Abstract
BACKGROUND
The study goal was to develop and test the effectiveness of a brief online education and support program for female infertility patients.
METHODS
A randomized-controlled trial was conducted. Using a Solomon-four group design, 190 female patients were recruited from three US fertility centers and were randomized into two experimental and two no-treatment control groups. The psychological outcomes assessed included infertility distress, infertility self-efficacy, decisional conflict, marital cohesion and coping style. Program dosage and satisfaction were also assessed at four weeks follow-up.
RESULTS
Women exposed to the online program significantly improved in the area of social concerns (P = 0.038) related to infertility distress, and felt more informed about a medical decision with which they were contending (P = 0.037). Trends were observed for decreased global stress (P = 0.10), sexual concerns (P = 0.059), distress related to child-free living (P = 0.063), increased infertility self-efficacy (P = 0.067) and decision making clarity (P = 0.079). A dosage response was observed in the experimental groups for women who spent >60 min online for decreased global stress (P = 0.028) and increased self efficacy (P = 0.024).
CONCLUSIONS
This evidence-based eHealth program for women experiencing infertility suggests that a web-based patient education intervention can have beneficial effects in several psychological domains and may be a cost effective resource for fertility practices.
Keywords: distress, eHealth, infertility, internet, self-efficacy
Introduction
The study presented here describes an innovative approach to patient education and preparation, using an online format, and is based on the theoretical and empirical literature regarding: (i) the psychosocial impact of infertility, (ii) the intervention approaches that appear to improve psychological distress and (iii) computer-tailored approaches in health promotion, currently referred to as eHealth [we use the definition for eHealth programming offered by Ahearn et al. (2006)], defined as ‘the use of emerging interactive technologies (e.g. internet, CD-ROMs, personal digital assistants, interactive television and voice response systems, computer kiosks, mobile commuting) to enable health improvement and health care services (p. 2)]’. The formative research for this program has been previously described (Cousineau et al., 2004).
On the whole, empirical evidence supports the benefits of patient preparation and psychological interventions for infertile patients (Boivin, 2003; Domar, 2006). However, in practice, the majority of information and support available to couples focuses on the medical and technical aspects of consultation and treatment, and there has been relatively little information available to help patients cope with the psychosocial aspects of fertility treatment, outside of text-based information. Further, few infertile patients perceive professional psychological services as important or intend to use such services (Boivin et al., 1999; Schmidt et al., 2003). In a large European survey of infertility patients on their satisfaction with treatment, results suggest that most patients: (i) assume that both the medical and psychosocial aspects of infertility will be addressed by medical staff and (ii) expect medical staff to espouse an emotionally supportive attitude. About half of the patients expect to receive ‘documentation’ regarding psychosocial aspects of infertility and few see the necessity for psychological counseling (Schmidt et al., 2003). Notably, many people go online in pursuit of medical information or support in chat rooms although few studies have empirically addressed the effectiveness of online information or support for infertility (Epstein et al., 2002; Himmel et al., 2005).
Online psychoeducation for infertile patients
Considerable interest has grown in the potential for web-based multimedia to educate and provide support to medical patients (Ritterband et al., 2003; Wantland, et al., 2004). There has been a surge in use of the Internet for health or medical information in the USA, with 95 million Americans (80% of the online population) having done so by October 2004; and middle-aged users (30–49 years) having significantly increased their interest in medical treatment information since the last survey in 2002 (58 versus 49%) (Fox, 2005). Uptake of Internet usage and positive attitudes toward its use for health information is also rapidly rising in the European Union (EU). Approximately, 23% of people within the EU use the Internet for health information with varied usage among countries ranging from 14 to 40% (European Opinion Research Group, 2003). In terms of health information specific to infertility, several surveys of Internet use by infertile individuals report that more than half of patients go online to gather fertility-related information, regardless of socioeconomic status (Weissman et al., 2000), and that the majority find Internet forums valuable for sharing treatment news (Epstein et al., 2002; Himmel et al., 2005). Therefore, the use of eHealth or computer-mediated patient education may be an effective adjunct to routine clinical care and may extend, rather than detract from, the ability of health providers to play an important role in educating and supporting their patients.
Building upon evidence-based psychosocial interventions
To address the need for accessible patient education and skill building, the goal of this study was to develop and test the effectiveness on an online patient education and support program for infertile patients, called ‘Infertility Source: Interactive Support Tools When Trying to Conceive’ (www.infertilitysource.com; See Fig. 1. Note that this site is not yet available for public use). The program was informed largely by the ‘Health Promotion Model’ (Pender et al., 2002), a multi-faceted, biopsychosocial approach, which seeks to expand the positive potential of individuals toward health (rather than inducing fear of health consequences). The clinical literature on infertility interventions supports the use of this model. In a review of over 380 published and unpublished psychological interventions for infertile patients, 25 studies used evaluation criteria (Boivin, 2003). Of these studies, it was concluded that interventions using cognitive behavioral skill building and stress management techniques were particularly effective in reducing patient distress (Domar et al., 2000a, b). The formative evaluation process of the pilot program indicated that such a computer-mediated support tool was feasible for both patients and providers, and that a fully-functional program may serve as an effective psychosocial intervention for infertility patients (Cousineau et al., 2004).
Figure 1.

Infertility source web images
In an effort to encourage active coping and self-efficacy, a key component of the ‘Infertility Source program’, patients are asked to complete a ‘Confidence Check’, based on the ‘Infertility Self-Efficacy Scale’ (ISE) (Cousineau et al., 2006). This assessment serves as the tailoring mechanism of the online program, resulting in targeted feedback based on a high, medium or low confidence level in the areas of: ‘Taking care of yourself’; ‘Managing your feelings’; ‘Your relationship with your partner’; ‘Managing your treatment’ and ‘Your relationship with your healthcare provider’. This assessment also results in a prioritization of program content so topics most relevant to areas of lower self-efficacy are presented first on the module topic pages. ‘Infertility Source’ has been objectively evaluated by the Health on the Net (HON) Foundation and displays the HON code insignia, a minimal standard for reliability and credibility of information presented on the world wide web.
Materials and Methods
Hypotheses
The current study examined the efficacy of Infertility Source with female fertility patients. The primary hypotheses of the study examined the extent to which female participants exposed to Infertility Source demonstrated: (ia) reductions in infertility-related stress and (ib) improvements in infertility self-efficacy. Secondary hypotheses examined the extent to which exposure to Infertility Source may have influenced (iia) marital cohesion and (iib) decisional conflict. We also were interested if potential moderating variables may have influenced the effect of exposure to Infertility Source, which included various (iiia) demographic variables as well as (iiib) coping styles. The study also assessed (iv) dosage of exposure and (v) satisfaction with the program among participants in the experimental group.
Participants
Because female partners represent the majority of information seekers in infertile couples and undergo the majority of treatment interventions, the hypotheses were examined with respect to the identified female patient. Recruitment took place from April 2005 through July 2006 at three US fertility centers: Fertility Centers of Illinois, Chicago, IL; Georgia Reproductive Specialists, Atlanta, GA and the New York Hospital, Cornell Medical Center, New York, NY. The study was approved by the Institutional Review Boards of Inflexxion, Inc., registered with the Department of Health and Human Services (for the former two sites), and Weill Medical College of Cornell University. Female patients attending the clinics during the study period were considered for potential study eligibility.
Procedure (Eligibility verification)
Designated health providers at the fertility centers were trained as site liaisons by the research team for female patient recruitment. Flyers and postings on the fertility clinic websites were used, which invited female patients to participate: ‘The purpose of this study is to investigate ways to improve support services for people who are experiencing infertility and infertility treatment’, followed by eligibility criteria and study contact information. In order to reduce expectancy, participants were not aware that part of condition involved a website, only that their participation would involve use of a computer.
The site liaisons met with interested patients to verify study eligibility. Inclusion criteria were: (i) female in a heterosexual marriage or co-habitation; (ii) at least 21 years old; (iii) a diagnosis of infertility and/or history of unsuccessfully trying to conceive for twelve months or more; (iv) if a participant had secondary infertility, the existence of no more than one previous child; (v) ability to read and write English; (vi) USA resident; (vii) access to a computer with Internet either at home, work, at a medical facility, or at a local school, library, or computer center and (viii) competence to make an informed consent decision. Exclusion criteria included: (i) current involvement in a professionally-led infertility support group or workshop (or planned involvement in the next two months and (ii) females experiencing infertility without a male partner, as the first version of Infertility Source targeted the majority of fertility treatment seekers (i.e. heterosexual couples). Current pregnancy status was not an exclusion criterion. We anticipated that a small percentage of women may have received a positive pregnancy test during the one month duration of the study, but expected that randomization procedures would balance this event across groups.
