen). Slides were examined at ×40 magnification via a Zeiss LSM 510 confocal microscope (Carl Zeiss Micro Imaging). Each condition was examined in triplicate, and values were obtained by counting the number of cells positive for CD31 or vWF in 15 randomly selected fields.
Colocalization of CD45 with WGA
CD45 staining was examined in LSEC labeled with wheat germ agglutinin (WGA) by in vivo perfusion or after in vitro labeling. Preferential uptake of WGA in periportal LSEC was confirmed by in vivo perfusion and confocal imaging. The livers were perfused through the portal vein with 10 ml tetramethylrhodamine isothiocyanate (TRITC)-conjugated lectin from Triticum vulgaris (10 μg/ml) at 2 ml/min. The livers were then cut into pieces and fixed by 4% PF, 4% sucrose solution followed by immersion in 30% sucrose overnight. The fixed livers were snap frozen in liquid nitrogen, cut into 10 μm sections, and mounted with Prolong Gold Antifade reagent (Invitrogen). Images were obtained at ×20 magnification via a Zeiss LSM 510 confocal microscope (Carl Zeiss Micro Imaging).
In vivo labeling.
One million LSEC, isolated from WGA-TRITC-perfused liver as described above, were incubated with FITC mouse anti-rat CD45 (1:50) at 4°C for 30 min.
In vitro labeling.
One million LSEC isolated from non-WGA-perfused rat liver were incubated with WGA-TRITC (10 μg/ml) at 37°C for 10 min followed by FITC-conjugated mouse anti-rat CD45 (1:50) at 4°C for 30 min.
Flow cytometry.
After gating for viable cells, expression of CD45 and uptake of WGA was analyzed on channels FL1 and FL2 using the FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). In some experiments, forward scatter was also analyzed; 20,000 cells were analyzed per sample.
Scanning Electron Microscopy
LSEC cultured on collagen-coated Thermanox coverslips (Thermo Fisher Scientific, Rochester, NY) were fixed with 2% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) in 0.1 M cacodylate buffer, PH 7.4 for 30 min. After washing, LSEC were treated with 1% tannic acid (Electron Microscopy Sciences) in 0.15 M cacodylate buffer for 1 h, postfixed with 1% osmium tetroxide (Ted Pella, Redding, CA) in 0.1 M cacodylate buffer for 30 min, dehydrated with graded alcohols, dried with hexamethyldisilazane (Ted Pella), sputter-coated with 10-nm gold, and examined via a JSM-6390LV scanning electron microscope (JEOL, Tokyo, Japan).
Quantitative Imaging
Scanning electron microscopy (SEM) images of 15 randomly selected LSEC per experiment were analyzed. Fenestral diameter, number of fenestrae per sieve plate and per cell, and porosity were determined by use of MetaMorph software (version 7.0; Molecular Devices, Downingtown, PA). Total LSEC surface area and the open area of individual fenestrae were quantified in square micrometers. These open areas were summed, divided by the total areas of the LSEC surface examined, multiplied by 100 to give the porosity as percent of open area, and averaged to give a single value per group. For cell diameter, one drop of freshly isolated cells was added to a glass slide, 15 random pictures were taken at ×20 magnification by light microscopy, and the diameter was measured by use of MetaMorph software.
Endocytosis
In vivo uptake of FITC-FSA.
Normal rat livers were perfused through the portal vein with 10 ml FITC-FSA (2 μg/ml) at 2 ml/min. The livers were then cut into pieces and fixed with 4% PF-4% sucrose solution for 4 h at room temperature followed by immersion in 30% sucrose overnight at room temperature. The fixed livers were snap frozen in liquid nitrogen, cut into 10-μm sections, and mounted with Prolong Gold Antifade reagent (Invitrogen). Images were obtained with a Zeiss LSM 510 confocal microscope (Carl Zeiss Micro Imaging).
In vitro uptake of FITC-FSA.
