Abstract
Although tumors express potentially immunogenic tumor-associated antigens (TAAs), cancer vaccines often fail because of inadequate antigen delivery and/or insufficient activation of innate immunity. The engineering of non-pathogenic bacterial vectors to deliver TAAs of choice may provide an efficient way of presenting TAAs in an immunogenic form. In this study, we used genes of Salmonella Pathogenicity Island 2 (SPI2) to construct a novel cancer vaccine, where a TAA, survivin was fused to SseF effector protein and placed under control of SsrB, the central regulator of SPI2 gene expression. This construct uses the type III secretion system (T3SS) of Salmonella and allows preferential delivery of tumor antigen into the cytosol of antigen-presenting cells for optimal immunogenicity. In a screen of a panel of attenuated strains of Salmonella we found that a double-attenuated strain of Salmonella typhimurium, MvP728 (purD/htrA) was not toxic to mice and effectively expressed and translocated survivin protein inside cytosol of murine macrophages. We also found that a ligand for CD1d-reactive Natural Killer T (NKT) cells, α-Glucuronosylceramide (GSL1) enhanced MvP728-induced IL-12 production in human DCs and that in vivo co-administration of a NKT ligand with MvP728-Llo or MvP728-survivin enhanced effector-memory CTL responses. Furthermore, combined use of MvP728-survivin with GSL1 produced anti-tumor activity in mouse models of CT26 colon carcinoma and orthotopic DBT glioblastoma. Therefore, the use of TAA delivery via SPI-2-regulated T3SS of Salmonella and NKT ligands as adjuvants may provide a foundation for new cancer vaccines.
Keywords: Cancer vaccine, Salmonella, Salmonella Pathogenicity Island 2 (SPI2), Natural Killer T (NKT) cells, Survivin
Introduction
One of the major reasons for tumor escape from immune control and for the failure of vaccination with tumor-associated antigens (TAA) is insufficient or defective activation of innate immunity that in turn prevents generation and/or execution of protective adaptive responses. The accumulating evidence suggests that an effective innate stimulation during vaccination can break tolerance to TAAs.1
Live attenuated Salmonella has been used in experimental cancer vaccines to deliver either TAA DNA (under control of eukaryotic promoters) or protein. Salmonella-based DNA vaccines conferred protection against various viral and other intracellular pathogens when they were co-delivered with immunostimulatory cytokines/chemokines or tumor-targeting immunocytokines.2 However, the low rate of somatic gene transfer inherent to DNA vaccines limits their therapeutic potential.3 Several approaches have been developed to deliver whole antigenic proteins or their immunogenic fragments.4 However, the intracellular location of Salmonella within the Salmonella-containing vacuole, which is linked to the endosomal compartment, routes antigens for the HLA class-II presentation and restricts their access to the cytosol and, therefore, to the HLA class-I presentation pathway.5 Moreover, Salmonella evolved yej gene, which product interferes with the MHC class I presentation, and yej mutants have been used in the design of Salmonella-based cancer vaccines as one of the approaches to enhance CTL generation to heterologous antigens.6,7 Alternatively, Salmonella carrier was designed to co-deliver a tumor antigen and CCL21, a potent chemoattractant both for mouse dendritic cells (DCs) and naive T cells that resulted in enhanced CD8 T cell generation and anti-tumor efficacy of the vaccine.8
The use of type III secretion system (T3SS) of Salmonella allows targeted delivery of antigenic proteins to the HLA class-I compartment of the antigen presenting cells (APC).9 For T3SS-mediated translocation of proteins, Salmonella has developed a specialized organelle known as the needle complex.10 The needle complex can form a pore in the mammalian cell membrane, through which a set of effector proteins pass to the cell cytosol.11 Some of these effector proteins or their secretion signals have been used in experimental vaccines to direct heterologous proteins expressed in Salmonella for secretion via T3SS. Such vaccines have been shown to be very effective in eliciting both CD8 and CD4 T cell-mediated immune responses in models of infectious diseases and in one model of cancer.12–14 One disadvantage of the expression systems used in these studies was that the antigen was constitutively expressed in Salmonella and could be transported inside any cell that was in contact with the bacteria. In contrast, some T3SS effector proteins that are encoded by the Salmonella Pathogenicity Island 2 (SPI2), are only expressed when Salmonella is inside host cells, preferentially DCs and macrophages.15–17 The efficacy and the safety of SPI2-T3SS-based Salmonella vaccines have recently been demonstrated in a murine model of listeriosis.18 However, SPI2-based regulated antigen delivery system has not been tested as a platform for cancer vaccines.
CD1d-restricted Natural Killer T (NKTs) cells play a critical role in bridging innate and adaptive immune responses and may be recruited for effective immunotherapy of cancer 19,20. Several recent studies indicate that NKTs may have been selected in evolution primarily for their role in antimicrobial defense.21–23 It has been demonstrated that NKTs are required for host protection from some forms of Gram-negative bacteria such as Sphingomonas spp.22 or Borrelia spp.24 that do not contain LPS and lipid A in their bacterial envelope. Instead of LPS, these microbes contain activating ligands for NKTs such as α-glucuronosylceramide (GSL1).22 Although NKTs are not required for host protection from LPS-positive bacteria including Salmonella,25 NKTs are activated during Salmonella infection by IL-12 and IL-18 produced by LPS-activated DCs25,26 and, possibly, by an endogenous ligand isoglobotrihexosylceramide (iGb3).27,28 Importantly, simultaneous stimulation of immature DCs by LPS or killed Salmonella with ligand-activated NKTs produced a synergistic effect on DC activation and maturation.29 Therefore, we hypothesized that NKT ligands may serve as adjuvants for Salmonella-based cancer vaccines by triggering CD1d- and TLR-mediated programs in DCs and inducing effector responses by NKT, NK, and T cells.
In this study, we have constructed a novel SPI2-based oral Salmonella vaccine against survivin and tested the vaccine immunogenicity and anti-tumor efficacy alone and in combination with a NKT cell ligand as an adjuvant.
Material and Methods
Cell lines, clones, gene transduction
Murine CD1d-negative CT26 colon carcinoma and DBT glioblastoma cell lines were obtained from Dr. Terabe (NIH/NCI) and Dr. Stohlman (USC, Los Angeles, CA), respectively. J774 macrophage, B16 melanoma, and GL261 glioblastoma cell lines were from Dr. Alan Epstein (USC). Cell lines were maintained in RPMI1640 medium that contained 2 mM L-glutamine and 10% heat inactivated fetal calf serum. To generate DBT/luc cells, we transduced DBT cells with firefly luciferase cDNA using a ViraPower™ T-REx™ lentiviral expression system (Invitrogen) as described.30
Strains of Salmonella typhimurium, plasmids, and constructs
Bacterial strains used in this study are listed in Supplemental Table 1. SL7207 (aroA) has been previously described31 and was kindly provided by Dr. B. Stocker. All other strains were isogenic to wild-type strain Salmonella enterica serovar Typhimurium (S. typhimurium) NCTC 12023. Virulence of the wild-type strain was attenuated by single deletions in strains MvP467 (htrA), MvP664 (galE) and MvP679 (galE), or by double mutations in strains MvP728 (purD/htrA), MvP729 (galE/htrA), or MvP740 (purD/galE). For construction of mutant strains, the 'One Step Deletion' approach was used to replace target genes by the aph resistance cassette.32 If required, the aph cassettes were removed by FLP-mediated recombination. Mutagenesis was repeated for a second locus to generate double mutant strains. The resulting strains were confirmed by PCR reactions specific for the deleted loci and growth characteristics in case of auxotrophic mutant strains. Bacteria were cultured in LB broth and on LB agar plates. If required for the selection of recombinants or to maintain plasmids, ampicillin (100 µg/ml), kanamycin and/or carbenicillin (50 µg/ml) was added. Synthetic minimal medium with limiting (PCN-P media) or non-limiting (PCN media) amounts of phosphate was used for the analyses of promoters under control of the SsrB regulatory system in vitro as described.33 Synthetic medium was supplemented with 1 mM adenine for growth of the auxotrophic purD strain.