Across the three sites we know that ~10 000 patients were seen at the clinics over the study period, suggesting that a large cohort of women could have been exposed to the flyers and study announcement on the clinics’ websites and e-newsletters. The average age of these fertility patients was 36 years, and the majority (90%) were married. Over 250 women expressed interest, 212 were assessed for eligibility, and of those eligible (n = 201) over 94% (n = 190) consented to participate (Fig. 2). Eligible volunteers who agreed to participate gave informed consent and were told that they would receive an email with a web link to the study questionnaires, located on a secure server accessible with a unique user identification number assigned by the research team. Recruitment aimed to include at least 10% minority patients, based on estimates of patients who seek treatment at fertility clinics (Ventura et al., 2000). We also know from the CDC’s National Survey of Family Growth data (Chandra et al., 2005) that of US women aged 15–44, 19.7% of non-White minorities reported an infertility specific service in the past year (e.g. consultation, tests, ovulation drugs, tubal surgery and ART).
Figure 2.
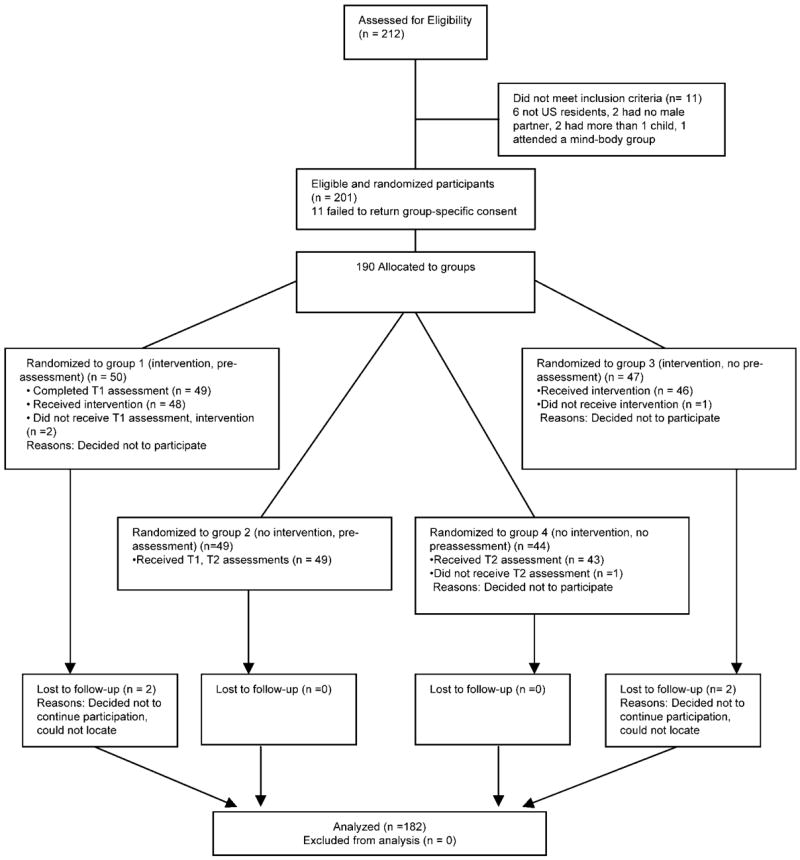
CONSORT diagram (Moher et al., 2001)
Study design
As the gold standard study design, this study employed a randomized-controlled trial comparing the online program to a no-treatment condition. Prior studies evidenced strong reactivity on the part of the target population (Domar et al., 2000a, b), wherein female participants assigned to the control condition (a waiting list for a mind-body group program for infertility) not only exhibited decreased stress but also increased pregnancy rates simply knowing that they would participate in a future program perceived to be beneficial. Moreover, we have found evidence for an effect of baseline assessments on control groups (Butler et al., 2000, 2003) in psychosocial intervention studies.
To address these concerns and to quantify reactivity, we opted to use the Solomon-four group design (Solomon, 1949; Campbell and Stanley, 1963). Specifically, the Solomon-four design calls for four group comparisons, illustrated by Campbell and Stanley (1963) (Fig. 3).
Figure 3.
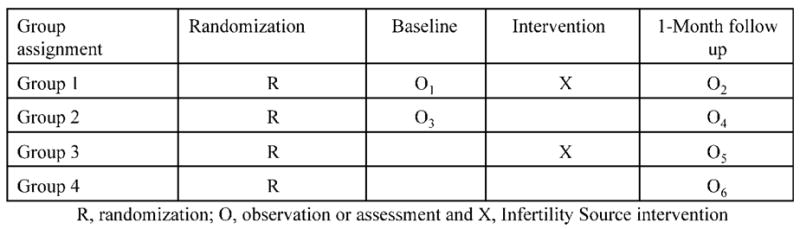
Solomon-four group design
Thus, two groups receive the experimental intervention, one with a baseline assessment and one group without. Likewise, one control group receives a baseline assessment and one does not. The Solomon-four design has been shown to address the concern of documented expectancy effects in studies on medical intervention (Koocher et al., 2002). Further justification for this approach includes: (i) there was an inherent need to control for expectancy to maximize the purity of program effects, (ii) we were not withholding beneficial support because we did not yet know if the program is effective and (iii) all participants would ultimately have access to the program at the end of the brief one-month study. Most importantly, this design permits examination of the effect of Infertility Source, the effect of the assessment/expectation of benefit, and the possible interaction of the assessment/expectation with the online intervention. In this study, the follow-up assessment occurred one month after randomization into the program, permitting the two experimental Groups (1 and 3) time to view Infertility Source.
Randomization procedure
Eligible female participants were randomized into either one of the experimental groups 1 and 3 (Infertility Source) or one of the control groups 2 and 4 (no program) (Fig. 2). Study eligibility was verified either in person or over the telephone. For those eligible, participant characteristics were collected on two stratification factors: (i) family income level (below $75K per year, at or above $75K per year) and (ii) presence of male factor infertility in the diagnosis (yes, no). The fertility clinic liaison contacted the Study Research Coordinator (M.S.) who determined group assignment for each participant via stratified block randomization using tables. Randomly permutated blocks of size four were used within each stratum for the randomization sequence. Once group assignment was determined, the clinic liaison ensured that each participant read and signed a group-specific consent form consistent with the Solomon-four study design. Participants were paid $100 for participation.
Experimental groups
The participants in Groups 1 and 3 were told that they were in a study to examine how couples cope with infertility and would receive a series of emails with web links to online questionnaires and a web program. Group 1 received the full baseline assessment; Group 3 completed the demographic information only at baseline and, after viewing the web program, they received a link to the full assessment (four weeks later). Participants were asked to view the program in a semi-structured way for two 45-min sessions or over several sittings over a four-week period in order to simulate naturalistic use of an online program. A target goal was viewing the content up to 90 min, based on an estimate of the amount of subject matter available. Participants were given general instructions to visit the Confidence Check (i.e. the tailoring mechanism of the program) and four main content areas. Web-based tracking allowed us to measure the number of visits and amount of time spent on the site, as a means of verifying the ‘dose’ received by the experimental group.
Control groups
The participants in Groups 2 and 4 were told that they were in a study to examine how couples cope with infertility and would receive a series of emails with web links to online questionnaires that they would be asked to complete. Group 2 received the full baseline assessment, whereas Group 4 only completed the demographic information at baseline and the full assessment four weeks later. Thus, by design of the Solomon-four, participants were not aware of the group to which they were randomized. After the one-month study all control participants were sent a link to Infertility Source and invited to view the program. A six-month follow up survey assessing the effects of the naturalistic use of the website, including possible relationship to pregnancy rates, is currently under analysis (manuscript in preparation).
Measures
Baseline questionnaire
Basic demographic information included: age, gender, ethnicity, marital status, education level, family income level, state of residence, cause of infertility, primary or secondary infertility, infertility treatments received for current attempt at pregnancy, resulting pregnancies and births, Internet usage for fertility problem, mental health service utilization and baseline state-trait anxiety (Spielberger, 1983). The following measures were administered at baseline and/or post-intervention following the Solomon-four design procedures. Time frame for all measures is ‘current’ unless otherwise indicated.