LSEC cultured on collagen-coated coverslips overnight as indicated were washed and incubated with DMEM-low glucose with 1% FBS for 60 min. LSEC were then incubated with FITC-FSA (0.5 μg/ml or 0.02 μg/ml) in DMEM-low glucose with 1% FBS for 10 min in a 37°C incubator. After washing, LSEC were incubated with DMEM-low glucose with 1% FBS for another 60 min. LSEC were then washed in cold PBS and fixed in 4% PF (4°C) for 30 min. Coverslips were washed with deionized water and mounted with Prolong Gold Antifade reagent (Invitrogen). Images were obtained at 40× magnification using a Zeiss LSM 510 confocal microscope (Carl Zeiss Micro Imaging). Percentage of uptake of FITC-FSA by LSEC was examined in triplicate, and values were obtained by counting the number of cells with positive labeling in 15 randomly selected fields.
Drug Studies
Studies were performed in freshly cultured cells exposed overnight to acetaminophen in DMEM with 10% FBS. Cells were allowed to adhere for at least 3 h before the drug incubation. Studies for toxicity testing were performed in 96-well plates, and incubations were performed in a dark 37°C CO2 incubator overnight. Cell viability was assessed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay as previously described (6). Viability is expressed as a percentage of untreated control cells.
Statistics
Numerical data represent means ± SE from at least three separate experiments. Values were compared by two-factor analysis of variance (ANOVA) with replication by use of the Microsoft Excel Analysis ToolPak (Microsoft, Redmond, WA). If ANOVA was significant, a posteriori analysis was done with least significant difference test. Results with P < 0.05 were considered significant.
RESULTS
Real-Time PCR of CD45 Expression by LSEC
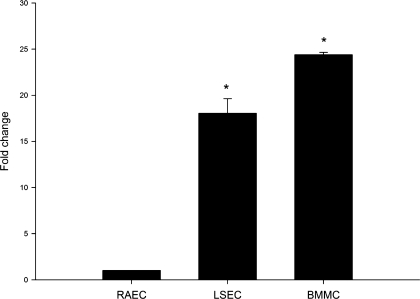
Our previous studies have shown by immunocytochemistry and immunohistochemistry that LSEC express CD45 (15). One possibility is that surface expression of CD45 is due to endocytosis of cell fragments containing CD45 and trogocytosis rather than due to endogenous expression of CD45 by LSEC. Trogocytosis is a phenomenon in which the cell extracts surface molecules from another cell and then expresses these antigens on its own cell surface (17); this process was first described in leukocytes and was subsequently recognized in various cell types including endothelial cells (14). To rule out that surface expression was simply due to trogocytosis, gene expression of CD45 was examined in LSEC. CD45 mRNA was examined in LSEC, RAEC and BMMC by reverse transcriptional PCR. There was almost no expression of CD45 in RAEC, but marked expression was found in both LSEC and BMMC (data not shown). CD45 levels were then compared in the three groups by real-time PCR, which showed a nearly 18- and 25- fold increase of CD45 mRNA level in LSEC and BMMC, respectively, compared with RAEC (Fig. 1).
Fig. 1.
Real-time PCR of CD45 expression in isolated liver sinusoidal endothelial cells (LSEC). CD45 mRNA expression in LSEC isolated from normal rat liver was quantified by real-time PCR. Rat bone marrow mononuclear cells (BMMC) were used as a positive control and rat aortic endothelial cells (RAEC) were used as a negative control. CD45 mRNA levels are significantly higher in LSEC and BMMC than RAEC (n = 4; P < 0.0001 for comparison of the 3 cell types by ANOVA; *P < 0.001 for comparison of LSEC and BMMC vs. RAEC by least significant difference test).