Plasmid p2810 for the SPI2-regulated expression of a sseF∷lisA∷HA fusion has been described before18 For the construction of a translocated fusion protein with Survivin, the human Survivin gene was amplified from plasmid pORF5-hSurvivin (InvivoGen, San Diego) using oligonucleotides hSurvivin-For-EcoRV (5'- tacGATATCGGTGCCCCGACGTTGCCCCC-3') and hSurvivin-HA-Rev-XbaI (5'-atttctagattaagcgtagtctgggacgtcgtatgggtaATCCATAGCAGCCAGCTGCTC-3'. The resulting DNA fragment was digested by EcoRV and XbaI and subcloned in EcoRV/XbaI-digested p2810. The resulting plasmid p3342 was confirmed by DNA sequencing and expression resulted in the synthesis of a HA-tagged SseF-hSurvivin fusion protein of the expected molecular weight.
Human dendritic and NKT cells
Human PBMC were purchased from Astarte Biologics. Monocytes were isolated by negative selection using Dynal Monocyte Negative Isolation Kit (Invitrogen) according to the manufacturer’s instructions. Immature dendritic cells were generated in 5-day monocyte culture with GM-CSF and IL-4 as previously described.30 The cell phenotype was analyzed by three-color flow cytometry using FITC-anti-CD86 2331, PE-anti-CD80 L307.4 mAbs (BD Biosciences) and DAPI for dead cell exclusion (Invitrogen). IL-12 production was measured with human IL-12 CBAPlex (BD Biosciences) according to manufacture’s instructions. Human NKT cells were from previously described human NKT cell lines.34 They were established from PBMC of healthy adults by culture in the presence of αGalactosylceramide (αGalCer, 100 ng/ml, Kirin Brewery Co, Japan) and IL-2. Expanded NKT cells are positively sorted using 6B11 PE-anti-Vα24Jα18 mAb (BD Biosinces) and anti-PE magnetic beads (Miltenyi Biotec, Auburn, CA) and maintained by periodic re-stimulation with αGalCer-pusled C1R/CD1d antigen-presenting cells and irradiated allogeneic PBMC as feeder cells. NKT cells used in this study were >99% CD3+Vα24Jα18+ cells, 40–50% CD4+ and 50–60% CD4/CD8-double negative (data not shown).
NKT ligand
α-Glucuronosylceramide (GSL1), a close analogue αGalCer was synthesized by Dr. Paul Savage’s lab35 and obtained from NIH Tetramer Facility (Germantown, MD) as a water-soluble powder. For in vivo experiments, GSL1 was injected i/p at 2 µg/mouse.
Immunofluorescent Microscopy
Salmonella strains were grown to stationary phase in LB with the appropriate antibiotic selection. The OD600 of the cultures was adjusted with LB to 0.2 and the bacteria were washed once with PBS, diluted in cell culture medium and added to the cells growing in 4-well tissue culture slides at a multiplicity of infection (MOI) of about 10. After infection for 25 min at 37°C in 5% CO2, the cells were washed three times with PBS and incubated for 1 h in cell culture medium containing 100 µg/ml gentamicin (Sigma). The medium was replaced with fresh medium containing 10 µg/ml gentamicin for the remainder of the experiment. The analysis of protein translocation was carried 16 h after infection. For immuno-fluorescence analysis, the cells were incubated in 4-well tissue culture slides. After infection and incubation for indicated time period, the cells were fixed with BD Cyofix/Cytoperm Fixation /Permeabilization buffer for 20 min at room temperature and then washed thee times with BD Cyofix/Cytoperm washing buffer. The antibodies were diluted in a BD Cyofix/Cytoperm washing buffer. The culture slides were incubated with various antibodies as detailed below and washed three times with BD Cyofix/Cytoperm washing buffer after each incubation step. If not otherwise stated, all antibody incubation steps were performed for 1 h at room temperature. The following antisera and antibodies were used at the indicated dilutions: mouse anti-LPS of S. typhimurium O-4 (1E6) conjugated with FITC (Santa Cruz, 1:200); rabbit anti-HA tag (Sigma, 1:200). For detection, sheep anti-rabbit Cy3-conjugate (Sigma, 1:500) was used. The culture slides were mounted on ProLong®Gold antifade reagent (Invitrogen) and sealed with Nail polish (Electron Microscopy Sciences). Fluorescent images were acquired on a Zeiss Axiovert 200 M with 100X Plan Neofluar objective with Zeiss filter sets: 3035B(GFP), and 4040B(Cy3) using IP Lab 4.0 software (Scanalytics, Inc.).
Multiparameter flow cytometry
Blood samples were collected 10, 21 and 36 days after immunization. After erythrocyte lysis with ammonium chloride solution, cells were stained with PE-labeled H-2Kd/LLO91–99 (GYKDGNEYI) Pro5 pentamer and rat anti-mouse CD8-FITC KT15 mAb (Proimmune). To identify CD8 T cell memory cells, cells were stained with APC-Cy7-anti-CD8 53-6.7, APC-anti-CD62L MEL-14 and FITC-anti-CD44 IM7 mAbs (BD Biosciences). To measure survivin-specific T cell response, isolated splenocytes were pulsed for 6 h with a mix of 18-mer overlapping survivin peptide library (kindly provided by Dr. S. Gottschalk, Baylor College of Medicine), adding brefeldin-A in the last 4 h of culture. Reactivity to mouse Fcγ receptors is blocked by 5-min pre-incubation with mouse Fc-block (1:1000, BD Biosciences). Surface antigens were stained with PreCP-anti-CD3 145-2C11 mAb, FITC-anti-CD4 GK1.5 mAb, and APC-Cy7-anti-CD8 53-6.7 mAb. For intracellular immunofluorescence, cells were fixed and permeabilized with Cytofix/Cytoperm™ kit according to the manufacture’s manual followed by 30 min (4° C) incubation with APC-anti-TNFα and PE-anti-FNγ (BD Biosciences). BD-suggested fluorochrome- and isotype-matching mAbs were used as negative controls. The analysis was performed on a LSR-II flow cytometer (BD Biosciences) using BD FACDiva software v. 6.0.
Vaccination experiments
BALB/c 4–6 week old female mice were purchased from the Jackson Laboratory and maintained under standard conditions in CHLA Animal Facility and were treated according to an IACUC approved protocol. For immunization, groups of mice (8 mice per group) were orally administrated with indicated doses of various S. typhimurium carrier strains (Supplemental Table 1) in 200 µl of 5% sodium bicarbonate using gastric gavage 20G needle (Popper & Sons, Inc). When indicated, a NKT ligand (2µg per mouse) was administrated at the time of primary vaccination. For CD8 T-cell depletion, we used rat anti-mouse CD8 mAb, 2.43 (a gift from Dr. Si-Yi Chen, USC) that was i/p injected 1 day before each vaccination, 300 µg/mouse. Rat serum’s IgG (Sigma) served as a control.
Brain Tumor Implantation
DBT/luc GBM tumor cells were implanted intracranially into 6–8 week old female BALB/c mice under ketamine/xylazine anesthesia, using a stereotaxic device as previously described.36,37 Briefly, using a needle, a hole was made in the skull 2 mm lateral and 0.5 mm anterior to bregma and 105 tumor cells in 1 µl PBS were injected via a 30 gauge needle at a 3.3 mm depth from the dural surface using a Harvard Precision delivery pump over 10 minutes. The needle was retracted slowly (10 minute) and the skull was closed with bone-wax. Tumor growth was assessed by bioluminescence imaging using the cooled IVIS® animal imaging system (Caliper Life Sciences, Hopkinton, MA) linked to a PC running the Living Image™ software along with IGOR (Wavemetrics, Seattle, WA) following intraperitoneal injection of luciferin (150 mg/kg, potassium salt), as described.37 A gray scale photograph of the mice was first collected in the imaging box under low-level LED illumination, followed by acquisition and overlay of the pseudocolor-scaled luminescent image. Following image acquisition, regions of interest were drawn around the heads of the animals and quantification of light output was obtained using the system software and expressed in units of photons per second per square centimeter per steradian (photons/sec/cm2/sr). Region sizes were the same in all animals. Mice were examined daily for signs of distress and euthanized when these were observed.