Primary measures Fertility problem inventory
The fertility problem inventory (FPI) is a 46-item validated questionnaire that assesses infertility related stress (Newton et al., 1999). It is one of the few extant infertility-specific measures and shows good reliability and validity (alpha = 0.93, Newton et al., 1999). Items fall into five subscales that assess distress, beliefs and attitudes related to infertility: social concern (sensitivity to comments about infertility by friends and family; feelings of social isolation); sexual concern (diminished sexual enjoyment due to scheduled sex); relationship concern (worries about the impact of infertility on the relationship); need for parenthood (close identification with role of parent or parenting as a goal in life) and rejection of childfree lifestyle (negative view of living without children or future happiness dependant on having a child/ren); and global stress, the total overall infertility related distress (based on a sum of all items), where higher scores reflect greater distress.
Infertility self-efficacy scale
The ISE was employed to measure a patient’s perception about his or her ability to engage in a set of cognitive, emotional and behavioral skills related to the medical treatment of infertility (Cousineau et al., 2006). The 10-item ISE short form was specifically validated with infertility patients and has demonstrated excellent reliability (Cronbach’s alpha = 0.94, test-retest reliability, Cousineau et al., 2006). Higher scores indicate a greater degree of self-efficacy. Sample items include: ‘I feel confident I can ignore or push away unpleasant thoughts that can upset me during medical procedures’; ‘Handle mood swings caused by hormonal treatments’; ‘Control negative feelings about infertility’ and ‘Cope with pregnant friends and family members’.
Secondary measures Ways of coping scale
The ways of coping scale (WOC) is a well-known measure designed to identify the thoughts and actions an individual has used to cope with a specific stressful encounter (Folkman and Lazarus, 1988). It has eight subscales with 66 items. The WOC scale has shown excellent reliability (Folkman and Lazarus, 1988) and modified versions have been used in other studies of infertility and coping (Klonoff-Cohen et al., 2001; Lancastle and Boivin, 2005). We were most interested in five subscales that approximate the various coping strategies for problem solving approaches relevant to managing a medical condition: escape-avoidance, planful problem solving, positive reappraisal, seeking social support and distancing.
Dyadic cohesion subscale of the RDAS
The revised dyadic adjustment scale is a frequently used instrument for measuring adjustment in relationships (Spanier, 1976; Busby et al., 1995). It has demonstrated good reliability (Guttman split half reliability coefficient = 0. 94). Higher scores indicate a greater degree of marital cohesion. The Cohesion subscale includes five questions using a five-point Likert scale on the frequency of the activity: ‘Do you and your partner engage in outside interests together; have a stimulating exchange of ideas; laugh together; calmly discuss something and work together on a project’.
Perceived negative support scale
The perceived negative support scale (PNSS) is a measure of spousal support and has been used with individuals with cancer and arthritis (Manne et al., 1999). The instructions and three items were adapted for the infertility population. It consists of 13 items rated on a 1–4 Likert scale in response to ‘How often has your partner responded in these ways during the past month while you have been in infertility treatment’. Higher scores indicate a greater degree of perceived negative support from a spouse. Sample items include: ‘seemed impatient with you; seemed not to enjoy being around you; complained about your infertility or about helping you with a task you found difficult to do yourself’. It has demonstrated good reliability (coefficient alpha = 0.86; Manne et al., 1999).
Decisional conflict scale
Decisional conflict scale (O’Connor, 1993; O’Connor et al., 2002) was also included; it is a 16-item scale measuring five decision making factors: informed (how informed or uninformed one feels regarding the decision at hand); values clarity (how clear one feels about the costs/benefits and risks about the decision); uncertainty (how sure one feels about the choice being made); support (how supported or pressured one feels about the decision making) and effective decisions (how satisfied one feels about the decision once it is made). This scale has been used for many kinds of health decisions; it has good reliability and validity (Bunn and O’Connor, 1996; Cranney et al., 2002; O’Connor, 1995). The scale has been adapted for this medical population as recommended by the scale’s author, to identify decision making related to the medical condition under study, i.e. infertility. It read: ‘are you currently facing or have you faced a medical decision around your infertility treatment?’ If yes, the participant proceeded to answer the 16-items based on a five-point Likert agreement scale (e.g. This decision is easy for me to make; I’m not sure what to do in this decision). Measurement of decisional conflict is posited as proximal to self-efficacy, where low decisional conflict promotes healthy decision making, influencing higher self-efficacy and better health outcomes (O’Connor, 1995, 2002). We also included a question on Anticipated Regret based on recommendations in the decision making literature (Janis and Mann, 1977) and based on clinical knowledge in working with this medical population. This final question read: ‘If I do not choose medical intervention (or drop out of a medical intervention) for our infertility I know that I will regret it’.
Program evaluation
Intervention participants also answered a brief satisfaction questionnaire, developed by the research team, after completion of the program. This 16-item questionnaire asked participants to respond on a 7-point Likert scale (1, not helpful, 7, extremely helpful) to a series of questions centered on overall satisfaction with content, as well as helpfulness related to coping, communication and decision making. A text field allowed participants to type in additional comments about the program.
Data analysis
Analyses were done on an intention-to-treat basis. Differences across the four study groups in sociodemographics were investigated using ANOVAs and chi-square tests, or their non-parametric equivalents. We took Braver and Braver’s (1988) suggestions for analysis of the Solomon-four design, following their specific analytic steps and culminating in the use of meta-analytic methods [briefly, Braver and Braver (1988) suggest a 2 (Group: Infertility Source, Control) × 2 (Condition: Pre-assessed, Not pre-assessed) between-group analysis of variance of the four post-intervention scores to detect the presence of the main and interaction effects. If pre-assessment effects exist (i.e. Group × Condition is significant [Test 1]),and a simple main effect for the intervention is significant in the Pre-assessed condition (Test 2) but not the Not pre-assessed condition (Test 2), one concludes that the observed effect is due entirely to pre-assessment; at this point analysis discontinues for outcomes with pre-assessment effects. If, however, there is both a statistically significant interaction (Test 1) and a significant main effect for the intervention group in the non pre-assessed condition (Test 3, i.e. those measured afterwards only), one concludes that the intervention interacts with pre-assessment by enhancing the intervention (Huck and Sandler, 1973). If no interaction is present, a main effects test for group (between-groups ANOVA) is conducted (Test 4). Lack of significance should not be considered conclusive evidence against the intervention. These analyses are followed by a two-group, independent t-test on mean ‘gain’ scores from pre-assessment to post-assessment (Test 5), then by an independent t-test on post-assessment scores for the non pre-assessed conditions (Test 6). The final test (Test 7) is a meta-analysis that combines the previous two statistical tests (i.e. meta-analyzing results from Tests 5 and 6). In this study, Zmeta = Zp1 + Zp2/√2, where Zp1 = the Z value corresponding to the P-value of Test 5, Zp2 = the Z value corresponding to the P-value of Test 6, and the 2 in the denominator indicates the number of tests involved in the meta-analysis]. This powerful analysis permits either (i) a conclusion that the intervention (in this case, Infertility Source) has an effect regardless of pre-assessment or (ii) the intervention shows no evidence of an effect. All tests were two-sided, conducted at the alpha = 0.05 level, though results that were marginally significant (i.e. P > 0.05 and <0.10) are also presented, given the tendency for Solomon-four studies to be inherently under-powered. Test statistics, test degrees of freedom, P-values and effect sizes (Cohen’s d) are reported for all outcomes. Confidence intervals around Cohen’s d for the primary and secondary outcomes were calculated when effect sizes exceeded 0.2, to highlight significant educational (≥0.25) and clinical (≥0.50) changes (Wolf, 1986).