Isolation of LSEC Subpopulations Based on CD45 Expression Level
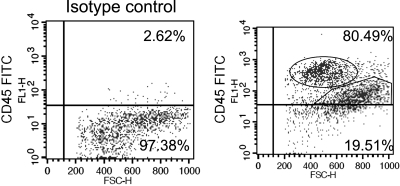
By flow cytometry 78.2 ± 2.3% of LSEC were CD45 positive by using cells stained with isotype-control antibody as control for gaiting (Fig. 2). LSEC could be divided into CD45 bright, CD45 dim, and CD45 negative populations (Fig. 2, Table 1). The CD45 bright population has smaller forward scatter (a parameter related to the cell size) than CD45 dim and CD45 negative populations (Fig. 2). Cells from the three subpopulations were also isolated by autoMACS and cell diameter was measured by light microscopy (Table 1), which confirmed the observation by flow cytometry that cell size differs between the three LSEC subpopulations. The increase in size of LSEC with decreased expression of CD45 in the three subpopulations is consistent with subpopulations that are periportal (CD45 bright), midlobular (CD45 dim), and centrilobular (CD45 negative) in origin.
Fig. 2.
Flow cytometry of CD45 expression in isolated LSEC. LSEC isolated from normal rat liver were stained with isotype control antibody (left) or CD45-FITC (right) and examined by flow cytometry. Scatter plots are gated on CD45 and are representative of 4 independent experiments. The percentage of LSEC expressing CD45 is shown in each plot. Three populations could be observed in the right plot: CD45 bright (oval in top right), CD45 dim (polygon in top right), and CD45 negative (bottom right).
Table 1.
Characterization of 3 LSEC subpopulations
| CD45 Bright (Periportal) | CD45 Dim (Midlobular) | CD45 Negative (Centrilobular) | |
|---|---|---|---|
| Percentage of total cells | 28.6 ± 2.7% | 49.6 ± 1.0% | 21.8 ± 2.3% |
| Yield of cells per liver (106) | 18.67 ± 2.54 | 35.42 ± 3.12 | 16.46 ± 2.06 |
| Cell diameter, μm | 6.0 ± 0.1 | 6.8 ± 0.0* | 7.1 ± 0.2* |
| Percentage of CD31-positive cells | 66.5 ± 1.2% | 90.5 ± 2.7%* | 92.0 ± 2.1%* |
| Percentage of vWF-positive cells | 11.5 ± 0.5% | 8.9 ± 1.8% | 15.1 ± 0.7% |
Three liver sinusoidal endothelial cell (LSEC) subpopulations were isolated as indicated. Flow cytometry of CD45 expression by unselected LSEC was used to determine the percentage of each population and light microscopy was used to measure the size of the cells in the 3 subpopulations. The percentage of CD31- and von Willebrand factor (vWF)-positive cells was determined as described in materials and methods. Values are means ± SE (n = 3). P < 0.0001 for comparison of cell diameter of the 3 subpopulations by ANOVA; P < 0.0005 for comparison of percentage of CD31-positive cells of the 3 subpopulations by ANOVA;
P < 0.001 for comparison of CD45 dim and negative versus CD45 bright by least significant difference test.
In Vivo CD45 Expression
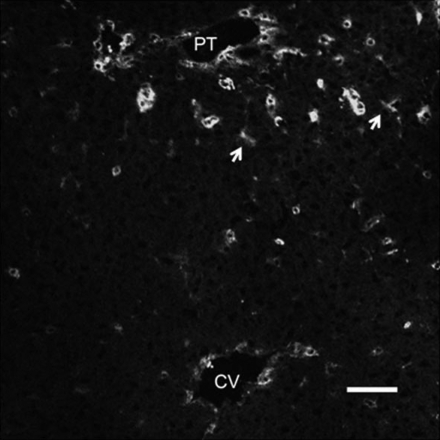
LSEC express CD45 in vivo (12) and CD45 staining colocalizes with CD31 staining. On immunohistochemistry of control liver sections, CD45 showed a lobular distribution (Fig. 3) with greater staining in the periportal than in the centrilobular regions.