Cytotoxicity Assay
Splenocytes were isolated from vaccinated or control mice and in vitro re-stimulated with a peptide mix from human survivin library followed by 7-day culture in the presence of 50 U/ml IL-2. CD8 T cells were isolated by negative selection using CD8a T cell isolation kit (Miltenyi Biotec, Auburn, CA). Cytotoxicity against tumor cells was assessed by a Calcein-AM assay as previously described.38,39 Tumor cells were pre-labeled with Calcein-AM (Invitrogen) and after co-culture with CD8 T cells for 4 h, viable target cells were quantified by FACS. The percentage of specific lysis was calculated as described.38
Statistical analyses
In the in vitro and in vivo experiments, comparisons between two groups were based on the two-sided unpaired Student's t-test or one-way ANOVA with the Tukey-Kramer post-test comparison of group means. Statistical computations were performed with GraphPad Prism™ 4.0 software (GraphPad Software).
Results
Construction of Salmonella-based survivin vaccine based on SPI2-regulated type-III secretion system
To generate survivin vaccine, we subcloned human survivin cDNA into p2810 to replace lisA that was previously used in the vaccine against Listeria monocytogenes.18 The resultant construct harbors bacterial sseA promoter that is controlled by SsrB, the central regulator of SPI2 gene expression, sscB, encoding a specific chaperone followed by a fusion sequence of sseF (encoding a translocated effector protein of the T3SS that directs the fusion protein into the host cell cytosol), survivin, and HA tag (Fig. 1A). Then we electroporated this survivin construct into strains of attenuated Salmonella typhimurium and tested the efficacy of target antigen expression and translocation. Salmonella expressed survivin in a SPI2-regulated manner as evidenced by lack of detectable survivin protein under non-inducing (PCN) culture conditions and its expression upon culture in inducing (PCN-P) medium, which mimics the intracellular conditions (Fig. 1B). To test the efficacy of target protein translocation into cell cytosol via the T3SS of Salmonella, we infected the murine macrophage cell line J774 with Salmonella carrying either vector control or the survivin construct. To examine whether attenuation affects T3SS-mediated translocation, we used wild-type Salmonella as a positive control. Fig. 1C demonstrates that a double-attenuated mutant strain MvP728 deficient in purD/htrA and MvP728 translocates survivin into cytosol of macrophages as effectively as the wild type Salmonella. Therefore, double-attenuated Salmonella express survivin in a SPI2-regulated manner and effectively translocates the target protein into the cytosol of APCs.
Figure 1. Salmonella-based vaccine that expresses and translocates human survivin in SPI2-regulated manner.
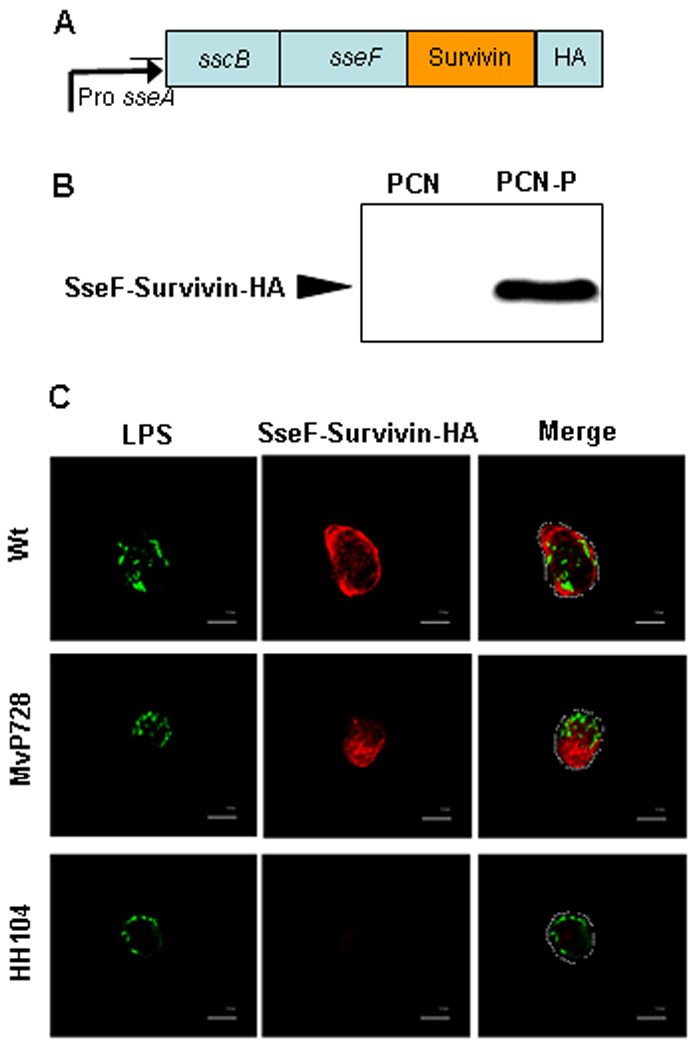
(A) Expression construct in p3342 plasmid with sseF-hSurvivin-HA fusion protein under control of sseA promoter and regulation by SsrB. (B) Regulated expression of the recombinant fusion protein by MvP728 strain of Salmonella: Western blot for HA tag performed 16 h after bacterial growth in non-inducing (PCN) or inducing (PCN-P) medium. (C) SPI2-T3SS-dependent translocation of the fusion protein by intracellular Salmonella. The murine macrophage-like cells, J774 were infected with wild type (Wt), purD/htrA-deficient (MvP728), or translocation-incapable sseC-deficient (HH104) strain of Salmonella, each carrying p3342 plasmid. At 16 h after infection, cells were fixed and processed for immunostaining of Salmonella LPS (green) and HA tag (red) followed by analysis with laser-scanning confocal microscopy. Representative images (100X magnification, scale bar is 10 µm) are shown from one of two experiments. The margins of the cells are outlined in the merged images.
NKT ligands and attenuated Salmonella synergistically activate DCs
Previous reports demonstrated that ligand-activated NKTs act in synergy with LPS or killed Salmonella to induce DC activation, maturation, and IL-12 production.27,29 This suggests that live attenuated Salmonella, our vector for the survivin vaccine, may cooperate with NKT ligand(s) in the induction of DC activation and maturation. To test this hypothesis, we cultured immature human monocyte-derived DCs (iDCs) with NKTs alone or with one of two activating NKT ligands, GSL1 or αGalactosylceramide (αGalCer). In addition, the same iDCs were cultured either with Salmonella alone or with Salmonella combined with ligand-activated NKTs. Fig. 2A demonstrates that a combination of Salmonella vector, MvP728 with GSL1-activated NKTs resulted in a stronger up-regulation of co-stimulatory molecules on the DC surface, CD80 and CD86, compared to either Salmonella or GSL1-activated NKTs. Next, we examined the functional response of DCs as measured by IL-12 production. Although GSL1-stimulated NKTs induced little IL-12 in iDCs (data not shown), we found that addition of GSL1 and NKTs to iDCs infected with Salmonella strongly enhanced IL-12 production compared to Salmonella alone, especially at low bacteria:DC ratios (Fig. 2B). Therefore, ligand-activated NKTs synergize with the vaccine strain of Salmonella for the induction of DC activation and IL-12 production, providing a rational for the potential use of NKT ligands as adjuvants for Salmonella-based vaccines.
Figure 2. NKT cells and MvP728 synergistically activate DCs.
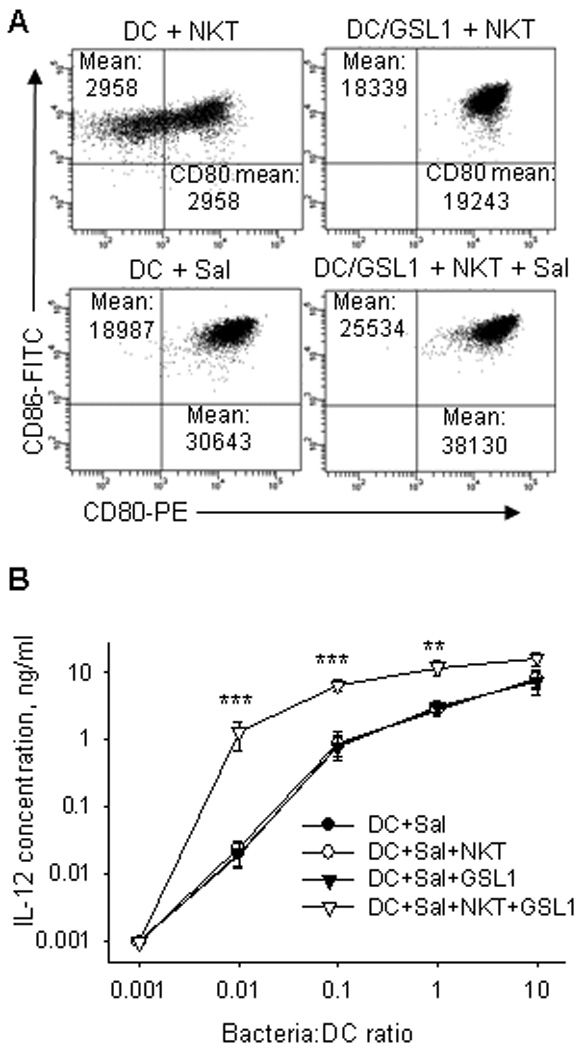
(A) Human monocyte-derived immature DCs were infected with MvP728 at indicated MOI. Extracellular bacteria were killed by gentamicin after 1 h. Human in vitro expanded NKT cells (1:1 NKT:DC ratio) and GSL1 (200 ng/ml) were added to culture when indicated. After 48 h, DC phenotype was analyzed by FACS. (B) IL-12 concentration in supernatants in the same experiments was determined by CBAPlex. Results are means ± SD from 4 experiments. * P<0.05, **P<0.01, ***P<0.001, one-way ANOVA.