There are several possible ways to analyze Solomon-four studies, (Braver and Braver, 1990a, 1990b; Sawilowsky and Markman, 1990a, b, c, d; Sawilowsky and Kelly, 1994). In this study, we applied the Braver and Braver approach, including classical parametric tests (i.e. ANOVA, gain score t-tests), with a meta-analysis performed on all tests unconditional on their statistical significance, thereby allowing the reader full review of all analytic approaches. To conduct the meta-analysis, any statistically significant findings of the individual parametric tests must be disregarded, a practice that increases the number of tests performed but, for illustrative purposes, provides a complete depiction of the analytic approaches considered. Following Sawilowsky and Kelley’s (1994) recommendations for optimizing power in Solomon-four hypothesis testing, when the treatment effect was moderate to large in size or when the pre-post assessment correlation was lower (i.e. rho ≤ 0.7), we focused interpretation on the meta-analytic results. When the effect size was small (and rho = 0.8) or when pre-post assessment correlation was high (i.e. rho ≥ 0.9), the classical parametric tests were highlighted.
As an exploratory analysis, we considered the effects of several moderating variables on the intervention’s efficacy, as measured by the two primary outcomes only. Moderating variables were median STAI score, family income, nature of infertility (explained versus unexplained fertility factor), median number of treatment cycles and a median split on three WOC subscales for which there was minimal intercorrelation in this sample: escape-avoidance, distancing and planful problem solving. The effect of dose of intervention, measured in total minutes spent on the site, was also investigated by comparing outcomes among those in the intervention group who spent more versus <60 min on the site. SPSS version 12.0 was used to conduct all data analyses.
Sample size calculations for our study assumed a moderate effect size for the post-assessment ANOVA intervention evaluation, defined by Cohen as d = 0.50 (Cohen, 1988). Calculations revealed that a sample size of 156 participants would result in power of 80.4%. To account for possible attrition (20%), we aimed to recruit 195 participants to yield sufficient power to detect the effects of interest.
Results
Randomization succeeded in balancing the four study groups on all baseline characteristics (Table I). Study participants averaged 34 years of age and most women were White and highly educated. A sizeable proportion of women had households with a combined income more than $75 000. Three quarters of the participants reported primary infertility (no previous biological children) and ~40% had female factor and 30% had male or male/female combined factor. This sample exhibited high anxiety, with a median STAI score above the 75th percentile for normal, age-matched females (Speilberger, 1983). Nearly equal proportions of study participants resided in states with mandated and non-mandated health insurance coverage for infertility treatments. In other words, half the sample likely had to self-pay for fertility services. Eight women dropped out of the study or were lost to follow up. There were no statistically significant differences between those who stayed and those who dropped out of the study based on group or any of the sociodemographics listed in Table I, except for a history of seeking psychological support service for infertility-related stress: 22% of those who remained in the study had sought such help compared with 80% (4 of the 5 for whom we had these data) of the women who did not complete the study (P = 0.01, Fisher’s exact test).
Table I.
Sociodemographics of female study participants.
| Characteristic | Group 1, n = 49 | Group 2, n = 49 | Group 3, n = 47 | Group 4, n = 43 |
|---|---|---|---|---|
| Age (mean, SD) | 34.53 (4.35) | 34.14 (4.29) | 34.26 (4.58) | 33.93 (4.30) |
| Ethnicity—White (%) | 39 (79.6) | 42 (85.7) | 40 (85.1) | 39 (90.7) |
| Education level | ||||
| Some/4 years college (%) | 24 (50.0) | 26 (53.1) | 31 (66.0) | 25 (58.1) |
| Graduate level (%) | 21 (42.9) | 21 (42.9) | 16 (34.0) | 18 (41.9) |
| Combined income >$75 000 (%) | 34 (69.4) | 41 (83.7) | 37 (78.7) | 34 (79.1) |
| Primary infertility factor (%) | 39 (79.6) | 36 (73.5) | 33 (70.2) | 35 (81.4) |
| Female factor (%) | 19 (38.8) | 21 (42.9) | 20 (42.6) | 14 (32.6) |
| Male factor (%) | 5 (10.2) | 6 (12.2) | 7 (14.9) | 7 (16.3) |
| Combined factor (%) | 9 (18.4) | 9 (18.4) | 5 (10.6) | 8 (18.6) |
| Unexplained factor (%) | 16 (32.7) | 13 (26.5) | 15 (31.9) | 14 (32.6) |
| Treatments | ||||
| IUI (%) | 32 (65.3) | 35 (71.4) | 21 (44.7) | 24 (55.8) |
| ART (%) | 25 (51.0) | 26 (53.1) | 20 (42.6) | 22 (51.2) |
| Mandated state health insurance coverage for infertility treatments (%) | 29 (59.2) | 32 (65.3) | 25 (53.2) | 22 (51.2) |
| Number of infertility treatment cycles pursued (mean, SD) | 3.41 (1.73) | 3.35 (1.75) | 2.62 (2.01) | 3.16 (1.80) |
| Baseline STAI score (mean, SD) | 45.55 (11.64) | 45.0 (10.09) | 42.60 (10.47) | 44.84 (11.55) |
| Hours per week using computer for personal use (mean, SD) | 4.65 (3.33) | 5.14 (5.38) | 3.55 (2.83) | 5.09 (5.03) |
| Ever sought a psychological support service related to infertility (%) | 15 (30.6) | 11 (22.4) | 9 (19.1) | 9 (20.9) |
| Ever searched for information on fertility on the internet (%) | 47 (95.9) | 48 (98) | 47 (100) | 43 (100) |
Pre-assessment effects (Tests 1–3)
The Solomon-four analytical results are presented in Table II; and means and standard deviations are presented in Table III. First, Tests 1 and 2 suggested that there were strong pre-assessment effects on two secondary outcomes: the PNSS (Test 1: F(1, 181) = 4.105, P = 0.044; Test 2: F(1,94) = 5.596, P = 0.02) and the Anticipated Regret item (Test 1: F(1181) = 3.184, P = 0.076; Test 2: F(1, 94) = 3.620, P = 0.06). Test 3 did not show intervention effects for either outcome (Test 3 PNSS: F(1,86) 0.409, P = 0.524; Anticipated regret: F(1, 86) = 0.294, P = 0.589), thus the improvement in these two measures was entirely attributed to pre-assessment and not to the intervention. No further testing for these outcomes was conducted.
Table II.
Solomon-four results.
| Primary outcomes | Test 1 | Test 2 | Test 3 | Test 4 | Test 5 | Test 6 | Test 7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group × Condition interaction | Simple main effect for Group, pre-assessed | Simple main effect for Group, not pre-assessed | Main effects test (for Group) | Independent t-test on gain scores (post- minus pre-assessment scores) | Independent t-test on post assessment scores for Groups × and 4 (not pre-assessed) | Meta-analyzed Z test | ||||||||
| FPI global stress (total score) | P = 0.10 d = 0.24 [−.15, 0.38] | |||||||||||||
| FPI subscales | ||||||||||||||
| Sexual concern | P = 0.059 d = 0.39 [−.02, 0.79] | P = 0.12 d = 0.22 [−0.17, 0.37] | ||||||||||||
| Relationship concern | ||||||||||||||
| Rejection of childfree lifestyle | P = 0.063 d = 0.38 [−0.02, 0.78] | P = 0.088 d = 0.25 [−0.22, 0.31] | ||||||||||||
| Need for parenthood | P = 0.038 d = 0.45 [0.02, 0.87] | P = 0.12 d = 0.23 [.−0.16, 0.37] | ||||||||||||
| Social concern | ||||||||||||||
| ISE | P = 0.067 d =0.27 [−0.02, 0.56] | P = 0.09 d = 0.25 [−0.15, 0.39] | ||||||||||||
| Secondary outcomes | ||||||||||||||
| Dyadic marital adjustment | ||||||||||||||
| Perceived negative spousal supporta | X P = 0.044 | X P = 0.02 | ||||||||||||
| Anticipated regreta | X P = 0.076 | X P = 0.06 | ||||||||||||
| Decisional conflict (total score) | ||||||||||||||
| DC subscales | ||||||||||||||
| Effective decision | ||||||||||||||
| Support | ||||||||||||||
| Values clarity | P = 0.079 d = 0.26 [−.14, 0.40] | |||||||||||||
| Informed | P = 0.037 d = 0.47 [0.03, 0.91] | P = 0.014 d = 0.37 [−0.03, 0.51] | ||||||||||||
| Uncertainty | ||||||||||||||
| WOC subscales | ||||||||||||||
Bold values highlight the specific tests yielding the greatest power to detect a true intervention effect, as recommended by Sawilowsky and Kelley (1994).