Fig. 3.
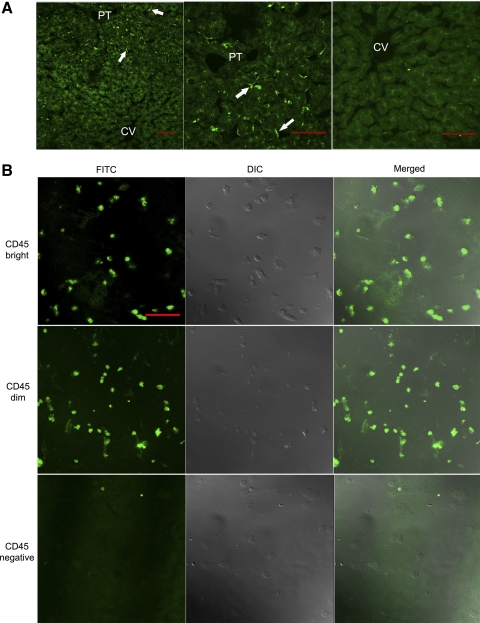
Immunohistochemistry of control rat liver stained for CD45. Frozen rat liver section was stained for CD45. Continuous staining was observed (arrow) along the sinusoids around the portal triad (PT) with no stain observed along the sinusoids around the central vein (CV). Scale bar: 50 μm.
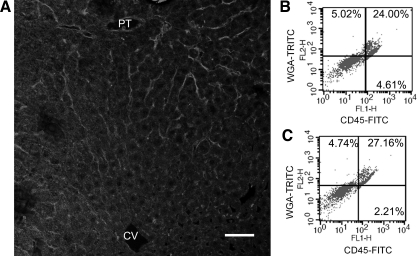
CD45 Bright LSEC Colocalize with WGA-Positive LSEC
WGA is a lectin that binds preferentially to mouse periportal LSEC (3) and that localized to rat periportal LSEC by immunohistochemistry (Fig. 4A). LSEC that took up WGA, after either in vivo perfusion or in vitro labeling, were stained CD45 bright (Fig. 4, B and C), confirming that CD45 bright cells are periportal LSEC.
Fig. 4.
Colocalization of wheat germ agglutinin (WGA) uptake with CD45 bright LSEC. A: livers were perfused with WGA- tetramethylrhodamine isothiocyanate (TRITC) and examined under confocal microscopy. Positive staining of LSEC, manifested as a continuous line along the sinusoids, was observed in the sinusoids around the PT with no staining observed in the sinusoids around the CV. Scale bar: 50 μm. B: LSEC isolated from WGA-TRITC-perfused rat liver were incubated in vitro with CD45-FITC and examined by flow cytometry. C: LSEC isolated from non-WGA-perfused rat liver were incubated in vitro with WGA-TRITC and CD45-FITC and examined by flow cytometry. Plots are gated on CD45 and WGA, and the percentage of cells positive for WGA only, for both CD45 and WGA, and for CD45 only are shown in top left, top right, and bottom right, respectively. The examples shown are representative of 3 independent experiments.
CD31 and vWF Expression
The three LSEC subpopulations were cultured overnight and stained for CD31 or vWF. Fewer CD45 bright LSEC expressed CD31 than the CD45 dim and negative populations (Table 1). vWF expression was similar in the three LSEC subpopulations (Table 1) and the percentage LSEC positive for vWF was consistent with previous findings (9).
CD45 Bright, Dim, and Negative LSEC Have Different Fenestral Patterns
Periportal and centrilobular LSEC differ in size of fenestrae, number of fenestrae per sieve plate and per cell, and porosity (24, 25, 28). The three LSEC subpopulations were cultured overnight and examined by SEM for these four parameters (Fig. 5). Quantification showed that CD45 bright LSEC have larger fenestrae, fewer fenestrae per sieve plate and per cell, and lower porosity than CD45 dim and negative populations (Table 2). CD45 dim LSEC have significantly different porosity than CD45 negative LSEC (Table 2). This is consistent with a separation into periportal, midlobular, and centrilobular LSEC.