Kinetics of antigen-specific CD8 T cell response induced by Salmonella vaccine alone and in combination with NKT ligand
To determine the kinetics of CTL induction by Salmonella vaccine, we used the p2810 construct with Listeria gene lisA (encodes listeriolysin O protein, Llo) instead of survivin because of the availability of H-2Kd-Llo pentamers that allow longitudinal measurement of the antigen-specific CTL response. In the initial screening experiments, we found that the MvP728 strain is the most efficient inducer of CTL response (data not shown). At day 10 after vaccination, MvP728-Llo induced 1.9 ± 0.3% pentamer-reactive CD8 T cells (Fig. 3A top). To test the adjuvant properties of NKT ligands, mice were vaccinated with MvP728-Llo with or without GSL1 followed by boost vaccinations with MvP728-Llo alone every two weeks. Surprisingly, in the first 10 days, co-administration of GSL1 as consistently inhibited CTL response, resulting in 0.7 ± 0.2% pentamer-reactive CD8 T cells (P < 0.01). However, at the later time points (days 21 and 36) the CTL response was greatest in mice that received the vaccine combination with an NKT ligand (Fig. 3A, middle, bottom). For example, at day 36 we detected 0.15 ± 0.07%, 3 ± 0.3%, and 4.7 ± 0.5% of Llo-reactive CD8 T cells in PBMC of mice that received MvP728-vector with GSL1 (specificity control), MvP728-Llo alone, and MvP728-Llo with GSL1, respectively (P < 0.001, one-way ANOVA, Fig. 3B). Therefore, despite an initial inhibition of CTL generation, addition of GSL1 to MvP728-based vaccine can increase the magnitude and/or duration of the specific CTL response.
Figure 3. Kinetics of antigen-specific CD8 T cell response induced by Salmonella vaccine alone and in combination with NKT ligand.
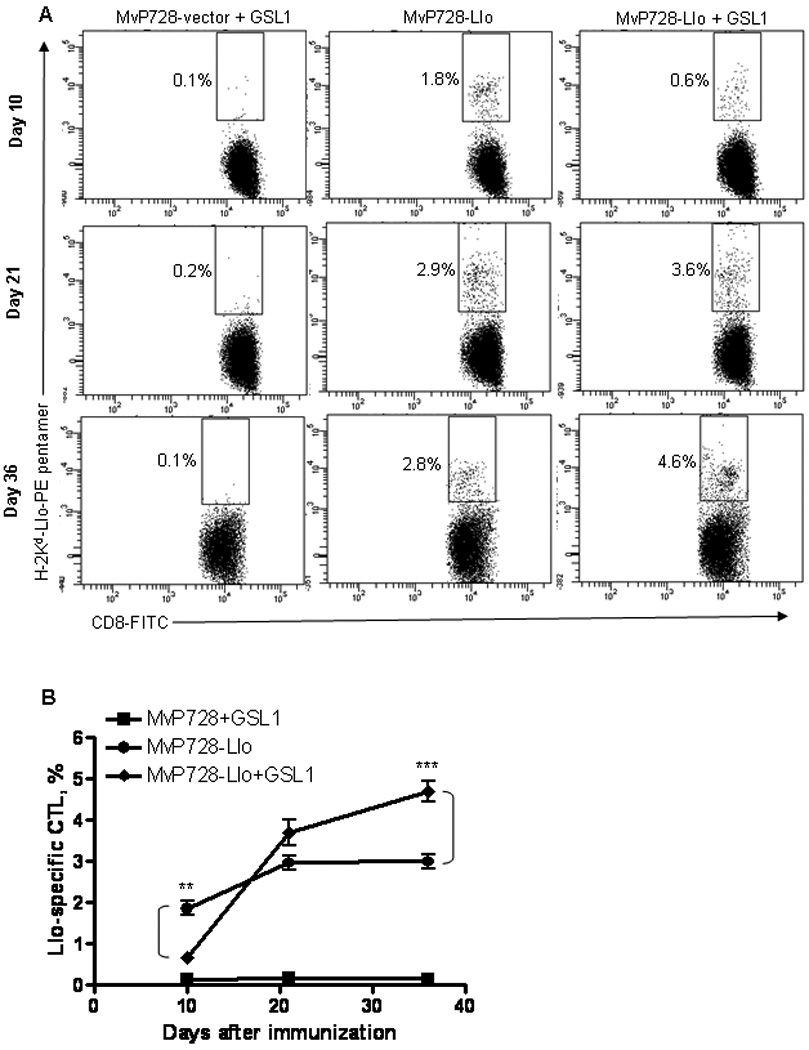
(A) Mice were administrated with either MvP728-vector, MvP728-Llo vaccine, or MvP728-Llo with GSL1. Boost vaccination was given on days 14 and 28 without GSL1. Blood was collected on days 10, 21, and 36, leukocytes were stained for CD8 and Llo-specific TCR expression. Shown are representative plots with numbers indicating percent Llo-specific CD8 T cells. (B) Means ± SD of Llo-specific CD8 T cells at indicated time points after vaccination from three experiments. **P<0.01, ***P<0.001, one-way ANOVA.
NKT ligand GSL1 enhances CTL generation in response to MvP728-survivin vaccine
Next, we examined specific CD8 and CD4 T cell responses to MvP728-survivin vaccine alone or with GSL1. Mice were vaccinated with MvP728-survivin with or without GSL1 and boosted with MvP728-survivin on day 14. On day 21, their splenocytes were examined for reactivity to a mix of overlapping 18-mer peptides from a human survivin library. Fig. 4A demonstrates that both IFNγ-producing CD8 and TNFα-producing CD4 survivin-specific T cells were clearly detectable (without ex vivo expansion) only in mice that received the MvP728-survivin and GSL1 combination compared to mice that were treated with either MvP728-survivin alone or with MvP728-vector and GSL1 (P<0.001, one-way ANOVA). Of note, in analogy with a well-described property of αGalCer to induce a long-lasting NKT-cell anergy,40 additional GSL1 injections (with boost vaccination) did not improve survivin-specific T-cell responses (data not shown). These results further support a hypothesis that NKT ligands may boost generation of antigen-specific T cells in response to a Salmonella-based vaccine.
Figure 4. Specific response to MvP728-survivin vaccine.