The outcome measure had pre-assessment reactivity and an X indicates that the interaction was significant. If the X falls in columns for tests 3–7, the result is interpretable as favoring the intervention. d, effect size, presented as Cohen’s d and their 95% confidence intervals.
Table III.
Pre/post assessment descriptive results.
| Primary outcomes | ||||||
|---|---|---|---|---|---|---|
| Pre-assessment, mean (SD)
|
Post-assessment, mean (SD)
|
|||||
| Group 1 | Group 2 | Group 1 | Group 2 | Group 3 | Group 4 | |
| FPI global stress (total score) | 162.73 (38.04) | 160.31 (34.03) | 153.74 (38.48) | 158.61 (36.07) | 155.18 (34.23) | 161.93(31.74) |
| FPI subscales | ||||||
| Sexual concern | 28.63 (9.76) | 26.53 (10.33) | 25.39 (10.00) | 26.00 (10.44) | 26.39 (9.71) | 26.98 (9.89) |
| Relationship concern | 24.92 (8.67) | 26.16 (8.84) | 24.63 (9.75) | 26.20 (9.63) | 25.48 (10.11) | 24.67 (8.99) |
| Rejection of childfree lifestyle | 29.49 (8.71) | 28.88 (9.38) | 27.39 (9.48) | 29.12 (9.29) | 28.95 (9.58) | 29.84 (7.55) |
| Need for parenthood | 42.71 (9.13) | 42.73 (7.59) | 40.39 (9.20) | 41.69 (6.97) | 40.59 (8.55) | 41.88 (7.35) |
| Social concern | 36.98 (12.67) | 36.00 (13.24) | 35.93 (12.09) | 35.59 (13.44) | 33.77 (11.14) | 38.56 (9.97) |
| ISE scale | 51.41 (16.92) | 50.10 (17.46) | 56.06 (17.80) | 52.14 (20.32) | 55.91 (15.63) | 50.09 (16.30) |
| Secondary outcomes | ||||||
| Pre-assessment, mean (SD) | Post-assessment, mean (SD) | |||||
| Group 1 | Group 2 | Group 1 | Group 2 | Group 3 | Group 4 | |
| Dyadic adjustment | 17.65 (3.14) | 17.12 (3.63) | 17.76 (3.33) | 17.16 (3.59) | 16.32 (3.55) | 17.09 (3.75) |
| PNSSa | 21.22 (7.82) | 22.10 (6.57) | 19.20 (6.10) | 22.53 (7.52) | 22.07 (7.96) | 21.00 (7.60) |
| Anticipated regreta | 1.90 (1.10) | 1.53 (.84) | 2.20 (1.20) | 1.76 (1.05) | 1.59 (1.00) | 1.70 (.83) |
| Decisional conflict (total score) | 2.34 (.73) | 2.24 (.83) | 2.16 (.64) | 2.17 (.79) | 2.07 (.80) | 2.24 (.65) |
| DC subscales | ||||||
| Effective decision | 2.05 (.71) | 2.01 (.88) | 2.01 (.71) | 1.85 (.79) | 1.86 (.86) | 1.88 (.60) |
| Support | 2.27 (.85) | 2.14 (.92) | 2.06 (.63) | 2.04 (.80) | 2.05 (.97) | 2.22 (.75) |
| Values clarity | 2.29 (.89) | 2.15 (1.09) | 2.13 (.77) | 2.22 (.96) | 2.00 (.92) | 2.20 (.81) |
| Informed | 2.16 (.82) | 1.87 (.72) | 1.91 (.69) | 1.99 (.88) | 1.81 (.81) | 2.09 (.90) |
| Uncertainty | 3.04 (1.16) | 3.13 (1.26) | 2.72 (1.06) | 2.87 (1.30) | 2.72 (1.14) | 2.91 (1.25) |
The outcome measure had pre-assessment reactivity.
Intervention effects for primary outcomes (Tests 4–7): hypotheses (ia and b)
Fertility problem index
The global FPI global stress scores were lower for intervention than control groups, a finding of marginal statistical significance (Test 7: Zmeta-analysis = 1.63, P = 0.10, d = 0.19). Three key subscales of the FPI indicated a statistically significant improvement for the intervention group compared with the control group. A reduction on the FPI sexual concern subscale was greater in the intervention than in the control group (Test 5: t94 = 1.92, P = 0.059, d = 0.39; Test 7: Zmeta-analysis = 1.53, P = 0.12, d = 0.22), an average of 2.25 points lower for those exposed to Infertility Source. The reduction in scores related to rejection of a childfree lifestyle was also greater (average 1.68 points lower) for intervention than control group participants (Test 5: t94 = 1.88, P = 0.063, d = 0.38; Test 7: Zmeta-analysis = 1.71, P = 0.088, d = 0.25). Finally, there was evidence of lower social concern for women exposed to the intervention compared with those in the control group (Test 6: F(1, 86) = 4.449, P = 0.038, d = 0.45; Test 7: Zmeta-analysis = 1.57, P = 0.12, d = 0.23), an average of 4.79 points lower.
Infertility self-efficacy scale
The intervention group demonstrated a marginally significant increase in self-efficacy compared with the control group (Test 4: F(1, 181) = 3.39, P = 0.067, d = 0.27; Test 7: Zmeta-analysis = 1.68, P = 0.093, d = 0.25).
Intervention effects for secondary outcomes (Tests 4–7): hypotheses (iia and b)
There were no changes over time for any of the groups on the dyadic cohesion subscale of the dyadic adjustment scale. Global scores for decisional conflict did not change among either the intervention or control group. However, changes over time in response to the intervention on two subscales are notable. Compared to the control group, those receiving Infertility Source improved their scores on the informed sub-scale indicating they felt less “uninformed” about an infertility-related decision they are struggling with (Test 5: t78 = 2.12, P = 0.037, d = 0.47; Test 7: Zmeta-analysis = 2.46, P = 0.014, d = 0.36) and increased the degree of clarity they feel about the benefits and risks of an infertility-related decision on the values clarity subscale (Test 7: Zmeta-analysis = 1.75, P = 0.079, d = 0.26). The subscales comprise two of the three modifiable factors related to uncertainty around a decision (O’Connor, 1993, 2002).
Moderators of the intervention (iiia and b)
The intervention had varying effects on the primary outcomes for women with certain demographic and clinically-relevant characteristics compared with their counterparts in the control group (Table IV).
Table IV.
Participant characteristics as moderators of the intervention.
| Moderator | Intervention result | Test statistic, degrees of freedom, P-value and effect size |
|---|---|---|
| Anxiety/higha | Lower sexual concern | t48 = −2.46, P = 0.018, d = 0.71 |
| Treatment cycles ≥4 | Lower sexual concern | t44 = −2.01, P = 0.05, d = 0.61 |
| Lower rejection of childfree lifestyle | t44 = −3.35, P = 0.002, d = 1.01 | |
| Explained infertilityb | Lower sexual concern | t65 = −2.01, P = 0.049, d = 0.50 |
| Higher self-efficacy | Z meta-analysis = 1.93, P = 0.05, d = 0.48 | |
| Higher income (>$75 000) | Higher self-efficacy | t66 = −2.45, P = 0.02, d = 0.60 |
| Lower sexual concern | t72 = −2.38, P = 0.02, d = 0.56 | |
| lower rejection of child free living | t53.49 = −2.60, P = 0.01, d = 0.71 | |
| Lower social concern | t66 = −1.97, P = 0.05, d = 0.48 | |
| Lower global FPI stress | t72 = −2.05, P = 0.04, d = 0.48 | |
| WOC/high escape-avoidance | Lower rejection of childfree lifestyle | t45 = −2.52, P = 0.047, d = 0.75 |
| Lower social concern | t28.1 = −1.98, P = 0.049, d = 0.75 | |
| WOC/low planful problem solving | Lower sexual concern | t54 = −2.86, P = 0.006, d = 0.78 |
| Lower social concern | t46 = −3.22, P = 0.002, d = 0.95 | |
| Higher self-efficacy | Zmeta-analysis = 2.14, P = 0.033, d = 0.63 | |
| WOC/high distancing | Higher global FPI stress | F(1, 181) = 8.51, P = 0.004, d = −0.42 |
| Higher sexual concern | F(1, 181) = 8.32, P = 0.004, d = −.39 | |
| WOC/low distancing | Lower global FPI stress | F(1, 181) = 8.51, P = 0.004 d = 0.48 |
| Lower sexual concern | F(1, 181) = 8.32, P = 0.004, d = 0.50 |
High anxiety, STAI score of 43.5 or greater;
Explained infertility, male, female, or male and female factor infertility known to study participant. WOC, Ways of coping subscales.