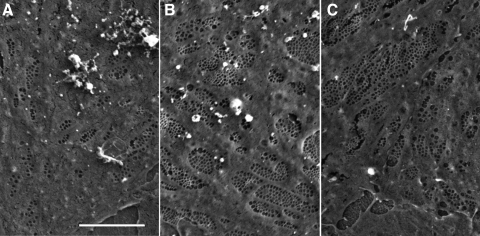
Fig. 5.
Scanning electron microscopy (SEM). LSEC were isolated and separated into CD45 bright, dim, and negative subpopulations as described in materials and methods. Cells were cultured overnight, processed, and examined by SEM at ×5,000 magnification. Note that the cells in the CD45 bright population (A) have fewer fenestrae than the cells in the CD45 dim (B) and the CD45 negative (C) populations. Scale bar: 5 μm.
Table 2.
Characterization of fenestration of 3 subpopulations of LSEC
| CD45 Bright (Periportal) | CD45 Dim (Midlobular) | CD45 Negative (Centrilobular) | |
|---|---|---|---|
| Diameter of fenestrae, nma | 155.4 ± 2.7 | 127.7 ± 2.4d | 118.0 ± 2.1e |
| No. of fenestrae per sieve plateb | 8.5 ± 0.6 | 23.1 ± 2.0e | 24.5 ± 1.8e |
| No. of fenestrae per cellb | 306.8 ± 36.8 | 731.3 ± 89.5d | 832.3 ± 56.9e |
| Porosityc | 2.8 ± 0.3% | 4.5 ± 0.3%e | 5.7 ± 0.3%e,f |
Three subpopulations of LSEC were isolated as indicated. Cells were cultured overnight, processed and examined by scanning electron microscopy at ×5,000 magnification. Data are mean ± SE from analysis of 15 images from each experiment (n = 3 for each group). Comparison of the 3 subpopulations by ANOVA:
P < 0.005,
P < 0.00001,
P < 0.0001,
P < 0.005;
P < 0.001 by least significant difference test compared with CD45 bright population,
P < 0.01 by least significant difference test compared with CD45 dim population.
Endocytosis
Endocytosis is a key functional characteristic of LSEC. Preferential uptake of FITC-FSA in periportal LSEC was confirmed by in vivo perfusion at low concentration and confocal imaging (Fig. 6A). The three subpopulations of LSEC were isolated, cultured overnight, and then exposed to FITC-FSA 0.5 or 0.02 μg/ml for 10 min. Uptake of FITC-FSA, 0.5 μg/ml, was ∼99% in the three subpopulations (data not shown). At a concentration of 0.02 μg/ml, there was no uptake of FITC-FSA in CD45 negative population whereas 86.9 ± 2.4% of CD45 bright and 84.0 ± 3.9% of CD45 dim LSEC took up FITC-FSA (Fig. 6B).
Fig. 6.
Uptake of FITC-labeled formaldehyde-treated serum albumin (FITC-FSA) by LSEC. A: livers were perfused with FITC-FSA and examined by confocal microscopy. Left, ×20 magnification; middle and right, ×40 magnification of the same field as left. Uptake of FITC-FSA (arrow) was observed in the sinusoids around the PT with no uptake was observed in the sinusoids around the CV. Scale bar: 50 μm. B: CD45 bright, dim, and negative populations were exposed to 0.02 μg/ml FITC-FSA as described in materials and methods. Uptake of FITC-FSA was observed in both CD45 bright and dim populations, whereas no uptake was observed in the CD45 negative population. DIC, differential interference contrast. Scale bar: 50 μm.