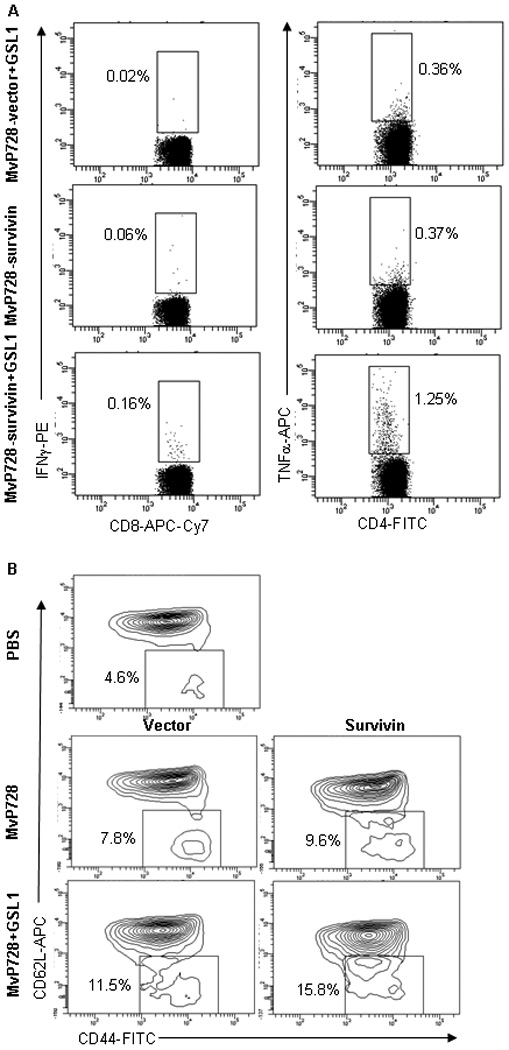
Mice were vaccinated in the same way as in Figure 3 using survivin instead of Llo vaccine. (A) On day 21, splenocytes were examined for reactivity to a mix of 18-mer overlapping peptides from human survivin library after staining with CD3, CD8, CD4, IFNγ, and TNFα. Shown are representative FACS plots with percent IFNγ- and TNFα-positive CD8 and CD4 T cells, respectively from one of two experiments. (B) Accumulation of effector-memory CTLs was measured by FACS analysis of splenocytes for surface expression of CD44 and CD62L in CD8 T cells. The indicated regions show percent effector-memory CD44highCD62Llow among CD8 T cells from one of two experiments with similar results.
To directly test the generation of effector-memory CTLs, splenocytes from mice on day 21 after vaccination were analyzed for expression of memory markers CD44 and CD62L within CD8+ T cells.41,42 We found that co-administration of GSL1 with either MvP728-vector or MvP728-survivin significantly enhanced accumulation of CD44high cells that included both CD62Llow effector memory and CD62Lhigh central memory subsets (P<0.01, t-test; Fig. 4B and data not shown). Therefore, NKT ligands may increase immunogenicity of Salmonella-based vaccines by boosting generation of memory CTLs.
Tumor growth inhibition induced by Salmonella-based survivin vaccine in CT26 colon cancer model
To test the anti-tumor potential of the survivin vaccine, we transformed seven strains of attenuated Salmonella with survivin plasmid and used CT26 colon carcinoma model in a prophylactic setting. The Salmonella strains harbored single or double mutations previously shown to attenuate virulence in mice (Table-1).31,43,44 Female BALB/c mice were orally immunized with one of the following survivin-expressing Salmonella mutant strains: SL7207 (aroA), MvP467 (htrA), MvP664 (galE), MvP679 (purD), MvP728 (purD/htrA), MvP729 (galE/htrA), or MvP740 (purA/galE). Mice were vaccinated twice with a two-week interval and then challenged with subcutaneous injection of CT26 cells one week after the last vaccination. Fig. 5A shows that MvP728 was the most effective strain in delaying the growth of CT26 tumor (P<0.01, one-way ANOVA). Based on these results and on the fact that a dose of at up to 1010 CFU of MvP728 was not toxic to mice (data not shown), we have chosen this strain as our vaccine carrier.
Table 1.
Bacterial strains and plasmids used in this study
| Strain | genotype, relevant characteristics | source/reference |
|---|---|---|
| Salmonella enterica Serovar Typhimurium | ||
| NCTC12023 | wild type | lab stock |
| SL7207 | ΔaroA | 31 |
| MvP467 | ΔhtrA | 44 |
| HH 104 | ΔsseC∷aphT, KanR | 65,66 |
| MvP664 | ΔgalE∷aph | this study |
| MvP679 | ΔgalE | this study |
| MvP680 | ΔpurD | this study |
| MvP728 | ΔpurD ΔhtrA | this study |
| MvP729 | ΔgalE ΔhtrA | this study |
| MvP740 | ΔpurD∷aph ΔgalE | this study |
| Plasmids | ||
| p2810 | pWSK29 PsseA sscB sseF1–263∷lisA51–363∷HA | 18 |
| p3342 | pWSK29 PsseA sscB sseF1–263∷hsurvivin∷HA | this study |
Figure 5. Anti-tumor efficacy of MvP728-survivin vaccine alone and with GSL1.
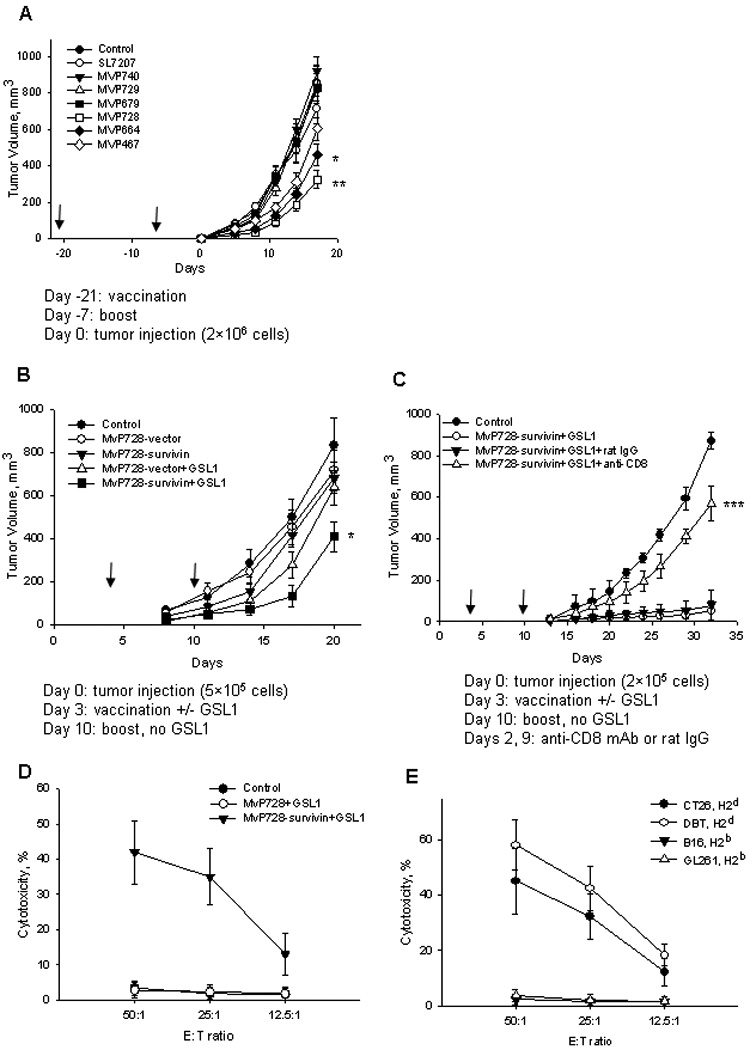
(A) Mice were vaccinated with survivin twice with a 2-week interval using one of the indicated strains of attenuated Salmonella. One week after the last vaccination, mice were s/c injected with 2×106 of CT26 cells. (B) Mice were s/c injected with 5 × 105 CT26 cells followed by vaccination with MvP728 with vector control or survivin construct with or without GSL1 at days 3 and 10 after tumor implantation. (C) Mice were s/c injected with 2 × 105 CT26 cells followed by vaccination with MvP728 with GSL1. When indicated, mice received anti-CD8 depleting mAb or rat IgG control. Non-vaccinated mice served as a control. Tumor size was measured every two-three days and plotted as means ± SD (8 mice per group). Experiments in A–C were repeated at least once with similar results. (D) Splenocytes from indicated groups of mice were in vitro stimulated with a survivin peptide library and IL-2 for 7 days followed by isolation of CD8 T cells using magnetic beads and evaluation of their cytotoxicity against Calcein-AM-labeled CT26 cells. (E) In vitro re-stimulated CD8 T cells from the vaccine+GSL1 group of mice were tested in the Calcein-AM cytotoxicity assay against two syngeneic and two allogeneic survivin-expressing tumor cell lines. Data are Mean ± SD from two experiments performed in triplicates.