Anxiety
Anxiety levels were split at the sample’s median STAI score (43.5) for these analyses since the scores were highly skewed and normal female adult median cut-offs were less meaningful for this sample. The results showed that for those participants with higher STAI scores, Infertility Source was effective in reducing women’s scores on the FPI sexual concern subscale (t48 = −2.46, P = 0.018, d = 0.71) compared with their counterparts in the control group.
Income
Income was not related to living in a state with mandated infertility treatment health care coverage, number of treatment cycles, nor cause of the infertility. However, women with higher income (>$75K) who were exposed to the intervention had lower scores on the FPI (t72 = −2.05, P = 0.04, d = 0.48) and higher ISE (F(1141)] = 3.79, P = 0.054, d = 0.33; t66 = −2.45, P = 0.02, d = 0.60). Additionally, bivariate analysis revealed that lower income level was associated with higher STAI scores (chi-square = 4.415, P = 0.036). In other words, income and anxiety are highly related.
Explained versus unexplained fertility diagnosis
Women with an explained fertility factor who received the intervention had lower FPI sexual concern scores (t65 = −2.01, P = 0.049, d = 0.50) and higher ISE scores (Zmeta-analysis = 1.93, P = 0.05, d = 0.48) compared with their counterparts in the control group.
Frequency of fertility treatment
For women who had ‘high treatment frequency’ (i.e. equal or greater than the median number of four treatment cycles), exposure to Infertility Source had the effect of decreasing their scores on the FPI sexual concern (t44 = −2.01, P = 0.05, d = 0.61) and rejection of childfree lifestyle subscales (t44 = −3.35, P = 0.002, d = 1.01).
Coping
Of the five WOC subscales measured, the most frequently endorsed coping styles in this sample of women were escape-avoidance (33%) and positive reappraisal (26.5%). However, no matter which coping strategy a participant endorsed most, it only accounted for one-third (30–35%) of all possible coping styles used. That is, participants used a variety of coping strategies. To reduce the number of tests undertaken, we chose the escape-avoidance, planful problem solving and distancing subscales to explore as possible moderators of the intervention effects, as these subscales were not highly inter-correlated in this sample, thereby reducing the redundancy of the moderator analyses and minimizing Type I errors.
The effects of the intervention were evident among those with higher median scores (>= 8) of the escape-avoidance subscale for the FPI rejection of childfree lifestyle (t45 = −2.52, P = 0.047, d = 0.75) and social concern (t28.1 = −1.98, P = 0.049, d = 0.75). Women who planful problem solve less often (i.e. score <8) and were exposed to Infertility Source had lower FPI sexual concern scores (t54 = −2.86, P = 0.006, d = 0.78), lower FPI social concern scores (t46 = −3.22, P = 0.002, d = 0.95) and had increased ISE (Zmeta-analysis = 2.14, P = 0.033, d = 0.63) than their counterparts in the control group. Finally, among women who tended to employ distancing coping style (i.e. scores>= 5), the FPI global stress scores increased significantly after exposure to Infertility Source (F(1181) = 8.51, P =0.004, d = 0.42), while the trend was reversed for those who were not using distancing coping as often. A similar pattern was repeated with the FPI sexual concern subscale (F(1181) = 8.32, P = 0.004, d = 0.39).
Dosage (iv)
The participants (n = 93) exposed to Infertility Source visited the program an average of four times and median time spent on the site was 63 min (mean = 76.53; SD = 60.08) over a range of 0–332 min. Of the intervention group, 36% spent the estimated appropriate ‘dose’ of 90 min or more on the site; 50% spent 60 min or more on the site’. An exploratory analysis among the intervention participants comparing women who spent <60 min on the site to those who spent more suggested a dose response. Compared with women who spent less time on the site, those who used Infertility Source for 60 or more minutes manifested lower FPI global stress scores (t44 = 2.27, P = 0.028, d = 0.68), lower FPI rejection of childfree lifestyle scores (t44 = 2.33, P = 0.025, d = 0.70), and had greater gains in their ISE (t44 = −2.34, P = 0.024, d = 0.71). Improvements on other outcomes (i.e. Decisional Conflict subscales of values clarity and uncertainty, FPI social concern and sexual concern subscales) were affected by exposure to but not amount of time spent on the site.
Program evaluation (v)
No adverse events were reported to site liaisons or to the researchers over the duration of the study. The satisfaction survey for Infertility Source revealed how acceptable the participants (n = 90) found the program content, using rating scales from 1 to 7. For instance, they found the medical information to be ‘informative’ [median rating 6.0 interquartile range (IOR) 3]; ‘helpful’ (median rating 5.0, IQR 2) ‘made efficient use of one’s time’ (median rating 6.0, IQR 3) and was more helpful ‘compared with other websites’ (median rating 6.0, IQR 3). Twelve percent of women reported that there were ways in which the program made them feel more distressed about their situation. Looking at their written explanations, participants typically wrote about their frustrations with their situation beyond the viewing of Infertility Source. For instance, a woman with secondary infertility wrote: ‘The only way that it made me feel more stressed is that I have had some distance from infertility and treatment since the pregnancy and birth of my son. However, we are getting ready to try for a second child, so I have had to think about what that means.’ Another woman wrote: ‘[I was] only distressed in that the site made me realize I’ve just begun what may be a very long journey. I don’t think I would want to do this for the length others have done this.’ And another: ‘I think just focusing on the feelings associated with my infertility is depressing—even if I’m doing something (or reading something) that will help me take action to feel better later. I don’t think this can be avoided.’ Such comments are expected for a psychosocial program targeted at a specific medical condition.
Discussion
Virtually all women in this study spent time searching for infertility information on the Internet, suggesting that a high-quality psychosocial program could be a viable resource for women struggling with fertility issues. With respect to the first hypothesis, we found that women exposed to Infertility Source significantly reduced their social concerns, an aspect of infertility problem distress related to how women negotiate relations with family and friends around their fertility problems. We attribute this to the program content which emphasized strategies and skills to help patients manage relationships, particularly in dealing with pregnant family and friends. Trends for a reduction in global fertility related distress, sexual concerns and negative perceptions about childfree living were also observed. Similarly, women exposed to Infertility Source exhibited a trend for improvement in self-efficacy related to the management of infertility treatment. Effect sizes for the primary and secondary outcomes ranged from small (d = 0.24) to moderate (d = 0.47). Even a small effect size of an intervention that reduces a prevalent problem in a patient population (i.e. fertility-related stress) can have a meaningful public health impact.
Importantly, Infertility Source facilitated aspects of decision-making, our second hypotheses. The findings suggest that participants exposed to Infertility Source perceived they were more informed and clear about the costs and benefits surrounding the decision they were grappling with compared with control participants. Given that patients are not likely to seek mental health services in spite of high levels of anxiety and infertility-related distress, this finding is highly relevant. A fairly non-threatening online program may be beneficial for patients contending with treatment-related distress and may help them persist in treatment or decide to end treatment sooner. Such a question would be of interest for future research. The program did not appear to have an effect on the dyadic marital measure. It may be that couples view their marriages as cohesive despite the current medical challenge.
It also appears that participant characteristics had moderating effects on the intervention. There were subgroups of women for whom Infertility Source was more advantageous. These were women who were more anxious, further along in their treatment experience, had higher incomes, and had an explained infertility diagnosis. It may be that the program was beneficial in helping the more anxious participants put in perspective the role of treatment and the burden on sexual relations (e.g. viewing treatment as a temporary disruption, having a sense of humor and empathy for spouse). The relationship between socioeconomic status and anxiety in this medical population warrants further inquiry, including how interventions may support these couples around their fertility treatment and financial burden. That the program was more effective for women with explained fertility problems suggests that a next version of the program more pointedly address the distress common to people with idiopathic infertility whose course of treatment is less clear (Covington and Burns, 2006). Infertility Source also had positive effects for participants who had completed four or more fertility treatment cycles and were thus farther along in their fertility treatment experience. In particular, the program reduced sexual concerns and negative perceptions related to living a childfree lifestyle.