Acetaminophen Toxicity
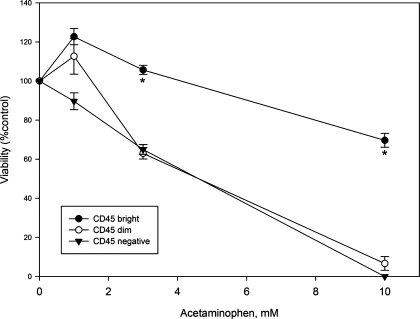
Acetaminophen is toxic to LSEC (11, 16, 26, 27) and causes centrilobular hemorrhagic necrosis. The three subpopulations of LSEC were isolated and exposed to acetaminophen, 1, 3, and 10 mM, overnight. Acetaminophen was significantly more toxic to CD45 dim and negative LSEC than to CD45 bright LSEC (Fig. 7).
Fig. 7.
Toxicity of acetaminophen to CD45 bright (periportal), CD45 dim (midlobular), and CD45 negative (centrilobular) LSEC. CD45 bright (●), CD45 dim (○), and CD45 negative (▾) LSEC were exposed to acetaminophen, 1, 3, and 10 mM, overnight as described in materials and methods. Acetaminophen is significantly more toxic to CD45 dim and CD45 negative cells than to CD45 bright cells (n = 3; P < 0.0001 for comparison of the 3 subpopulations by ANOVA; *P < 0.001 for comparison of CD45 dim and negative vs. CD45 bright at 3 and 10 mM by least significant difference test).
DISCUSSION
The present study demonstrates high CD45 gene expression in LSEC, establishes and validates a simple method to separate LSEC into periportal, midlobular, and centrilobular subpopulations on the basis of CD45 expression level, and demonstrates that these subpopulations can be used to study zonated LSEC-dependent processes. The three groups selected by CD45 expression were identified on the basis of differences in size of cell, size of fenestrae, numbers of fenestrae per sieve plate and per cell, porosity, uptake of WGA, and endocytosis, which were previously reported to be different in periportal and centrilobular LSEC (3, 24, 25, 29). The cell sizes and fenestral findings differ quantitatively from previous reports, since the cells under study were isolated cells that had been cultured overnight and previous descriptions examined LSEC in liver sections.
CD45 is a transmembrane protein tyrosine phosphatase usually expressed on leukocytes. The present study confirms the endogenous expression of CD45 in LSEC, which suggests that LSEC may be of bone marrow origin. Immunohistochemistry also demonstrates that CD45 expression in LSEC has a lobular gradient distribution. CD45 is highly expressed in periportal LSEC, low in midlobular LSEC, and negative in centrilobular LSEC. The method described is novel in that it isolates cells from the three regions of the lobule, obtained from a single liver and adequate for functional studies. The morphological characteristics of the three subpopulations change as a continuum, which suggests that the phenotypic differences might be due to either maturation of the LSEC as they stream across the lobule or to the effect of oxygen tension on LSEC phenotype.
The present study confirms that periportal and midlobular LSEC are CD45 positive. Some of the more recently developed protocols to isolate LSEC begin with exclusion of CD45+ cells to exclude Kupffer cells, but the present finding suggests that these approaches will yield a subpopulation of centrilobular LSEC.
In the present study, the CD45 dim and CD45 negative populations are similar in size, CD31 and vWF expression, and susceptibility to acetaminophen toxicity. However, the CD45 dim population has lower porosity and higher endocytic activity than CD45 negative populations, demonstrating that the CD45 dim and negative are distinct populations.