In the next set of experiments, we tested whether MvP728-survivin can be effective in a therapeutic setting of the CT26 model and whether its anti-tumor activity can be enhanced by co-administration of GSL1. Mice were vaccinated on days 3 and 10 after tumor inoculation, and tumor growth was monitored by measuring tumor volume every 2–3 days. Fig. 5B demonstrates that by day 20, a significant delay in tumor growth was only achieved when MvP728-survivin was combined with GSL1 (P<0.05, one-way ANOVA). GSL1 alone (not shown) and in combination with MvP728-vector showed a modest inhibition of tumor growth up to day 17, which was expected from a well-known property of NKT ligands to activate innate anti-tumor responses.45 The protective effect of MvP728-survivin/GSL1 vaccine was more pronounced when the dose of injected tumor cells was decreased from 5 to 2×105 (Fig. 5C). This anti-tumor activity was mediated by CD8 T cells since it was strongly inhibited by pre-treatment with rat anti-mouse CD8 depleting mAb but not by rat IgG control (P<0.001, one-way ANOVA). To directly examine generation of survivin-specific CTL to our vaccine, we compared in vitro cytotoxicity against CT26 cells with ex-vivo re-stimulated CD8 T cells from mice that were treated with MvP728-survivin/GSL1, MvP728-vector /GSL1, or PBS. Figure 5D shows that only CD8 T cells from mice vaccinated with the survivin-containing vaccine exhibited dose-dependent cytotoxicity against tumor cells, indicating that the cytotxicity is survivin-specific. To test, MHC restriction of CTLs induced by MvP728-survivin/GSL1 vaccination, we tested their cytotoxicity against two syngeneic, H2d (CT26 and DBT) and two allogeneic, H2b (B16 and GL261) tumor cell lines. Both B16 and GL261 cell lines have been reported to express high levels of survivin (confirmed by western blot, data not shown) and be sensitive to survivin-reactive CTLs from C57BL/6 mice.46,47 Figure 5E demonstrates that only H2d syngeneic tumor cells were sensitive to CTL-mediated cytotoxicity, indicating that this cytotoxicity is MHC class-I-restricted.
GSL1 strongly enhances the anti-tumor efficacy of MvP728-survivin vaccine in a DBT orthotopic glioma model
Survivin is broadly over-expressed in cancer, and, in some tumors, survivin over-expression has been linked to aggressive tumor behavior and poor outcome in patients. For example, high level of survivin expression is predictive of poor outcome in patients with glioblastoma, the most common brain tumor and one of the most deadliest forms of cancer.48–51 Therefore, we decided to test the therapeutic potential of our vaccine against a well-established mouse orthotopic model using DBT glioblastoma cells.52 DBT cells were stably transduced with firefly luciferase (DBT/luc) and intra-cranially injected into the caudate/putamens in brains of BALB/c mice. Because the intra-cranial tumor injection is a relatively stressful surgical procedure that may affect immune responses, we vaccinated mice 3 days before tumor injection. Mice received oral vaccination with 1010 CFU of MvP728-vector, MvP728-survivin, MvP728-vector + GSL1, or MvP728-survivin + GSL1. Tumor growth was monitored by bioluminescence imaging. Fig. 6A demonstrates that while the vaccine inhibited tumor growth in the first 11 days, its combination with GSL1 had a significantly stronger anti-tumor activity (P<0.001). The anti-tumor effect of MvP728-survivin + GSL1 was also stronger than the innate effect of MvP728-vector + GSL1, indicating that the survivin-specific response contributed to the overall anti-tumor efficacy of the vaccine/ligand combination. These results suggest that NKT ligands may serve as potent adjuvants for recombinant Salmonella-based cancer vaccines.
Figure 6. Anti-tumor efficacy of MvP728-survivin with GSL1 in glioblastoma model.
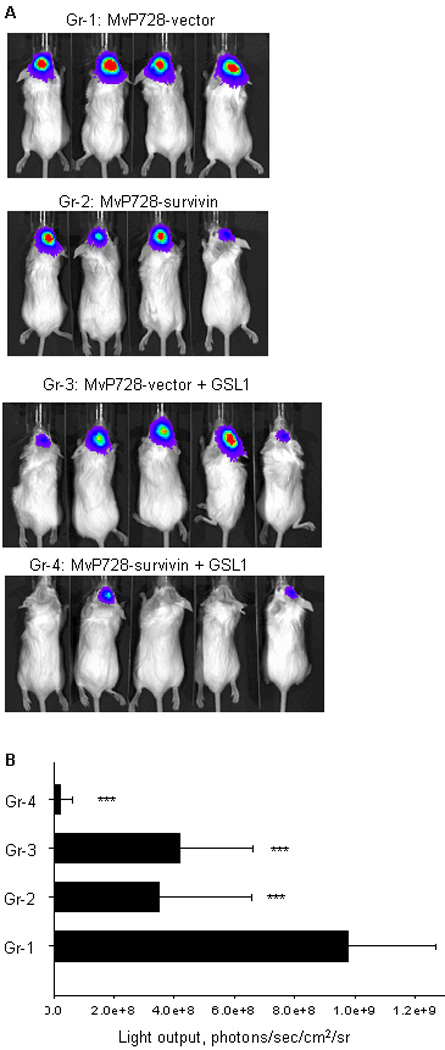
(A) Mice were intra-cranially injected with 105 DBT/luc cells 3 days after they were treated with: 1. MvP728-vector; 2. MvP728-survivin; 3. MvP728-vector + GSL1; 4. MvP728-survivin + GSL1. Bioluminescence imaging was performed on day 11 after tumor injection. (B) Results are expressed in units of photons per second per square centimeter per steradian (photons/sec/cm2/sr). Bar chart shows means ± SD from one of two experiments.
Discussion
We constructed a novel cancer vaccine that uses SPI2-regulated T3SS of attenuated Salmonella to express and translocate human survivin as a tumor antigen of choice into the cytosol of host APCs. Among seven strains tested, we found that MvP728 (purD/htrA) is the most effective in eliciting antigen-specific memory CD8 T cells and in inhibiting tumor growth. The combined use of MvP728-survivin with NKT ligands (GSL1 or αGalCer) enhances generation of antigen-specific effector-memory CD8 and CD4 T cells that results in increased anti-tumor efficacy of the vaccine in murine models of colon cancer and glioblastoma.
Since the discovery that attenuated Shigella can deliver DNA for expression in mammalian cells,53 attenuated strains of several types of intracellular bacteria (Shigella, Salmonella, Listeria, and Yersinia) have been studied as delivery vehicles for subunit vaccines against a range of infectious diseases and cancer.3 From the clinical application prospective, Salmonella-based vectors might have an advantage because of excellent safety record of an FDA-approved oral Salmonella vaccine (S. typhi strain Ty21a, Vivotif®) in children and in adults54,55 and low toxicity in cancer patients of tumor-targeting strains of Salmonella in recent clinical trials.56,57 In this study, we demonstrate for the first time that the SPI2-regulated expression system can be used as a platform for cancer vaccines. Our data demonstrate that Salmonella transformed with p3342 plasmid expresses survivin in a tightly regulated manner and effectively translocates the target protein into the cytosol of macrophages and DCs. The new vaccination platform retains the well-documented advantages of oral vaccines based on live attenuated Salmonella58 and uses the unique properties of SPI2-regulated system to selectively deliver a target antigen into the cytosol of host APCs in situ.
Using a well-established experimental system with Llo antigen to track antigen-specific CTL response, we demonstrated that orally administrated MvP728-Llo vaccine alone elicited significant Llo-specific CTL response within the first 10 days. However, subsequent boost vaccinations had little effect on the magnitude of the CTL response. The effect of boost vaccinations was significantly greater when a NKT ligand, GSL1 was co-administrated with the first dose of vaccine despite the fact that CTL frequency was decreased in the first 10 days after ligand administration. Such paradoxical bimodal effect of NKT ligands on vaccine activity has recently been reported in a study of αGalCer as an adjuvant for an inactivated influenza virus A vaccine.59 The authors showed that αGalCer diminished the primary CTL response while at the same time augmented virus-specific CD8+ memory T cell number, especially those of central memory CD62L+IL7R+ CTL subset. They found that the initial reduction of CTL number could be explained by NKT cell-mediated high levels of IFNγ production that led to a transient IDO up-regulation in T cells and disruption of tryptophan metabolism. This explanation is consistent with the observations in our model that NKT-mediated CTL reduction is transient and that NKT ligands increase the magnitude and duration of vaccine-induced memory CTL response as it was evidenced by increased accumulation of CD44highCD62Llow CD8+ T cells after boost vaccinations.