Coping style also moderated the intervention effects. We know from prior infertility studies that women tend to use more coping styles relative to men (Klonoff-Cohen et al., 2001; Peterson et al., 2006) and that some coping styles are more health promoting than others depending on the circumstances. The program was beneficial for those women who tended to use escape-avoidance coping often or who did not frequently employ planful problem solving. For women who often employed distancing, the program heightened their infertility-related distress levels. We suggest that for those engaged in strategies to put the infertility experience out of their minds, the program essentially forced them to face various issues, raising their infertility distress. Further refinement of computer-tailored approaches can target these subgroups of women with individualized messaging and direct them to various skills building and ameliorating activities. Use of Infertility Source in conjunction with counseling and stress management may enhance positive effects in these domains and suggests a future avenue of study.
This study has several methodologically relevant findings. Sawilowsky and Kelley (1994) recommended a more nuanced application of Braver and Braver’s approach in analyzing Solomon-four designs. This study provides illustration of their recommendations, as different outcomes required interpretation of different statistical tests to maximize power. The pre-post assessment decisional conflict scale scores did not correlate >0.7, hence, the meta-analytic findings are more powerful. However, the FPI and ISE scale had higher pre-post assessment correlations, larger effect sizes (i.e. large Cohen’s d) or both, supporting the interpretation of the more statistically powerful classic parametric findings.
We also explored if the study was underpowered. Post hoc power calculations on the outcomes of marginal significance revealed that, under the tests that used half of the sample (Tests 5 and 6), power ranged from 0.053 to 0.57. Sample sizes would need to have at least doubled for outcomes of marginal significance and small effect size to be detected at the P < .05 level. Informed by these findings, analyses of intervention effects in two hypothetical scenarios were conducted: (i) doubling the Solomon four’s sample size and 2) a pre-post test design (simulations available upon request). As expected, a larger sample size reduced the standard errors of the means, making it easier to detect a true difference if one existed, and a hypothetical full pre-post test design returned significant results for those scales with high pre-post correlations. In both cases, the findings reported in this study would have exhibited greater statistical significance, indicating that the design was underpowered. Finally, because confidence intervals are a function of the standard error and influenced by sample size employed in the specific test, a larger sample size or a full pre-post design would have rendered tighter confidence intervals around the parametric test’s effect sizes and smaller P-values in general. Given that Infertility Source is a web-based intervention and could potentially reach a large constituency of patients or persons struggling with infertility in a relatively short time, we are hopeful that this intervention will prove beneficial in the practical and naturalistic environment of online help-seeking.
There are both strengths and limitations to this study. This study is limited to infertility patients actively seeking medical care, and does not attend to a large cohort of subfertile women who do not seek treatment (White et al., 2006), thereby limiting the generalizability of our findings. However, this program was specifically designed for treatment seekers as an adjunct psychoeducational support program in medical settings. In fact, this cohort likely reflects those who will eventually be users of an online program like Infertility Source. The participants also reported a range of fertility issues over time, and we do not know if the program may have different outcomes for individuals with specific diagnoses, e.g. first-time IVF patients and patients with complicating medical conditions. Various program elements may be more relevant at different points in fertility treatment. Although we used no-treatment comparison groups to control for the effect of the online intervention, it does not rule out demand characteristics (e.g. help-seeking).
Additional limitations include the study incentive, the self-report nature of the data and the relatively short follow-up duration period that limited the use of biological outcomes such as pregnancy attainment. The study was likely underpowered to test several of the outcomes, though simulations support interpretation of the findings. The study was likely also under-powered to test moderators other than those variables used in the stratified randomization (i.e. explained/unexplained fertility and income). Nevertheless, such analyses were undertaken and presented to explore intervention effects among subgroups of clinical relevance: women employing different coping styles, with high anxiety, and of varied treatment experience. Replication of these exploratory subgroup findings in a study adequately powered to test them is preferable. Since participants could use the program at home or work to approximate natural day-to-day settings in which one may use the Internet, (versus a controlled environment), we cannot be certain the intervention dosage was in fact accurate (e.g. participant may have left their computers to do other things).
There was a high participation rate in this study. This may be in part due to the short duration of the study and/or the incentive. However, the program evaluation findings suggest women were highly motivated and enjoyed the site in spite of any distress that such focused attention on the program may have engendered. An additional strength was that participants came from demographically and geographically diverse groups. That the entire sample had an equal proportion of participants residing in states with and without health insurance mandates for fertility treatment is also a strength, diminishing the likelihood that treatment effects were influenced by this variable. Notably, the 15% minority sample is one of the largest in infertility-related intervention studies to date and may be more representative of the diversity of women who seek infertility services and treatments (Chandra et al., 2005). Thus, the study results are likely externally valid.
The positive effects on women who used Infertility Source are promising. This study appears to be the first randomized, controlled online eHealth study for women experiencing infertility. Previous work in the area of infertility support interventions has focused primarily on group and couple interventions. Comparatively, as a self-guided program, Infertility Source is less resource intensive and potentially more cost-effective than face-to-face interventions. Given the literature that infertility patients are not apt to seek supportive services until prolonged and failed treatment cycles ensue, Infertility Source may be of interest to patients not yet ready to seek counseling or to those uncomfortable with group or couple formats. Psychological interventions can produce beneficial outcomes including reduction of distress (Boivin, 2003), and it appears that Infertility Source represents one such viable intervention. Future analyses will address the effects of the program on male partners, couple interactions, and personal belief systems, and will contribute to further refinement of Infertility Source.
Acknowledgments
We thank Rosalind King, NICHD Project Officer for her support of this program. The authors are grateful for the expertise of our consultants: diane Clapp, BSN, RN; Alice D. Domar, PhD of the Domar Center for Mind-Body Health Complementary Healthcare; Carole Lesser, MSN, RNP and Brian Berger, MD, Boston IVF; and James Clyde Sellman, PhD. We thank the clinical site research coordinators: Jennifer Clair, BSN, MS at Georgia Reproductive Specialists; Jennifer Brown at the Fertility Centers of Illinois; and Andrea Felton, Janelle Lamoreaux and Penny Peterson, at The Center for Reproductive Medicine and Infertility, The New York Hospital—Cornell Medical Center.
Funding
This project was supported by the National Institute of Child Health and Human Development, a Division of the National Institutes of Health (NIH), grant R44HD39066-02.