It is well established that acetaminophen is toxic to LSEC in vitro (11, 16), although the contribution of LSEC injury to in vivo liver injury still requires exploration. The experiments with acetaminophen presented here are meant as proof of principle that the cells obtained by CD45 immunomagnetic sorting are healthy enough to perform drug toxicity studies. Acetaminophen-induced liver injury causes centrilobular hemorrhagic necrosis. Consistent with this, the findings here demonstrate that acetaminophen is indeed more toxic to LSEC in the midlobular and centrilobular region of the liver, consistent with a role for LSEC in determining the centrilobular localization of acetaminophen-induced liver injury. One of the important questions that can be answered by separating LSEC into subpopulations is whether lobular differences in CYP2E1 and glutathione turnover in LSEC determine the zonation of injury. In some mouse strains acetaminophen is P450 activated by LSEC, whereas in other mouse strains the LSEC is simply a target of acetaminophen metabolically activated by neighboring hepatocytes (11). The present experiments demonstrate that acetaminophen is metabolically activated by rat LSEC rather than requiring P450 activation by hepatocytes.
In summary, the present study describes and validates a simple method to isolate periportal, midlobular, and periportal LSEC. The cells isolated by this method show lobular differences in endocytosis and susceptibility to acetaminophen injury. This method will permit future studies of zonated, LSEC-dependent processes.
GRANTS
This work was supported by NIH grant DK66423 and DK46357, by the Histology and Microscopy subcore of the USC Research Center for Liver Diseases (P30DK048522), and the Non-parenchymal Liver Cell subcore of the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases and Cirrhosis (R24AA012885).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors gratefully acknowledge Michelle MacVeigh-Aloni for invaluable assistance with immunostaining and confocal microscopy.
REFERENCES
- 1.Aarnoudse JG, Houthoff HJ, Weits J, Vellenga E, Huisjes HJ. A syndrome of liver damage and intravascular coagulation in the last trimester of normotensive pregnancy. A clinical and histopathological study. Br J Obstet Gynaecol 93: 145–155, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Asumendi A, Alvarez A, Martinez I, Smedsrød B, Vidal-Vanaclocha F. Hepatic sinusoidal endothelium heterogeneity with respect to mannose receptor activity is interleukin-1 dependent. Hepatology 23: 1521–1529, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Barberá-Guillem E, Rocha M, Alvarez A, Vidal-Vanaclocha F. Differences in the lectin-binding patterns of the periportal and perivenous endothelial domains in the liver sinusoids. Hepatology 14: 131–139, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Caldwell-Kenkel C, Thurman RG, Lemasters JJ. Selective loss of nonparenchymal cell viability after cold ischemic storage of rat livers. Transplantation 45: 834–837, 1988 [PubMed] [Google Scholar]
- 5.Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. Hepatology 10: 292–299, 1989 [DOI] [PubMed] [Google Scholar]
- 6.DeLeve LD. Dacarbazine toxicity in murine liver cells: a novel model of hepatic endothelial injury and glutathione defense. J Pharmacol Exp Ther 268: 1261–1270, 1994 [PubMed] [Google Scholar]
- 7.DeLeve LD, Ito I, Bethea NW, McCuskey MK, Wang X, McCuskey RS. Embolization by sinusoidal lining cell obstructs the microcirculation in rat sinusoidal obstruction syndrome. Am J Physiol Gastrointest Liver Physiol 284: G1045–G1052, 2003 [DOI] [PubMed] [Google Scholar]
- 8.DeLeve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 48: 920–930, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is under paracrine and autocrine control. Am J Physiol Gastrointest Liver Physiol 287: G757–G763, 2004 [DOI] [PubMed] [Google Scholar]
- 10.DeLeve LD, Wang X, Kanel GC, Tokes ZA, Tsai J, Ito Y, Bethea NW, McCuskey MK, McCuskey RS. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology 38: 900–908, 2003 [DOI] [PubMed] [Google Scholar]