Consistent with the results obtained with MvP728-Llo vaccine, we found that combined use of MvP728-survivin vaccine with NKT ligand, GSL1 enhanced generation of survivin-reactive CTLs (by day 21) compared with the vaccine alone. Moreover, we also found that only the vaccine/ligand combination resulted in the generation of survivin-specific CD4 T cells. A recent report from Dr. Steinman’s group suggested that induction of CD4 T cell-mediated immune responses against survivin is important to improve the quality of future survivin vaccines 60. Indeed, antigen-specific CD4 T cells can help CD8 T cells during the priming and maintenance of memory response so that an effective cancer vaccine may require a balanced response by both CD8 and CD4 T cells.61
Carrier strains for the use of T3SS for translocation of recombinant antigens have to fulfill specific requirements. The strains need to be sufficiently attenuated to be safe in the vaccinated individual, but should regain the ability to express SPI2-regulated genes after phagocytosis by APCs. Among seven examined attenuated strains of Salmonella typhimurium, MvP728 was found to mediate the strongest anti-tumor activity of survivin vaccine in the prophylactic setting of CT26 model as measured by tumor growth delay. The combination of two independent attenuating mutations in S. typhimurium carrier strains was successful in the murine model used here. We previously observed that certain mutations such as defects in aroA, although resulting in a sufficient attenuation of the carrier strain, prevent sufficient expression of SPI2-regulated fusion constructs.62 The low performance of the aroA strain SL7207 in this study was in line with these observations.
Although MvP728-survivin vaccine alone had little effect on CT26 growth in the therapeutic setting, addition of GSL1 as an adjuvant significantly delayed tumor growth. Since CT26 cells are CD1d-negative63 (and data not shown), the GSL1 effect cannot be due to induction of direct NKT cell cytotoxicity against tumor cells. Using CD8 T cell depletion, we demonstrated that the anti-tumor effect of MvP728-survivin/GSL1 vaccination nearly completely depended on CD8 T cells. Indeed, CD8 T cells from vaccinated mice exhibited direct cytotoxicity against syngeneic tumor cells. Therefore, in addition to the ability of NKT ligands to activate innate anti-tumor immunity via induction of IFNγ and activation of NK cells,34,45 they can increase anti-tumor efficacy of Salmonella-based vaccines via enhancement of protective CTL responses.
The anti-tumor efficacy of MvP728-survivin/GSL1 combination was not limited to the CT26 colon cancer model. GSL1 also strongly enhanced the vaccine anti-tumor efficacy in the highly aggressive orthotopic model of DBT glioblastoma. The observed potency of our survivin vaccine in a glioblastoma model is of particular importance to the potential clinical translation because the current therapy against glioblastoma multiforme is largely ineffective while survivin is a biologically and clinically relevant target in this disease.48–51 The antitumor efficacy of our vaccine in a brain tumor model is consistent with demonstrated ability of antigen-experienced tumor-specific T cells to localize to CNS tumors and function within the brain parenchyma both in mouse models and in patients with glioblastoma.64
Thus, the described vaccination approach against a tumor-associated antigen using a SPI2-regulated T3SS system of attenuated Salmonella typhimurium may provide a foundation for clinical trials of new oral vaccines against glioblastoma and other types of cancer that will be relatively easy to manufacture, standardize, and administer to patients.
Supplementary Material
Acknowledgments
This work was supported in part by the Pediatric Brain Tumor Foundation of the US (L.S.M, A.E.E, R.C.S, G.M.S.); the Neil Bogart Memorial Fund of the T.J. Martell Foundation for Leukemia, Cancer, and AIDS Research (R.C.S, L.S.M, A.E.E., G.M.S.); Jean Perkins Foundation (G.M.S.), Bavaria California Technology Center (L.S.M. and M.H.); and the Deutsche Forschungsgemeinschaft (HE 1964/7-3) as a part of the priority program 1089 “Novel strategies for vaccination” (MH). The authors gratefully acknowledge the excellent support of Barbara Bodendorfer for strain construction.
Abbreviations
- TAAs
tumor-associated antigens
- SPI2
Salmonella Pathogenicity Island 2
- T3SS
type III secretion system
- NKT
Natural Killer T
- GSL1
α-Glucuronosylceramide
- αGalCer
α-Galactosylceramide
Footnotes
Novelty and Impact
This is the first demonstration that genes of Salmonella Pathogenicity Island 2 (SPI2) can be used in the design of cancer vaccines, where a tumor antigen of choice is expressed in a regulated fashion inside the host APCs and translocated into cell cytosol for processing and presentation via HLA class-I .
Demonstrated anti-tumor efficacy of a SPI2-based survivin vaccine in models of colon cancer and glioblastoma suggests that SP2-based vaccination approach may have broad applicability and lead to development of effective immunotherapy against various forms of cancer.
Reference List
- 1.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoen C, Stritzker J, Goebel W, Pilgrim S. Bacteria as DNA vaccine carriers for genetic immunization. Int J Med Microbiol. 2004;294:319–335. doi: 10.1016/j.ijmm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Vassaux G, Nitcheu J, Jezzard S, Lemoine NR. Bacterial gene therapy strategies. J Pathol. 2006;208:290–298. doi: 10.1002/path.1865. [DOI] [PubMed] [Google Scholar]
- 4.Gentschev I, Dietrich G, Spreng S, Kolb-Maurer A, Brinkmann V, Grode L, Hess J, Kaufmann SH, Goebel W. Recombinant attenuated bacteria for the delivery of subunit vaccines. Vaccine. 2001;19:2621–2628. doi: 10.1016/s0264-410x(00)00502-8. [DOI] [PubMed] [Google Scholar]
- 5.Verjans GM, Janssen R, UytdeHaag FG, van Doornik CE, Tommassen J. Intracellular processing and presentation of T cell epitopes, expressed by recombinant Escherichia coli and Salmonella typhimurium, to human T cells. Eur J Immunol. 1995;25:405–410. doi: 10.1002/eji.1830250215. [DOI] [PubMed] [Google Scholar]
- 6.Qimron U, Madar N, Mittrucker HW, Zilka A, Yosef I, Bloushtain N, Kaufmann SH, Rosenshine I, Apte RN, Porgador A. Identification of Salmonella typhimurium genes responsible for interference with peptide presentation on MHC class I molecules: Deltayej Salmonella mutants induce superior CD8+ T-cell responses. Cell Microbiol. 2004;6:1057–1070. doi: 10.1111/j.1462-5822.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 7.Hummel S, Apte RN, Qimron U, Vitacolonna M, Porgador A, Zoller M. Tumor vaccination by Salmonella typhimurium after transformation with a eukaryotic expression vector in mice: impact of a Salmonella typhimurium gene interfering with MHC class I presentation. J Immunother. 2005;28:467–479. doi: 10.1097/01.cji.0000170359.92090.8b. [DOI] [PubMed] [Google Scholar]
- 8.Xiang R, Mizutani N, Luo Y, Chiodoni C, Zhou H, Mizutani M, Ba Y, Becker JC, Reisfeld RA. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65:553–561. [PubMed] [Google Scholar]
- 9.Russmann H, Shams H, Poblete F, Fu Y, Galan JE, Donis RO. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281:565–568. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 10.Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 11.Marlovits TC, Kubori T, Sukhan A, Thomas DR, Galan JE, Unger VM. Structural insights into the assembly of the type III secretion needle complex. Science. 2004;306:1040–1042. doi: 10.1126/science.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shams H, Poblete F, Russmann H, Galan JE, Donis RO. Induction of specific CD8+ memory T cells and long lasting protection following immunization with Salmonella typhimurium expressing a lymphocytic choriomeningitis MHC class I-restricted epitope. Vaccine. 2001;20:577–585. doi: 10.1016/s0264-410x(01)00363-2. [DOI] [PubMed] [Google Scholar]