References
- Ahearn DK, Kreslake JM, Phalen JM. What is eHealth (6): perspectives on the evolution of eHealth research. J Med Internet Res. 2006;8:e4. doi: 10.2196/jmir.8.1.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin J. A review of psychosocial interventions in fertility. Soc Sci Med. 2003;57:2325–2341. doi: 10.1016/s0277-9536(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Boivin J, Scanlan LC, Walker SM. Why are infertile patients not using psychosocial counselling? Hum Reprod. 1999;14:1384–1391. doi: 10.1093/humrep/14.5.1384. [DOI] [PubMed] [Google Scholar]
- Braver MCW, Braver SL. Statistical treatment of the Solomon four-group design: a meta-analytic approach. Psychol Bull. 1988;104:150–154. [Google Scholar]
- Braver SL, Walton Braver MC. Meta-analysis for Solomon four-group designs reconsidered: A reply to Sawilowsky and Markman. Perceptual and Motor Skills. 1990a;71:321–322. [Google Scholar]
- Braver SL, Walton Braver MC. Switching replications research design: What, why, when, and how. Paper presented at the annual meeting of the American Educational Research Association; Boston. April, 1990b. [Google Scholar]
- Bunn H, O’Connor A. Validation of client decision-making instruments in the context of psychiatry. Can J Nurs Res. 1996;28:13–27. [PubMed] [Google Scholar]
- Busby DM, Christiansen C, Crane DR, Larson JH. The revision of the Dyadic Adjustment Scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. J Marital Fam Ther. 1995;21:289–308. [Google Scholar]
- Butler SF, Budman SH, Beardslee WR. Risk reduction in children from families with parental depression: a videotape psychoeducation program. National Academies of Practice Forum: Issues in Interdisciplinary Care. 2000;2:267–276. [Google Scholar]
- Butler SF, Chiauzzi E, Bromberg JI, Budman SH, Buono DP. Computer-assisted screening for alcohol problems in primary care. J Tech Hum Serv. 2003;21:1–19. [Google Scholar]
- Campbell D, Stanley J. Experimental and Quasi-experimental Designs for Research. Chicago: Rand McNally; 1963. [Google Scholar]
- Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 2005;23:25. [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. New York: Academic Press; 1988. [Google Scholar]
- Cousineau TM, Lord SE, Seibring AR, Corsini EA, Viders JC, Lakhani SR. A multimedia psychological support program for couples receiving infertility treatment: a feasibility study. Fertil Steril. 2004;81:532–538. doi: 10.1016/j.fertnstert.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Cousineau TM, Green TC, Corsini EA, Barnard T, Seibring AR, Domar AD. Development and validation of the Infertility Self-Efficacy scale. Fertil Steril. 2006;85:1684–1696. doi: 10.1016/j.fertnstert.2005.10.077. [DOI] [PubMed] [Google Scholar]
- Covington SN, Burns LH. Infertility Counseling: A Comprehensive Handbook for Clinicians. 2. New York: Cambridge University Press; 2006. [Google Scholar]
- Cranney A, O’Connor AM, Jacobsen MJ, Tugwell P, Adachi JD, Ooi DS, Waldegger L, Goldstein R, Wells GA. Development and pilot testing of a decision aid for postmenopausal women with osteoporosis. Patient Educ Couns. 2002;47:245–255. doi: 10.1016/s0738-3991(01)00218-x. [DOI] [PubMed] [Google Scholar]
- Domar AD. Psychological stress and infertility. In: Rose BD, editor. UpToDate. Wellesley, MA: UptoDate; 2006. [Google Scholar]
- Domar A, Clapp D, Slawsby EA, Dusek J, Kessel B, Freizinger M. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril. 2000a;73:805–811. doi: 10.1016/s0015-0282(99)00493-8. [DOI] [PubMed] [Google Scholar]
- Domar A, Clapp D, Slawsby EA, Kessel B, Orav J, Freizinger M. The impact of group psychological interventions on distress in infertile women. Health Psychol. 2000b;19:568–575. doi: 10.1037//0278-6133.19.6.568. [DOI] [PubMed] [Google Scholar]
- Epstein YM, Rosenberg HS, Grant TV, Hemenway N. Use of the Internet as the only outlet for talking about infertility. Fertil Steril. 2002;78:507–514. doi: 10.1016/s0015-0282(02)03270-3. [DOI] [PubMed] [Google Scholar]
- European Opinion Research Group. [2003, last date accessed];European Union Citizens and Sources of Information about Health. http://ec.europa.eu/health/ph_information/documents/eb_58_en.pdf.
- Folkman S, Lazarus RS. The Ways of Coping Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1988. [Google Scholar]
- Fox S. [2005, last date accessed];Internet Health Resources. Pew Internet & American Life Project May 17, 2005. http://www.pewinternet.org.
- Himmel W, Meyer J, Kochen MM, Michelmann HW. Information needs and visitors’ experience of an internet expert forum on infertility. J Med Internet Res. 2005;7:e20. doi: 10.2196/jmir.7.2.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck S, Sandler HM. A note on the Solomon 4-group design: appropriate statistical analyses. J Exper Educ. 1973;42:54–55. [Google Scholar]
- Janis IL, Mann L. Decision Making: A Psychological Analysis of Conflict, Choice, and Commitment. New York: Free Press; 1977. [Google Scholar]
- Klonoff-Cohen H, Chu E, Natarajan L, Sieber W. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76:675–687. doi: 10.1016/s0015-0282(01)02008-8. [DOI] [PubMed] [Google Scholar]
- Koocher GP. Using the CABLES Model to Assess and Minimize Risk in Research: Control group hazards. Ethics & Behavior. 2002;12:75–86. doi: 10.1207/S15327019EB1201_5. [DOI] [PubMed] [Google Scholar]
- Lancastle D, Boivin J. Dispositional optimism, trait anxiety, and coping: unique or shared effects of biological response to fertility treatment. Heath Psychol. 2005;24:171–178. doi: 10.1037/0278-6133.24.2.171. [DOI] [PubMed] [Google Scholar]
- Manne SL, Pape SJ, Taylor KL, Doherty J. Spouse support, coping, and mood among individuals with cancer. Ann Behav Med. 1999;21:111–121. doi: 10.1007/BF02908291. [DOI] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]
- Newton CR, Sherrard W, Glavac I. The Fertility Problem Inventory: measuring perceived infertility distress. Fertil Steril. 1999;72:54–62. doi: 10.1016/s0015-0282(99)00164-8. [DOI] [PubMed] [Google Scholar]
- O’Connor AM. Decisional conflict. In: Thompson JM, McFarland GK, Hirsch JE, Tucker JS, editors. Mosby’s Clinical Nursing. 3. Toronto, Ontario: The CV Mosby Co; 1993. pp. 1554–1555. [Google Scholar]
- O’Connor AM. Validation of the Decisional Conflict Scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Jacobsen MJ, Stacey D. An evidence-based approach to managing women’s decisional conflict. J Obstet Gynecol Neonatal Nurs. 2002;31:570–581. doi: 10.1111/j.1552-6909.2002.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Pender NJ, Murdock CL, Parsons M. Health Promotion in Nursing Practice. Upper Saddle River, NJ: Prentice Hall; 2002. [Google Scholar]
- Peterson BD, Newton CR, Rosen KH, Skaggs GE. Gender differences in how men and women who are referred for IVF cope with infertility stress. Hum Reprod. 2006;21:2443–2449. doi: 10.1093/humrep/del145. [DOI] [PubMed] [Google Scholar]
- Ritterband LM, Gonder-Frederick LA, Cox DJ, Clifton AD, West RW, Borrowitz SM. Internet interventions: in review, in use, and into the future. Prof Psychol: Res Prac. 2003;34:527–534. [Google Scholar]
- Sawilowsky SS, Kelley DL. Meta-analysis and the Solomon four-group design. J Exp Educat. 1994;62:361. [Google Scholar]
- Sawilowsky SS, Markman BS. Meta-~analysis and the Solomon four-group design. Paper presented at the annual meeting of the American Educational Research Association; Boston. 1990a. [Google Scholar]
- Sawilowsky SS, Markman BS. ERIC Document Reproduction Service No. ED 316 556. 1990b. Power of meta-analysis in the Solomon four-group design. [Google Scholar]
- Sawilowsky SS, Markman BS. Another look at the power of meta-analysis in the Solomon four-group design. Perceptual and Motor Skills. 1990c;70:177–178. [Google Scholar]
- Sawilowsky SS, Markman BS. Rejoinder to Braver and Braver. Perceptual and Motor Skills. 1990d;71:424–426. [Google Scholar]
- Schmidt L, Holstein BE, Boivin J, Tjornhoj-Thomsen T, Blaabjerg J, Hald F, Rasmussen PE, Nyboe Anderson A. High ratings of satisfaction with fertility treatment are common: findings from the Copenhagen Multi-Centre Psychosocial Infertility Research Programme. Hum Reprod. 2003;18:2638–2646. doi: 10.1093/humrep/deg505. [DOI] [PubMed] [Google Scholar]
- Solomon RL. An extension of control group design. Psychol Bull. 1949;46:137–150. doi: 10.1037/h0062958. [DOI] [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38:15–28. [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Ventura S, Mosher W, Curtin S, Adma J, Henshaw S. Trends in pregnancies and pregnancy rates by outcome: estimates for the United States, 1976–1996. National Center for Health Statistics. Vital Health Stat. 2000;21:65. [PubMed] [Google Scholar]
- Wantland DJ, Portillo C, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of Web-based vs. non-Web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res. 2004;6:e40. doi: 10.2196/jmir.6.4.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A, Gotlieb L, Ward S, Greenblatt E, Casper RF. Use of the Internet by infertile couples. Fertil Steril. 2000;73:1179–1182. doi: 10.1016/s0015-0282(00)00515-x. [DOI] [PubMed] [Google Scholar]
- White L, McQuillan J, Greil AL. Explaining disparities in treatment seeking: the case of infertility. Fertil Steril. 2006;85:853–857. doi: 10.1016/j.fertnstert.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Wolf FM. Meta-analysis: Quantitative Methods for Research Synthesis. Beverly Hills, CA: Sage; 1986. [Google Scholar]