- 11.DeLeve LD, Wang X, Kaplowitz N, Shulman HM, Bart JA, van der Hoek A. Sinusoidal endothelial cells as a target for acetaminophen toxicity: direct action versus requirement for hepatocyte activation in different mouse strains. Biochem Pharmacol 53: 1339–1345, 1997 [DOI] [PubMed] [Google Scholar]
- 12.DeLeve LD, Wang X, McCuskey MK, McCuskey RS. Rat liver endothelial cells isolated by anti-CD31 immunomagnetic sorting lack fenestrae and sieve plates. Am J Physiol Gastrointest Liver Physiol 291: G1187–G1189, 2006 [DOI] [PubMed] [Google Scholar]
- 13.DeLeve LD, Wang X, Tsai J, Kanel GC, Strasberg SM, Tokes ZA. Prevention of sinusoidal obstruction syndrome (hepatic venoocclusive disease) in the rat by matrix metalloproteinase inhibitors. Gastroenterology 125: 882–890, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Grau GER. Importance of microvesiculation in the immunopathology of cerebral malaria (Abstract). BMC Proc 2, Suppl 1: S19, 2008 [Google Scholar]
- 15.Harb R, Xie G, Lutzko C, Guo Y, Wang X, Hill C, Kanel G, DeLeve LD. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology 137: 704–712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito Y, Bethea NW, Abril ER, McCuskey RS. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation 10: 391–400, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol 4: 815, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto Y, McCaughan GW, Painter DM, Bishop GA. Evidence that portal tract microvascular destruction precedes bile duct loss in human liver allograft rejection. Transplantation 56: 69–75, 1993 [DOI] [PubMed] [Google Scholar]
- 19.McCloskey TW, Todaro JA, Laskin DL. Lipopolysaccharide treatment of rats alters antigen expression and oxidative metabolism in hepatic macrophages and endothelial cells. Hepatology 16: 191–203, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Pritchard DJ, Butler WH. Apoptosis—the mechanism of cell death in dimethylnitrosamine-induced hepatotoxicity. J Pathol 158: 253–260, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Rocha M, Lentini A, Asumendi A, Falasca L, Autuori F, Dini L, Vidal-Vanaclocha F. In situ and in vitro correlation between mannose receptor expression and fenestration pattern in endothelial cells selected from different zones of liver lobule. In: Cells of the Hepatic Sinusoids, edited by Knook DL, Wisse E. Leiden, The Netherlands: The Kupffer Cell Foundation, 1993, p. 470–473 [Google Scholar]
- 22.Scoazec JY, Racine L, Couvelard A, Flejou JF, Feldmann G. Endothelial cell heterogeneity in the normal human liver acinus: in situ immunohistochemical demonstration. Liver 14: 113–123, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 12: 642–649, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Vidal-Vanaclocha F, Barbera-Guillem E. Fenestration patterns in endothelial cell of rat liver sinusoids. J Ultrastruct Res 90: 115–123, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Wack KE, Ross MA, Zegarra V, Sysko LR, Watkins SC, Stolz DB. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology 33: 363–378, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Walker RM, Racz WJ, McElligott TF. Acetaminophen-induced hepatotoxic congestion in mice. Hepatology 5: 233–240, 1985 [DOI] [PubMed] [Google Scholar]
- 27.Walker RM, Racz WJ, McElligott TF. Scanning electron microscopic examination of acetaminophen-induced hepatotoxicity and congestion in mice. Am J Pathol 113: 321–330, 1983 [PMC free article] [PubMed] [Google Scholar]
- 28.Wisse E. An ultrastructural characterization of the endothelial cell in the rat liver sinusoid under normal and various experimental conditions, as a contribution to the distinction between endothelial and Kupffer cells. J Ultrastruct Res 38: 528–562, 1972 [DOI] [PubMed] [Google Scholar]
- 29.Wisse E, De Zanger RB, Charels K, van der Smissen P, McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestra, the sinusoidal wall and the space of Disse. Hepatology 5: 683–692, 1985 [DOI] [PubMed] [Google Scholar]
- 30.Xie G, Kanel GC, DeLeve LD. cGMP Signaling regulates liver sinusoidal endothelial cell (SEC) phenotype and accelerates reversal of cirrhosis (Abstract). Gastroenterology 138: S773, 2010 [Google Scholar]