- 13.Evans DT, Chen LM, Gillis J, Lin KC, Harty B, Mazzara GP, Donis RO, Mansfield KG, Lifson JD, Desrosiers RC, Galan JE, Johnson RP. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J Virol. 2003;77:2400–2409. doi: 10.1128/JVI.77.4.2400-2409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, Ritter G, Jager E, Nomura H, Kondo S, Tawara I, Kato T, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensel M, Shea JE, Gleeson C, Jones MD, Dalton E, Holden DW. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 16.Hensel M. Salmonella pathogenicity island 2. Mol Microbiol. 2000;36:1015–1023. doi: 10.1046/j.1365-2958.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- 17.Abrahams GL, Hensel M. Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol. 2006;8:728–737. doi: 10.1111/j.1462-5822.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- 18.Husseiny MI, Wartha F, Hensel M. Recombinant vaccines based on translocated effector proteins of Salmonella Pathogenicity Island 2. Vaccine. 2007;25:185–193. doi: 10.1016/j.vaccine.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Swann JB, Coquet JM, Smyth MJ, Godfrey DI. CD1-restricted T cells and tumor immunity. Curr Top Microbiol Immunol. 2007;314:293–323. doi: 10.1007/978-3-540-69511-0_12. [DOI] [PubMed] [Google Scholar]
- 20.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 21.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 22.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 23.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 24.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 25.Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur J Immunol. 2005;35:2100–2109. doi: 10.1002/eji.200425846. [DOI] [PubMed] [Google Scholar]
- 26.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 27.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 28.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 29.Hermans IF, Silk JD, Gileadi U, Masri SH, Shepherd D, Farrand KJ, Salio M, Cerundolo V. Dendritic cell function can be modulated through cooperative actions of TLR ligands and invariant NKT cells. J Immunol. 2007;178:2721–2729. doi: 10.4049/jimmunol.178.5.2721. [DOI] [PubMed] [Google Scholar]
- 30.Song L, Ara T, Wu HW, Woo CW, Reynolds CP, Seeger RC, DeClerck YA, Thiele CJ, Sposto R, Metelitsa LS. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J Clin Invest. 2007;117:2702–2712. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 32.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 34.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, Kronenberg M, Seeger RC. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 35.Long X, Deng S, Mattner J, Zang Z, Zhou D, McNary N, Goff RD, Teyton L, Bendelac A, Savage PB. Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat Chem Biol. 2007;3:559–564. doi: 10.1038/nchembio.2007.19. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, Laug WE. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48:151–157. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 37.Burgos JS, Rosol M, Moats RA, Khankaldyyan V, Kohn DB, Nelson MD, Jr, Laug WE. Time course of bioluminescent signal in orthotopic and heterotopic brain tumors in nude mice. Biotechniques. 2003;34:1184–1188. doi: 10.2144/03346st01. [DOI] [PubMed] [Google Scholar]
- 38.Metelitsa LS, Gillies SD, Super M, Shimada H, Reynolds CP, Seeger RC. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcgammaRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood. 2002;99:4166–4173. doi: 10.1182/blood.v99.11.4166. [DOI] [PubMed] [Google Scholar]
- 39.Metelitsa LS. Flow cytometry for natural killer T cells: multi-parameter methods for multifunctional cells. Clin Immunol. 2004;110:267–276. doi: 10.1016/j.clim.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mobley JL, Rigby SM, Dailey MO. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J Immunol. 1994;153:5443–5452. [PubMed] [Google Scholar]
- 42.Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 43.Husseiny MI, Hensel M. Construction of highly attenuated Salmonella enterica serovar Typhimurium live vectors for delivering heterologous antigens by chromosomal integration. Microbiol Res. 2008;163:605–615. doi: 10.1016/j.micres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Husseiny MI, Hensel M. Rapid method for the construction of Salmonella enterica Serovar Typhimurium vaccine carrier strains. Infect Immun. 2005;73:1598–1605. doi: 10.1128/IAI.73.3.1598-1605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 46.Lladser A, Ljungberg K, Tufvesson H, Tazzari M, Roos AK, Quest AF, Kiessling R. Intradermal DNA electroporation induces survivin-specific CTLs, suppresses angiogenesis and confers protection against mouse melanoma. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciesielski MJ, Kozbor D, Castanaro CA, Barone TA, Fenstermaker RA. Therapeutic effect of a T helper cell supported CTL response induced by a survivin peptide vaccine against murine cerebral glioma. Cancer Immunol Immunother. 2008;57:1827–1835. doi: 10.1007/s00262-008-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, Loeffler JS. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20:1063–1068. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 49.Grunda JM, Nabors LB, Palmer CA, Chhieng DC, Steg A, Mikkelsen T, Diasio RB, Zhang K, Allison D, Grizzle WE, Wang W, Gillespie GY, et al. Increased expression of thymidylate synthetase (TS), ubiquitin specific protease 10 (USP10) and survivin is associated with poor survival in glioblastoma multiforme (GBM) J Neurooncol. 2006;80:261–274. doi: 10.1007/s11060-006-9191-4. [DOI] [PubMed] [Google Scholar]
- 50.Uematsu M, Ohsawa I, Aokage T, Nishimaki K, Matsumoto K, Takahashi H, Asoh S, Teramoto A, Ohta S. Prognostic significance of the immunohistochemical index of survivin in glioma: a comparative study with the MIB-1 index. J Neurooncol. 2005;72:231–238. doi: 10.1007/s11060-004-2353-3. [DOI] [PubMed] [Google Scholar]
- 51.Shirai K, Suzuki Y, Oka K, Noda SE, Katoh H, Suzuki Y, Itoh J, Itoh H, Ishiuchi S, Sakurai H, Hasegawa M, Nakano T. Nuclear survivin expression predicts poorer prognosis in glioblastoma. J Neurooncol. 2009;91:353–358. doi: 10.1007/s11060-008-9720-4. [DOI] [PubMed] [Google Scholar]
- 52.Jost SC, Wanebo JE, Song SK, Chicoine MR, Rich KM, Woolsey TA, Lewis JS, Mach RH, Xu J, Garbow JR. In vivo imaging in a murine model of glioblastoma. Neurosurgery. 2007;60:360–370. doi: 10.1227/01.NEU.0000249264.80579.37. [DOI] [PubMed] [Google Scholar]
- 53.Sizemore DR, Branstrom AA, Sadoff JC. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science. 1995;270:299–302. doi: 10.1126/science.270.5234.299. [DOI] [PubMed] [Google Scholar]
- 54.Ivanoff B, Levine MM, Lambert PH. Vaccination against typhoid fever: present status. Bull World Health Organ. 1994;72:957–971. [PMC free article] [PubMed] [Google Scholar]
- 55.Gentschev I, Spreng S, Sieber H, Ures J, Mollet F, Collioud A, Pearman J, Griot-Wenk ME, Fensterle J, Rapp UR, Goebel W, Rothen SA, et al. Vivotif--a 'magic shield' for protection against typhoid fever and delivery of heterologous antigens. Chemotherapy. 2007;53:177–180. doi: 10.1159/000100515. [DOI] [PubMed] [Google Scholar]
- 56.Thamm DH, Kurzman ID, King I, Li Z, Sznol M, Dubielzig RR, Vail DM, MacEwen EG. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res. 2005;11:4827–4834. doi: 10.1158/1078-0432.CCR-04-2510. [DOI] [PubMed] [Google Scholar]
- 57.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, Freezer LJ, Morton KE, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiang R, Luo Y, Niethammer AG, Reisfeld RA. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev. 2008;222:117–128. doi: 10.1111/j.1600-065X.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 59.Guillonneau C, Mintern JD, Hubert FX, Hurt AC, Besra GS, Porcelli S, Barr IG, Doherty PC, Godfrey DI, Turner SJ. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charalambous A, Oks M, Nchinda G, Yamazaki S, Steinman RM. Dendritic cell targeting of survivin protein in a xenogeneic form elicits strong CD4+ T cell immunity to mouse survivin. J Immunol. 2006;177:8410–8421. doi: 10.4049/jimmunol.177.12.8410. [DOI] [PubMed] [Google Scholar]
- 61.Yu P, Spiotto MT, Lee Y, Schreiber H, Fu YX. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J Exp Med. 2003;197:985–995. doi: 10.1084/jem.20021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Husseiny MI, Hensel M. Evaluation of an intracellular-activated promoter for the generation of live Salmonella recombinant vaccines. Vaccine. 2005;23:2580–2590. doi: 10.1016/j.vaccine.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 63.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masson F, Calzascia T, Di Berardino-Besson W, de TN, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007;179:845–853. doi: 10.4049/jimmunol.179.2.845. [DOI] [PubMed] [Google Scholar]
- 65.Shea JE, Hensel M, Gleeson C, Holden DW. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang FC, Holden DW. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


